ABSTRACT
This study investigates the correlation between sperm morphology and the incidence of embryo aneuploidy in an oocyte donation program. A total of 1,165 embryos from 103 patients have been analyzed by fluorescent in situ hybridization (FISH) for numerical abnormalities in chromosome numbers 13, 18, 21, X, and Y. Data has been evaluated in five groups according to sperm morphology, which has been assessed according to the Kruger’s strict criteria. The results did not show any difference in paternal (p = 0.878), maternal (p = 0.873), and donor ages (p = 0.871), sperm counts (p = 0.782) and motility (p = 0.124), and fertilization rate (p = 0.080) among the groups. However, total aneuploidy rate (p < 0.001) and its derivatives (trisomy p = 0,042, monosomy p = 0,004) differed significantly and they were reversibly correlated with sperm morphology (rho correlation test; total aneuploidy p < 0.001, trisomy p < 0.001, monosomy p = 0.004). Therefore, these results suggested that diminished sperm quality is correlated to the aneuploidy rate in preimplantation embryos.
Abbreviations: FISH: fluorescence in situ hybridization; ICSI: intracytoplasmic sperm injection; HCG: human chorionic gonadotropin
Introduction
The high incidence of aneuploidy observed in preimplantation embryos is one of the most significant factors affecting the clinical outcomes in assisted reproduction treatments. Aneuploidies are related to maternal and/or paternal factors and they are affected by age, diet, and lifestyle, which can lead to chromosomal abnormalities occurring during gametogenesis, with an impact on fertilization and cell divisions (Potapova and Gorbsky Citation2017). Abnormal chromosomal segregation occurring during meiosis can lead to aneuploidy in both male and female gametes by deleting or including an extra copy of chromosomes (Hassold and Hunt Citation2001; Lamb et al. Citation2005). Nevertheless, there is a difference in frequency of aneuploidy observed in spermatozoa (~4.5%) and oocytes (~20%) (Donate et al. Citation2016). The difference in the incidence of aneuploidy among the male and female gametes can be attributed to the earlier completion of meiosis in the spermatozoa than its counterpart, and that it is equipped with more effective check-points before fertilization. However, it has been suggested that aneuploidy may cause meiotic arrest and cease gamete production, which may result in oligozoospermia or even azoospermia (Andreescu et al. Citation2016).
The gamete selection mechanisms occurring in vivo or in vitro reduce the incidence of aneuploidy in fertilized oocytes and embryos (Berkovitz et al. Citation2006). It is likely that the composition of the tubal fluid, cumulus cells, and zona pellucida contribute to filtering morphologically and chromosomally abnormal spermatozoa. Yet, in vitro fertilization techniques bypass most of the natural selection mechanisms. Sperm selection during intra cytoplasmic sperm injection (ICSI) relies vastly on morphological assessment and motility, where present. It has been reported that ICSI can lead to higher incidence of chromosomal abnormalities in pre-implantation embryos than conventional IVF possibly due to the relative ineffectiveness of the utilized sperm selection methods (Van Steirteghem et al. Citation1993). In order to evaluate the impact of sperm morphology to the genetic composition of the embryo, and hence, indirectly to its viability, the present study assessed a donor oocyte program, which minimized the impact of aneuploidies arising from the female gamete to those observed in autologous cycles.
