ABSTRACT
Chromosomal polymorphisms involve heterochromatic regions and occur in the general population. However, previous studies have reported a higher incidence of these variants in infertile patients. The aim of this study was to examine the relationship between polymorphic variants and infertility and their association with aneuploidies in male gametes and embryos. We retrospectively considered 1,551 cytogenetic studies involving infertile patients (study group; n=866) and oocyte/sperm donors as the control group (n=685). We had detected 168 polymorphisms in the study group and 92 in the control group. An increase in the frequency of polymorphic variants was observed among infertile patients (19.4% study group vs. 13.4% control group; P < 0.01). Sperm aneuploidies among 145 infertile men were evaluated by fluorescent in situ hybridization (FISH). The frequency of infertile men with increased rates of sperm aneuploidy was higher among polymorphism carriers. Twenty men showed an abnormal rate of sperm aneuploidy in the carrier group (n=53) vs. 15 in the non-carrier group (n=92) (37.7% vs. 16.3%, respectively; P < 0.01). Finally, aneuploidies in blastocysts (n=301) resulting from donated oocytes were also examined by array comparative genomic hybridization (array-CGH). Significant differences were reported in the embryo aneuploidy rate between female carriers and non-carriers in oocyte donation cycles (50.0% vs. 27.6%; P < 0.001). This study suggests that polymorphic variants have an impact on fertility. Moreover, our results show a relationship between polymorphisms and aneuploidy in spermatozoa and embryos.
Abbreviations: FISH: fluorescent in situ hybridization; CGH: comparative genomic hybridization; ESHRE: European Society of Human Reproduction and Embryology; ASRM: American Society for Reproductive Medicine; RPL: recurrent pregnancy loss; WHO: World Health Organization; ISCN: International System for Human Cytogenetic Nomenclature guidelines; WGA: whole genome amplification; SPSS: Statistical Package for Social Sciences
Introduction
Structural chromosomal abnormalities are responsible for reproductive conditions such as infertility and recurrent pregnancy loss [Düzcan et al. Citation2003]. In addition to Robertsonian and reciprocal translocations and inversions, there are also polymorphic variants on chromosomes that are considered ‘normal’ by cytogeneticists because they involve heterochromatic regions and occur in the general population without apparent clinical significance [Borgaonkar Citation1997]. However, previous studies have reported that the incidence of these variants is higher in infertile patients [Madon et al. Citation2005], couples suffering from recurrent miscarriage [Iyer et al. Citation2007], and in men with poor sperm quality [Nakamura et al. Citation2001; Nagvenkar et al. Citation2005; Collodel et al. Citation2006]. These variants are quantitative/positional modifications of constitutive heterochromatin. Constitutive heterochromatin consists of highly repetitive satellite DNA sequences located on chromosomes 1, 9, and 16 (centromeric region); the distal end of the Y chromosome long arm; and the short arms of acrocentric chromosomes (D/G group).
The heterochromatic variants most frequently associated with infertility are variants of chromosomes 9 and the Y chromosome. Chromosome 9 presents the highest degree of morphological variation among non-acrocentric human chromosomes as the pericentromeric region (between 9p11-12 and 9q11-12/13) is rich in heterochromatin [Humphray et al. Citation2004]. The most common polymorphisms are pericentric inversions followed by 9qh+ and 9qh- [Verma et al. 1978]. Chromosome 9 pericentric inversions are the most frequent inversions in the general population [Ferguson-Smith Citation1974]. There are varying published opinions regarding their association with infertility [Teo et al. Citation1995], recurrent miscarriages, and congenital abnormalities [Uehara et al. Citation1992]. Recent compelling evidence has proven that the presence of a pericentric inversion on chromosome 9 is correlated with an increased rate of sperm aneuploidy. It seems to affect the meiotic segregation in spermatogenesis [Collodel et al. Citation2006]. It also seems to be related to poor seminal quality and infertility in men [Mozdarani et al. Citation2007]. Variant 9qh+ is also frequent in humans [Codina-Pascual et al. Citation2006], and a statistically significant increase in the frequency of this variant was observed in infertile patients [Minocherhomji et al. Citation2009]. Likewise, Y chromosome polymorphisms (Yqh+ and Yqh-) are believed to be insignificant variants. However, recent studies show a higher frequency of Y chromosome polymorphisms among azoospermic and oligozoospermic men compared to fertile men [Penna Videaú et al. Citation2001; Nagvenkar et al. Citation2005].