Results
A total of 1,165 embryos obtained from 103 patients were allocated to five groups according to sperm morphology (scored from 1 to 5, corresponding to Kruger’s strict criteria). There were 131, 200, 382, 168, and 284 embryos in Groups 1–5, respectively. Forty-five embryos (3.90%) in the groups were not analyzed due to loss of nuclei or insufficient signal. Results showed that paternal, maternal, and donor ages, sperm parameters (i.e., count and motility), fertilization, and live birth rates did not differ among groups (). However, there were significant differences among groups with regard to the incidence of aneuploidy (p < 0.001, ) and its derivatives (trisomy p = 0.042, monosomy p = 0.004, ), which were not reflective of live birth rates, as they remained similar (p = 0.705, ). Although a gradual increase in fertilization rates have been observed with improved sperm morphological score, the difference among groups was not significant (Group 1 = 70.1%, Group 2 = 74.6%, Group 3 = 73.7%, Group 4 = 77.5%, Group 5 = 82.9%, p = 0.080). The mean incidence of aneuploidy in Group 5 was lower than other groups (1 vs. 5 p < 0.001, 2 vs. 5 p < 0.001, 3 vs. 5 p = 0.001, 4 vs. 5 p = 0.003). Furthermore, the percentage of trisomy and monosomy in Group 5 were lower than Group 2. Group 1 also differed from groups 3, 4, and 5 in the percentage of normal embryos, which was significantly lower (p ≤ 0.001, p = 0.004, p < 0.001, respectively). Similarly, lower values were observed in Group 2 vs. Groups 3 and 5, Groups 3 and 4 vs. Group 5 ().
Table 1. Descriptive statistics in groups.
Table 2. Sub-group analyses.
Figure 1. Aneuploidy and normal embryo rates among groups. Ascending numbers from left to right on the X-axis represent the study groups (corresponding to Kruger’s morphological scores). Blue bars show percentage of normal embryos. Lines represent percentage of total aneuploidy (Total AE, Red), Trisomy (Green), and Monosomy (Purple). Values of each line and bars are shown in the bottom of the graph.
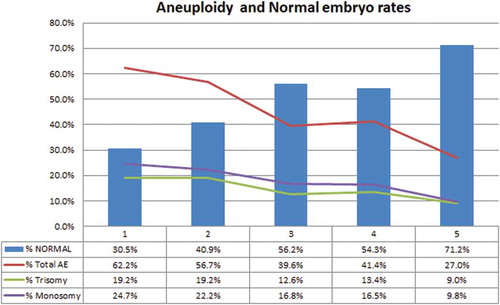
The correlations between total aneuploidy rates, trisomy, monosomy, and sperm morphology were analyzed by the rho correlation test. The results showed a negative correlation between the Kruger’s score and the aneuploidy rate observed in preimplantation embryos (total aneuploidy rates to Kruger score was −0.589, p < 0.001, to trisomy −0.280, p < 0.004 and to monosomy −0.360, p < 0.001, ). Although fertilization rates were similar among groups (p = 0.080, ), this test has shown a negative correlation between the fertilization rate and the morphologic score (p = 0.014, ). Data showed no correlation between paternal age and total aneuploidy (p = 0.202), trisomy (p = 0.290), monosomy (p = 0.079), the incidence of normal embryos (p = 0.824) and the fertilization rate (p = 0.848) (Details are listed in ).
Table 3. Correlation analyses (rho test).
Discussion
The present study assessed the potential correlation between sperm quality and the incidence of aneuploidy observed in preimplantation embryos in a donor oocyte program, which enabled focusing on paternal variables by minimizing the impact of female factors on the results. Embryo mosaicism presents a challenge in the analysis of genetic abnormalities. It has been shown that the diagnosis by single cell analysis can enhance the false diagnosis in mosaic embryos (Michiels et al. Citation2006). In comparison, it is obvious that mosaic embryos may be diagnosed as normal as well as abnormal depending on the level of mosaicism (Harton et al. Citation2017). This can be important for the investigation of the rate of misdiagnosed and/or discarded normal embryos but its effect on our results will be less due to possible misdiagnoses of either normal or abnormal embryos.
The incidence of sperm chromosomal aneuploidy in humans and its association with semen parameters (Vegetti et al. Citation2000; Calogero et al. Citation2001), with paternal age (Luetjens et al. Citation2002) and with infertility (Baart et al. Citation2006) have been reported. It has been reported that the incidence of chromosomally abnormal sperm is less than 10% (Andreescu et al. Citation2016) and it is increased in patients with abnormal semen parameters (Calogero et al. Citation2001) indicating a correlation between semen parameters and numerical chromosome abnormalities in sperm. Sperm preparation methods are able to filter most of the abnormal sperm (morphological and chromosomal) (Tomlinson et al. Citation2001), which may lower the expected rate of paternally derived numerical chromosome abnormalities in preimplantation embryos. However, the observed incidence of aneuploid embryos in earlier (Garcia-Ferreyra et al. Citation2015) and in the current study is more than 50%, which may suggest that the meiotic events occurring during fertilization have an impact on chromosomal impairment. The present study showed a negative correlation between sperm morphology to chromosomal aneuploidy of the embryos. These results are of significance due to the minimal impact of the oocyte factor on embryo aneuploidy rates in a donor program and focusing more on the semen parameters, particularly to sperm morphology.