Heterochromatin variants on acrocentric chromosomes are another type of frequent chromosome polymorphism. These heteromorphisms show increased heterochromatin at the short arm of the chromosome that is close to nucleolar organizer regions (NORs), which contains ribosomal genes associated with histone proteins. Therefore, changes in chromatin or proteins and other epigenetic variations such as methylation of DNA could affect gene expression and lead to meiotic arrest [Liu et al. Citation2008]. Even so, the relationship between the presence of isolated heterochromatin variants on acrocentric chromosomes and infertility is still a very controversial topic, and more data are necessary to resolve this matter. The aim of this work was to study the relationship between chromosomal polymorphic variants and infertility and to analyze the association between these polymorphic variants and aneuploidies in male gametes and embryos.
Results
MII cytogenetic results
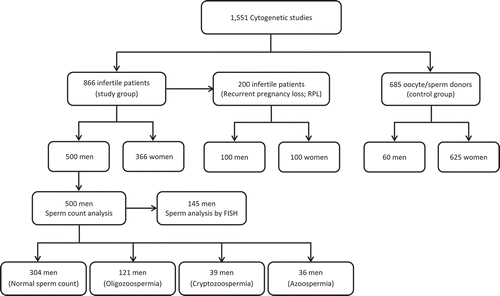
The study design of this retrospective study is outlined in the . A total of 1,551 cytogenetic analyses were performed from January 2009 to December 2012 and retrospectively reviewed in this study; this involved 866 infertile patients (study group) and 685 oocyte/sperm donors (control group). One hundred sixty-eight variant carriers were reported in the study group (92 males and 76 females), and 92 were reported in the control group (8 males and 84 females). A statistically significant increase in the frequency of polymorphic variants in the study group was observed (19.4%, compared with 13.4% in the control group; P < 0.01). No significant differences were reported among infertile male and female polymorphism carriers (18.4% in men vs. 20.8% in women), and there were no differences between male and female carriers in the control group (13.3% in men vs. 13.4% in women). The frequencies of polymorphic variant 9qh+ and acrocentric polymorphisms (ps+) were higher in the study group (). In the study group, the patients with recurrent pregnancy loss (RPL; n=200) showed a higher incidence of polymorphic variants compared to controls (26.5% for the RPL group vs. 13.4% for the control group, P < 0.001). Polymorphisms 9qh+ and ps+ remained increased in the RPL group. Differences in the frequency of variants between the RPL and control groups are shown in . No differences were observed in all other groups (i.e., unexplained infertility, poor sperm quality, and low ovary response groups).
Table 1. Frequency of chromosome variants in control and study/RPL groups.
Sperm aneuploidy and sperm count analysis
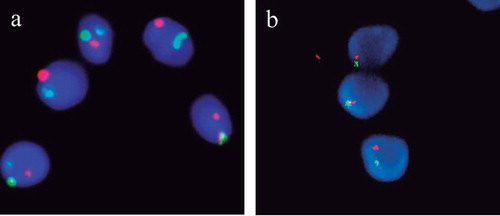
Sperm fluorescent in situ hybridization (FISH) analysis from 145 infertile men was compared in both groups of men (i.e., 53 polymorphism carriers and 92 non-carriers). FISH was performed with probes specific for chromosomes X, Y, 13, 18, and 21. An illustrative example is shown in . Statistically significant differences were observed for the frequency of abnormal sperm between polymorphism carriers and non-carriers; 20 men showed an abnormal rate of sperm aneuploidy in the carrier group vs. 15 in the non-carrier group (37.7% vs. 16.3%; P < 0.01). In the carrier group, we reported the frequency of each polymorphism in men with normal and abnormal sperm, as detected by FISH (). The percentage of men carrying variants on acrocentric chromosomes (ps+) was increased among the abnormal sperm group (40.0% compared to 16.4% in the normal sperm group; P < 0.01). 21ps+ was the most frequent variant in men with an abnormal sperm aneuploidy rate ().
Table 2. Frequency of chromosome variants in men with abnormal and normal sperm FISH results.
Figure 2. Example of sperm FISH result. (A) Triple color FISH for chromosomes X (green), Y (red), and 18 (blue). (B) Double color FISH for chromosomes 13 (green) and 21 (red). FISH: fluorescent in situ hybridization.
To analyze the relationship between polymorphic variants and the sperm count, men from the study group (n=500) were classified into four groups (men with normal sperm count and oligozoospermic, cryptozoospermic, and azoospermic men). The percentages of carrier and non-carrier men within the different groups are shown in . Polymorphism carriers and non-carriers displayed similar frequencies in the four groups. According to the sperm count, among the patients who performed sperm FISH, there were no differences in the percentages of men with normal sperm count, oligozoospermic, and cryptozoospermic men between carrier and non-carrier groups ().
Embryo aneuploidy analysis
The association between female chromosomal polymorphisms and the oocyte aneuploidy rate was tested by analyzing embryo aneuploidies in 301 embryos from oocyte donation cycles. In these instances, oocyte donors were divided into polymorphism carriers and non-carriers. We selected only oocyte donation cycles because donors were young women with normal ovarian function. In 75 analyzed cycles, 28 oocyte donors carried a polymorphic variant, and 47 donors were non-carriers. The data from the cycles are shown in . There were no significant differences with respect to the number of retrieved oocytes, MII oocytes, and embryos biopsied between carrier and non-carrier groups. Differences were found in the embryo aneuploidy rate between groups. The embryo aneuploidy rate was higher in cycles in which the oocyte donor carried a polymorphic variant (50.0% in carrier group vs. 27.6% in non- carrier group; P < 0.001). Differences in female age between groups were also reported (). As the donor age was higher in the carrier group than the non-carrier group, we adjusted the statistical analysis using logistic regression with female age as the confounding factor. The adjusted analysis remained significant among carrier and non-carrier groups (OR=2.747, 95% CI=1.039-7.264; P < 0.05). According to the type of aneuploidy (monosomy or trisomy), the percentage of embryos with a single aneuploidy was different between the groups (). The monosomies were more frequent among the embryos from female carriers than those from non-carriers, however the trisomies were more frequent among the non-carrier group. The percentage of the embryos with complex aneuploidies was similar in both groups.
Table 3. Frequency of carriers and non-carriers among infertile men (a) and infertile men who performed sperm FISH (b) in the different groups of sperm count.
Table 4. Data from oocyte donation cycles among carrier group and non-carrier group.
Discussion
The relationship between chromosomal polymorphic variants and infertility is a controversial topic. Numerous studies have been performed to clarify how these variants could affect different parameters of human fertility. Some research has shown an increased incidence of these polymorphisms in infertile couples [Madon et al. Citation2005; Minocherhomji et al. Citation2009; Caglayan et al. Citation2010; Mierla and Stoian Citation2012], but these data needed to be confirmed with additional studies in larger populations. The present study, carried out on 866 infertile patients and 685 gamete donors attending Instituto Bernabeu, shows a higher incidence of polymorphisms among infertile populations. This observation suggests a possible association between the presence of these polymorphisms and infertility and supports previously published data. This study differs from others because it incorporates the use of fertile gamete donors as a control population. Unlike other studies [Madon et al. Citation2005; Minocherhomji et al. Citation2009], that found a higher incidence of variants among infertile men than among infertile women, our study shows that this difference between sexes is not statistically significant. As has been observed in previous studies, the analysis of each variant separately showed that 9qh+ was increased among our infertile patients. This suggested a mechanism whereby meiotic segregation on that chromosome could be affected as heterochromatin increased, disturbing normal gametogenesis. Acrocentric short arm variants (D/G group) are another type of polymorphism that was increased among our study population. These data agree with recent publications [Madon et al. Citation2005; Minocherhomji et al. Citation2009; Hong et al. Citation2011], supporting the hypothesis that heterochromatic changes on acrocentric chromosomes could also disturb meiosis and result in reduced fertility [Codina-Pascual et al. Citation2006]. Moreover, recurrent pregnancy loss seems to be related to the presence of polymorphic variants because 26.5% of the RPL patients included in this work carried variants compared to 13.4% of controls. These data agree with many reports that have demonstrated a higher incidence of polymorphisms among couples who have experienced spontaneous recurrent miscarriages [Tsenghi et al. Citation1976; Nielsen Citation1978; Caglayan et al. Citation2010].