Although the necessity of preimplantation screening for aneuploidy in embryos from patients of advanced paternal age (≥50) (Garcia-Ferreyra et al. Citation2015), our data did not show a significant correlation between paternal age to total aneuploidy rates and to sperm morphology (). In comparison, our results suggested that diminished sperm quality/morphology are a potential origin for aneuploidy in early embryos.
Although the present study utilized donor oocytes to minimize the impact of age related maternal contribution to the incidence of embryo aneuploidy, which has been found to be high in earlier studies (Martini et al. Citation1998), the precise inference about source of aneuploidy could be complicated. The contribution of other factors, like meiotic chromosomal rearrangements and embryo mosaicism depending on abnormal chromosomal segregation during cell division (mitosis), need to be assessed in order to support these findings. Furthermore, factors such as gamete production, fertilization, embryonic development, age, and many others should be considered during the investigation of the source of numerical chromosome abnormalities in preimplantation embryos. At this juncture our results suggested that sperm low quality/morphology is associated with the incidence of embryo aneuploidy.
Material and methods
Study design
Informed consent was obtained from all patients who were involved in this study subsequent to the study approval by the Ethics Committee of Research and Development Department of Cyprus IVF Centre. Data obtained between January to December 2013 from donor cycles that had been treated due to advanced maternal age and/or ovarian failure have been analyzed retrospectively. Embryo aneuploidy rates were assessed in correlation to sperm morphology which were grouped according to Kruger’s strict criteria (Kruger et al. Citation1988).
Sperm morphology assessment and ICSI procedures
The morphological assessment of sperm was performed according to Kruger’s strict criteria at the day of semen collection and were grouped into five groups, as follows: (1) equal or less than Kruger’s score 1, n = 13 patients; (2) Kruger’s score 2, n = 20 patients; (3) Kruger’s score 3, n = 32 patients; (4) Kruger’s score 4, n = 16 patients; and (5) Kruger’s score equal or more than 5, n = 22 patients. All samples were prepared through a three-layer density gradient centrifugation (PureSperm, Nidacon, Sweden), which was followed by washing in HTF medium (Irvine Scientific, Santa Ana, California, USA).
Oocytes were collected 35 h after HCG injection. Oocyte cumulus complexes were denuded after two h in HTF (Irvine Scientific) containing 5% hyaluronidase (Irvine Scientific). ICSI was performed under inverted microscope with x400 magnification (Zeiss, Axiovert, Germany). Fertilization was checked 16 h after ICSI.
Embryo biopsy and fluorescence in-situ hybridization (FISH)
Embryo biopsy was performed on 6–8 cell embryos on day three (day 0 = oocyte retrieval) by removing a single cell and the embryos were cultured until day five. The cytoplasm was removed in a drop of spreading solution (1% HCL, 10% PBS) after that cell was washed with PBS. These steps were repeated for all cells, and slides were left to dry. Dried slides were washed in PBS for five min and dehydrated in gradually increasing concentrations of alcohol (70%, 85%, and 100%) for two min each. Slides were left to dry at room temperature and the presence of nuclei was confirmed before sending them to the genetic laboratory. Hybridization was performed as described before (Smith et al. Citation1998).
Chromosomes 13, 18, 21, and sex chromosomes X and Y were analyzed. With these selections, it was possible to screen embryos for some common chromosomal diseases such as Down syndrome (Trisomy 21), Edward’s syndrome (Trisomy 18), Patau (Trisomy 13), and Kleinefelter syndrome (XXY).