Because these polymorphic variants could affect spermatogenesis in men, this study evaluated the relationship between polymorphisms and seminal parameters, such as sperm count and sperm aneuploidy rate. Our results showed that sperm count was not affected by the presence of polymorphic variants in infertile males. These data are in agreement with that of previous studies [Kalantari et al. Citation2001; Yakin et al. Citation2005; Liang et al. Citation2014]. However, other studies show a higher frequency of chromosomal variants among azoospermic and oligozospermic men [Penna Videaú et al. Citation2001; Nagvenkar et al. Citation2005]. Therefore, further studies would be needed to clarify the relationship between these two factors. We also examined the relationship between polymorphic variants and sperm aneuploidy rate using FISH. There was a clear relationship between polymorphisms and sperm chromosome aneuploidy. Infertile men carrying polymorphisms showed a higher incidence of sperm aneuploidy, independent of their sperm count. Only two studies have evaluated the rate of aneuploidy in the spermatozoa of men carrying polymorphisms. Collodel et al. [Citation2006] reported an increased incidence of diploidy in men with chromosome 9 inversions, and in a study by Yakin et al. [Citation2005], FISH analysis in oligoasthenoteratozoospermic men with 9qh+ showed an increased rate of sperm aneuploidy. However, an interesting finding from this study that has not been previously reported is that the sperm aneuploidy is associated with polymorphisms on acrocentric chromosomes, specifically on chromosome 21. Twenty percent of men with abnormal sperm detected by FISH carried the variant 21ps+ compared with 4.5% of men with normal FISH results (P < 0.01). Following this finding, we postulate that all of these variants could affect meiotic segregation in male gametes and lead to a higher rate of aneuploid embryos.
To determine if polymorphisms could affect meiotic segregation in female gametes, whole chromosome imbalances in 301 embryos from oocyte donation cycles were analyzed. The influence of male factor in embryo aneuploidies was excluded because we selected only cycles in which the male partner had a normal karyotype and sperm FISH data. As the average donor age in the carrier group was higher than that in the non-carrier group (27.5 vs. 25.6; P < 0.05), the statistical analysis was adjusted by logistic regression using female age as a confounding factor, and differences in embryo aneuploidy rates among groups were observed (50.0% in the carrier group vs. 27.6% in the non-carrier group; P < 0.001). Data reported by Franasiak et al. [Citation2014] showed that female age is not associated with an increase in the prevalence of embryo aneuploidy in women 25 to 30 years old. According to our data there is a higher incidence of monosomies in embryos from female carriers compared with embryos from non-carriers. These results suggest that the presence of a polymorphic variant in females increases the probability of embryo aneuploidies, especially embryo monosomies. Interestingly, a study performed by Liang et al. [Citation2014] showed that female polymorphism carriers had a lower embryo cleavage rate after IVF cycles compared to non-carrier women. The results obtained in our study could be an explanation for the effect found by Liang et al. [Citation2014]. The higher rate of embryo aneuploidies in female carriers compared with non-carriers could lead to lower cleavage rates in these women.
Our data show an association between polymorphisms and aneuploidies in spermatozoa, and for the first time, this study reveals that female polymorphism carriers have a higher risk for embryo aneuploidy than females with a normal karyotype. Therefore, the application of a comprehensive chromosome screening (array-CGH) to select euploid embryos could improve IVF success rates among infertile male and female chromosomal polymorphism carriers. Moreover, to ensure a good success rate in an assisted reproduction procedure with donated gametes, we should consider withdrawing the oocyte donors with polymorphic variants from the donation program, and analyzing the rate of sperm aneuploidy in the sperm donors carrying a polymorphism in order to accept these males into the sperm bank.
Materials and methods
Study and control populations
We retrospectively included all cytogenetic studies performed at the Instituto Bernabeu from January 2009 to December 2012 that involved infertile patients (study group) and oocyte/sperm donors (control group). This study involved only retrospective analysis of anonymous medical records and it was approved by the Instituto Bernabeu Institutional Review Board. A total of 866 individuals formed the study group: 500 men and 366 women. The control group included 685 donors: 60 men and 625 women. To be included in the study, female and male donors had to be between the age of 18 and 35 and meet the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine [ESHRE Citation2002; ASRM Citation2013] guidelines for oocyte/sperm donation and the Instituto Bernabeu donation program requirements, which include extensive chromosomal and genetic screening. The fertility study performed on all patients included ultrasound scans to assess antral follicle count and uterine morphology, hormonal analysis, spermiogram, and karyotyping. The clinical indications for karyotyping in the study group were unexplained infertility (n=403; 240 men and 163 women), RPL (n=200; 100 men and 100 women), poor sperm quality (n=146 men), low ovarian response (n=103 women), and other indications (n=14 men). In 145 men from the study group, a sperm analysis by FISH was also conducted to evaluate the rate of aneuploidy in the spermatozoa.
To analyze the relationship between sperm count and polymorphic variants, infertile men (n=500; chromosomal polymorphism carriers and non-carriers) were classified into four groups according to WHO [Citation2010] sperm count criteria: men with normal sperm count (n=304), oligozoospermic men (n=121), cryptozoospermic men (<1x106 spermatozoa/ml) (n=39), and azoospermic men (n=36). The study design of this retrospective study is outlined in the .
To study the possible influence of female polymorphisms on the embryo aneuploidy rate, we included the array-CGH (comparative genomic hybridization) results of 301 embryos from oocyte donation cycles performed between 2013 and 2014, in which the oocyte donors were divided into polymorphism carriers and non-carriers (male karyotype and sperm FISH data were normal in all cases). Twenty-eight and 47 oocyte donors were included in the carrier and non-carrier groups, respectively. The main outcome measure was embryo aneuploidy rate.
Cytogenetic analysis
Chromosome analysis was carried out according to the International System for Human Cytogenetic Nomenclature guidelines [Shaffer et al. Citation2009]. Lymphocytes from heparinized blood samples were cultured using Chang MF medium (Gibco, Life Technologies, Madrid, Spain) supplemented with phytohaemagglutinin and incubated for 72 h at 37ºC. Metaphases were obtained by GTG banding using standard protocols. At least 20 metaphases were analyzed for each case using light microscopy. The banding resolution was 400-550 bands per haploid set. All karyotypes were evaluated by two geneticists to avoid variable results.
Polymorphic variants
The reported polymorphisms were variations in the length of the centromeric heterochromatin on the long arms of chromosomes 1, 9, and 16 (1qh+, 9qh+, 9qh-, 16qh+) and the distal heterochromatin on Y chromosome (Yqh+). Increased lengths of the satellites (ps+) of acrocentric D and G group chromosomes (13, 14, 15, 21, and 22) were also reported. Polymorphisms were included as a variant when the chromosome region was greater or smaller than the same region on the homologous chromosome (at least twice the size of the corresponding region on the other homologue). The Y chromosome was compared with chromosome 22. The pericentric inversion of chromosome 9 was also counted as a heteromorphic variant.