Statistical analyses
For statistical analyses SPSS 15.0 for Windows was used. Parametric and non-parametric tests were done according to the distribution of data. Descriptive statistics for the numerical variables were given as mean and standard deviation. Numerical variables were not satisfied with normal distribution so Kruskal Wallis test for more than two groups were conducted. Sub-group analyses were done by using Mann Whitney-U test followed by Benferroni correction. Numerical variables were analyzed by Spearman Correlation and statistical alfa significance was accepted as 0.05. Rho correlation test was used to analyze percentage of aneuploidies, normal embryo, fertilization, and their correlation with Kruger’s score and paternal age.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Nedime Serakinci
Substantial contributions to design, acquisition, analysis, and interpretation of data, drafting the article, critical revision of manuscript, and approval of final version of the manuscript: OC; Substantial contributions to conception and design of project, interpretation of data, critical revision of manuscript, and approval of final version of the manuscript: MS; Substantial contributions to analysis of data, critical revision of manuscript, and approval of final version of the manuscript: ZOS, EMB; Substantial contributions to conception and design of project, interpretation of data, critical revision of manuscript, and approval of final version of the manuscript: NS.
References
- Andreescu NI, Cosma M, Farcas SS, Stoian M, Amzar DG, Puiu M. 2016. Assessment of chromosomal aneuploidies in sperm of infertile males by using FISH technique. Rom J Morphol Embryol. 57(1):173–178.
- Baart EB, Martini E, Van Den Berg I, Macklon NS, Galjaard RJ, Fauser BC, Van Opstal D. 2006. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod. 21(1):223–233.
- Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, Bartoov B. 2006. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 12(5):634–638.
- Calogero AE, De Palma A, Grazioso C, Barone N, Burrello N, Palermo I, Gulisano A, Pafumi C, D’Agata R. 2001. High sperm aneuploidy rate in unselected infertile patients and its relationship with intracytoplasmic sperm injection outcome. Hum Reprod. 16(7):1433–1439.
- Donate A, Estop AM, Giraldo J, Templado C. 2016. Paternal age and numerical chromosome abnormalities in human spermatozoa. Cytogenet Genome Res. 148(4):241–248.
- Garcia-Ferreyra J, Luna D, Villegas L, Romero R, Zavala P, Hilario R, Duenas-Chacon J. 2015. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of Sperm DNA Fragmentation. Clin Med Insights Reprod Health. 9:21–27.
- Harton GL, Cinnioglu C, Fiorentino F. 2017. Current experience concerning mosaic embryos diagnosed during preimplantation genetic screening. Fertil Steril. 107(5):1113–1119.
- Hassold T, Hunt P. 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2(4):280–291.
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. 1988. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 49(1):112–117.
- Lamb NE, Sherman SL, Hassold TJ. 2005. Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet Genome Res. 111(3–4):250–255.
- Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. 2002. Sperm aneuploidy rates in younger and older men. Hum Reprod. 17(7):1826–1832.
- Martini E, Von Bergh AR, Coonen E, De Die-Smulders CE, Hopman AH, Ramaekers FC, Geraedts JP. 1998. Detection of structural abnormalities in spermatozoa of a translocation carrier t(3;11)(q27.3;q24.3) by triple FISH. Hum Genet. 102(2):157–165.
- Michiels A, Van Assche E, Liebaers I, Van Steirteghem A, Staessen C. 2006. The analysis of one or two blastomeres for PGD using fluorescence in-situ hybridization. Hum Reprod. 21(9):2396–2402.
- Potapova T, Gorbsky GJ. 2017. The consequences of chromosome segregation errors in Mitosis and Meiosis. Biology (Basel). 6(1):12.
- Smith SE, Toledo AA, Massey JB, Kort HI. 1998. Simultaneous detection of chromosomes X, Y, 13, 18, and 21 by fluorescence in situ hybridization in blastomeres obtained from preimplantation embryos. J Assist Reprod Genet. 15(5):314–319.
- Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, Sakkas D. 2001. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 16(10):2160–2165.
- Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, Wisanto A, Devroey P. 1993. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 8(7):1061–1066.
- Vegetti W, Van Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Van Steirteghem A. 2000. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 15(2):351–365.