FISH
Sperm samples were treated with a hypotonic solution (KCl 0.56%) followed by Carnoy fixative. Fixed cells were dropped onto wet slides and air-dried (two slides per sample). For sperm membrane permeabilization and nuclei decondensation, the slides were washed twice in 2x saline-sodium citrate solution (SSC) for 3 min, dehydrated in an ethanol series (70%, 90%, 100%) for 2 min each, and incubated in 5 mmol dithiothreitol (DTT) with 1% Triton X-100 for 10 min at 37ºC. The slides were washed twice in 2x SSC, dehydrated for 2 min, and air dried. A double and a triple color FISH were performed on each sample, using DNA probes specific for chromosome X (CEP X SpectrumGreen, Xp11.1-q11.1), for chromosome Y (CEP Y SpectrumOrange, Yp11.1-q11.1), and for chromosome 18 (CEP 18 SpectrumAqua, 18p11.1-q11.1), and DNA probes specific for chromosome 13 (LSI 13 SpectrumGreen, 13q14) and chromosome 21 (LSI 21 SpectrumOrange, 21q22.13-q22.2), using the commercial kit AneuVysion (CEP 18, X, Y-alpha satellite, LSI 13 and 21; Vysis, Abbott Laboratories, IL, USA). Probe mixtures were added to the corresponding slide, and the slides were placed into a pre-warmed 73ºC hybridization chamber (HYBrite™) for 3 min to denature the sperm sample and the probes and incubated for a minimum of 12 h at 37ºC in a wet chamber. After incubation, the slides were rinsed in 0.4x saline citrate solution and 0.3% NP-40 at 73°C for 2 min and then in 2x standard saline citrate solution and 0.1% NP-40 at room temperature for 30 s. Preparations were counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; Vysis) and observed using an Olympus fluorescence microscope (), following the assessment criteria described previously by Blanco et al. [Citation1996]. The hybridization rate was above 90% in all the analyzed samples. Manual analysis was performed, and a minimum of 500 spermatozoa were evaluated for each patient and probe mixture. The rates of disomic and diploid spermatozoa were calculated and compared with those of the control population. A FISH result was considered abnormal when the diploid or disomic rate for at least one of the five analyzed chromosomes was higher than that observed in the control group (the control group consisted of 10 healthy sperm donors with normal karyotype and proven fertility). The mean disomy frequency in the control group was 0.1% for chromosomes 13 and 18, 0.14% for chromosome 21, and 0.45% for sex chromosomes, and the mean frequency for diploid spermatozoa was 0.3%. These values are according to the sperm aneuploidy levels in males from the general population [Egozcue et al. Citation1997].
Array-CGH
Whole chromosome imbalances were detected by array-CGH in trophectoderm cells from D5 embryos. Array-CGH analysis was performed using Agilent SurePrint G3 8x60K CGH microarrays with previous whole genome amplification (WGA) of genomic DNA. Trophectoderm cells were first lysed and the genomic DNA was amplified with the use of the PicoPLEX WGA system (Rubicon Genomics, MI, USA) according to the manufacturer’s protocol. WGA products were fluorescently labelled (Cy5) and competitively hybridized to 8x60K CGH microarrays with a PicoPLEX-amplified reference (XY male) for 16 h at 65ºC. Reference DNA samples were prepared according to sample preparation methods, amplified using the procedure specified in the PicoPLEX protocol and fluorescently labelled with Cy3. Data extracted from the scanned microarray image were analyzed with Agilent CytoGenomics Software to detect whole chromosome aneuploidies for all chromosomes.
Statistical analysis
The statistical analysis was performed with Statistical Package for Social Sciences software, version 20.0 (SPSS; Chicago, IL, USA). Values are reported as percentages for categorical variables and averages ± SD for continuous data. Differences between groups were evaluated using the Fisher’s exact statistical test for categorical variables and Student’s t-test for continuous data. Logistic regression using donor age as a possible confounding factor was performed for aneuploidy rate. All p-values obtained were two-tailed. P < 0.05 was considered significant; P < 0.01 was considered very statistically significant; P < 0.001 was considered highly statistically significant.
Declaration of interest
IB Biotech is a subsidiary of Instituto Bernabeu. The authors report no declarations of interest.
Acknowledgments
We want to thank everyone who participated in the study. Virginia Alarcon, Erin Moore, and Elsevier Language Editing Services helped us with the language.
Additional information
Notes on contributors
Ruth Morales
Involved in the acquisition, analysis, and interpretation of data: RM, BL, JAO; Responsible for reviewing the article: BL, JAO, JT, JL, RB. All authors contributed to the design of the study, drafting and revising the manuscript, and approval of the final version to be published: RM, BL, JAO, JT, JL, RB.
Belén Lledó
Involved in the acquisition, analysis, and interpretation of data: RM, BL, JAO; Responsible for reviewing the article: BL, JAO, JT, JL, RB. All authors contributed to the design of the study, drafting and revising the manuscript, and approval of the final version to be published: RM, BL, JAO, JT, JL, RB.
José A. Ortiz
Involved in the acquisition, analysis, and interpretation of data: RM, BL, JAO; Responsible for reviewing the article: BL, JAO, JT, JL, RB. All authors contributed to the design of the study, drafting and revising the manuscript, and approval of the final version to be published: RM, BL, JAO, JT, JL, RB.
Jorge Ten
Involved in the acquisition, analysis, and interpretation of data: RM, BL, JAO; Responsible for reviewing the article: BL, JAO, JT, JL, RB. All authors contributed to the design of the study, drafting and revising the manuscript, and approval of the final version to be published: RM, BL, JAO, JT, JL, RB.
Joaquin Llácer
Involved in the acquisition, analysis, and interpretation of data: RM, BL, JAO; Responsible for reviewing the article: BL, JAO, JT, JL, RB. All authors contributed to the design of the study, drafting and revising the manuscript, and approval of the final version to be published: RM, BL, JAO, JT, JL, RB.
Rafael Bernabeu
Involved in the acquisition, analysis, and interpretation of data: RM, BL, JAO; Responsible for reviewing the article: BL, JAO, JT, JL, RB. All authors contributed to the design of the study, drafting and revising the manuscript, and approval of the final version to be published: RM, BL, JAO, JT, JL, RB.
References
- ASRM. (2013) Recommendations for gamete and embryo donation: A committee opinion. Fertil Steril 99:47–62.
- Blanco, J., Egozcue, J. and Vidal, F. (1996) Incidence of chromosome 21 disomy in human spermatozoa as determined by fluorescent in-situ hybridization. Hum Reprod 11:722-726.
- Borgaonkar, D.S. (1997) Chromosomal Variation in Man: A Catalog of Chromosomal Variants and Anomalies. New York: Wiley-Liss.
- Caglayan, A.O., Ozyazgan, I., Demiryilmaz, F. and Ozgun, M.T. (2010) Are heterochromatin polymorphisms associated with recurrent miscarriage? J Obstet Gynaecol Res 36:774-776.
- Codina-Pascual, M., Navarro, J., Oliver-Bonet, M., Kraus, J., Speicher, M.R., Arango, O., et al. (2006) Behaviour of human heterochromatic regions during the synapsis of homologous chromosomes. Hum Reprod 21:1490-1497.
- Collodel, G., Moretti, E., Capitani, S., Piomboni, P., Anichini, C., Estenoz, M., et al. (2006) TEM, FISH and molecular studies in infertile men with pericentric inversion of chromosome 9. Andrologia 38:122-127.
- Düzcan, F., Atmaca, M., Cetin, G.O. and Bagci, H. (2003) Cytogenetic studies in patients with reproductive failure. Acta Obstet Gynecol Scand 82:53-56.
- Egozcue, J., Blanco, J. and Vidal, F. (1997) Chromosome studies in human sperm nuclei using fluorescence in-situ hybridization (FISH). Hum Reprod Update 3:441-452.
- ESHRE Task Force on Ethics and Law. (2002) III. Gamete and embryo donation. Hum Reprod 5:1407-1408.
- Ferguson-Smith, M.A. (1974) Autosomal polymorphisms. Birth Defects Orig Artic Ser 10:19-29.
- Franasiak, J.M., Forman, E.J., Hong, K.H., Werner, M.D., Upham, K.M., Treff, N.R., et al. (2014) The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 101:656-663.
- Hong, Y., Zhou, Y.W., Tao, J., Wang, S.X. and Zhao, X.M. (2011) Do polymorphic variants of chromosomes affect the outcome of in vitro fertilization and embryo transfer treatment? Hum Reprod 26:933-940.
- Humphray, S.J., Oliver, K., Hunt, A.R., Plumb, R.W., Loveland, J.E., Howe, K.L., et al. (2004) DNA sequence and analysis of human chromosome 9. Nature 429:369-374.
- Iyer, P., Wani, L., Joshi, S., Lakshmi, J., Dalvi, R., Chavan, D., et al. (2007) Cytogenetic investigations in couples with repeated miscarriages and malformed children: report of a novel insertion. Reprod Biomed Online 14:314-321.
- Kalantari, P., Sepehri, H., Behjati, F., Ashtiani, Z.O. and Akbari, M.T. (2001) Chromosomal studies in infertile men. Tsitol Genet 35:50-54.
- Liang, J., Zhang, Y., Yu, Y., Sun, W., Jing, J. and Liu, R. (2014) Effect of chromosomal polymorphisms of different genders on fertilization rate of fresh IVF-ICSI embryo transfer cycles. Reprod Biomed Online 29:436-444.
- Liu, L., Li, Y. and Tollefsbol, T. (2008) Gene environment interactions and the epigenetic basis of human diseases. Curr Issues Mol Biol 10:25-36.
- Madon, P.F., Athalye, A.S. and Parikh, F.R. (2005) Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod Biomed Online 11:726-732.
- Mierla, D. and Stoian, V. (2012) Chromosomal polymorphisms involved in reproductive failure in the romanian population. Balkan J Med Genet 15:23-28.
- Minocherhomji, S., Athalye, A.S., Madon, P.F., Kulkarni, D., Uttamchandani, S.A. and Parikh, F.R. (2009) A case-control study identifying chromosomal polymorphic variations as forms of epigenetic alterations associated with the infertility phenotype. Fertil Steril 92:88-95.
- Mozdarani, H., Meybodi, A.M. and Karimi, H. (2007) Impact of pericentric inversion of Chromosome 9 [inv (9) (p11q12)] on infertility. Indian J Hum Genet 13:26-29.
- Nagvenkar, P., Desai, K., Hinduja, I. and Zaveri, K. (2005) Chromosomal studies in infertile men with oligozoospermia & non-obstructive azoospermia. Indian J Med Res 122:34-42.
- Nakamura, Y., Kitamura, M., Nishimura, K., Koga, M., Kondoh, N., Takeyama, M., et al. (2001) Chromosomal variants among 1790 infertile men. Int J Urol 8:49-52.
- Nielsen, J. (1978) Large Y chromosome (Yq+) and increased risk of abortion. Clin Genet 13:415-416.
- Penna Videaú, S., Araujo, H., Ballesta, F., Ballescá, J.L. and Vanrell, J.A. (2001) Chromosomal abnormalities and polymorphisms in infertile men. Arch Androl 46:205-210.
- Shaffer, L., Slovak, M., and Campbell, L. (2009) An International System for Human Cytogenetic Nomenclature: Recommendations of the International Standing Committee on Human Cytogenetic Nomenclature. Karger Press.
- Teo, S.H., Tan, M., Knight, L., Yeo, S.H. and Ng, I. (1995) Pericentric inversion 9–incidence and clinical significance. Ann Acad Med Singapore 24:302-304.
- Tsenghi, C., Metaxotou-Stavridaki, C., Strataki-Benetou, M., Kalpini-Mavrou, A. and Matsaniotis, N. (1976) Chromosome studies in couples with repeated spontaneous abortions. Obstet Gynecol 47:463-468.
- Uehara, S., Akai, Y., Takeyama, Y., Takabayashi, T., Okamura, K. and Yajima, A. (1992) Pericentric inversion of chromosome 9 in prenatal diagnosis and infertility. Tohoku J Exp Med 166:417-427.
- Verma, R.S., Dosik, H. and Lubs, H.A. (1978). Size and pericentric inversion heteromorphisms of secondary constriction regions (h) of chromosomes 1, 9, and 16 as detected by CBG technique in Caucasians: classification, frequencies, and incidence. Am J Med Genet 2:331-339.
- WHO. (2010). Laboratory manual for the examination and processing of human semen. 5th ed. WHO Press, Switzerland.
- Yakin, K., Balaban, B. and Urman, B. (2005) Is there a possible correlation between chromosomal variants and spermatogenesis? Int J Urol 12:984-989.