ABSTRACT
In this study, we sought to investigate the effect of lifestyle and demographic factors on classic and functional semen parameters. Three hundred and twenty-eight subjects who underwent semen analysis were recruited. Routine SA, sperm vitality, acrosome reaction (AR) assay and sperm DNA fragmentation index (DFI) were analyzed. Demographic and lifestyle information, including (1) BMI, (2) current smoking and alcohol drinking frequency, (3) sleep habits, (4) daily fluid intake, (5) weekly meat intake, (6) sports frequency, (7) trouser cell phone use, (8) age, and (9) abstinence time, were collected. Generalized additive models were used to analyze the possible non-linear association. The results showed that total sperm count (TSC) was significantly associated with age (P = 0.001), abstinence time (P = 0.001) and daily coffee intake (P = 0.044). Semen volume was significantly associated with age (P < 0.001) and daily coffee intake (P < 0.001). Sperm concentration was significantly associated with abstinence time (P = 0.011) and average sleep duration (P = 0.010). Sperm motility was significantly associated with age (P = 0.002) and daily juice intake (P = 0.001). Total motile sperm count was significantly associated with age (P = 0.003) and abstinence time (P = 0.009). DFI was significantly associated with age (P = 0.002), irregular sleeping habit (P = 0.008) and abstinence time (P = 0.032). The percentage of AR sperm was significantly associated with daily juice intake (P = 0.013). In conclusion, DFI and TSC were the most sensitive semen parameters for demographic and lifestyle features, whereas age had more influence on semen parameters than other demographic and lifestyle features.
Abbreviations: BMI: body mass index; SA: semen analysis; AR: acrosome reaction; DFI: DNA fragmentation index; GAM: generalized additive model; TSC: total sperm count; TMC: total motile sperm count; IUI: intrauterine insemination; SCSA: sperm chromatin structure assay; SD: standard deviation; IQR: interquartile range; CBAVD: congenital bilateral absence of vas deferens; NEQAS: national external quality assessment service; HTF: human tubal fluid; HSA: human serum albumin.
Introduction
During the past decades, several investigators described a global downward trend in human semen quality (Rolland et al. Citation2013), although this is not universally agreed (Merzenich et al. Citation2010). The exact underlying cause for the downward trend is unclear but the environment and lifestyles factors, such as body mass index (BMI), cigarette smoking, alcohol intake, diet, physical activity, trouser cell phone use and sleep duration have all been considered to be risk factors for a reduction in poor semen quality (Povey et al. Citation2012; Collodel et al. Citation2014; Eisenberg et al. Citation2014; Jurewicz et al. Citation2014a; Gaskins et al. Citation2015; Mikkelsen et al. Citation2016; Sharma et al. Citation2016a). However, supporting evidence from previous studies is often inconclusive (Pacey et al. Citation2014; Jurewicz et al. Citation2014a; Bandel et al. Citation2015; Yang et al. Citation2015). In addition, most previous studies concentrated only on the association between various demographic and lifestyle factors and traditional semen parameters (volume, density, motility and morphology); a few studies included sperm DNA fragmentation and sperm aneuploidy (Jurewicz et al. Citation2014a, Citation2014b; Bandel et al. Citation2015; Yang et al. Citation2015) but none attempted to examine the association between demographic and lifestyle factors and different aspects of sperm function. Although traditional semen analysis (SA) can provide useful information regarding spermatogenesis and sperm maturation, the discriminative power of traditional SA in predicting the outcome of natural and assisted reproductive cycles is limited (Oehninger et al. Citation2014; Zhu et al. Citation2016). The sequential analysis of extended sperm tests, such as sperm vitality and sperm acrosome reaction (AR) assessment has been recognized as additional tests to assist the diagnosis and treatment of male infertility (Barratt et al. Citation2011; Oehninger et al. Citation2014). In this cross-sectional study, we examined the association between various demographic and lifestyle factors and traditional semen parameters, as well as sperm vitality, sperm DNA fragmentation index (DFI) and sperm AR, with a view to understanding to what extent lifestyle and demographic factors would affect sperm function.
Results
Study population
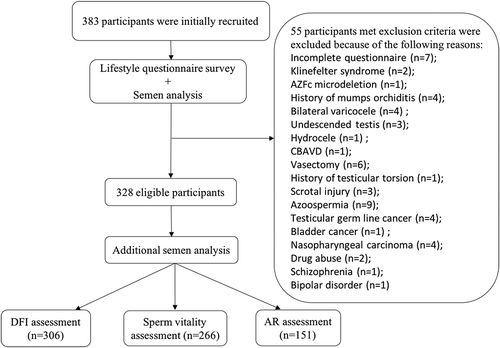
A total of 383 men were initially recruited in this study on the day of their SA. However, after checking the SA record and medical records of the participants, 55 subjects were excluded for the reasons outlined in . All of the eligible participants underwent routine SA, with 93.2% of subjects undergoing one or more additional sperm tests () depending on the sample volume and concentration after clinical use.
The demographic and semen parameters of study population
The demographic characteristics and semen parameters of the total participants are shown in . There are no significant differences (P ≥ 0.05) in the age, BMI, abstinence time and morphology between participants who did or did not have additional sperm tests (Supplementary Table 1). However, the semen volume, concentration, motility, total sperm count and total motile count of participants who had additional sperm tests were significantly higher (P < 0.05) than participants who did not have additional sperm tests (Supplementary Table 1).
Table 1. Demographic characteristics and semen parameters of participants.
The lifestyle factors of study population
The lifestyle characteristics of the study population are shown in . There were no significant differences (P ≥ 0.05) in any of the lifestyle factors between participants who did or did not have additional sperm tests (Supplementary Table 2).
Table 2. Lifestyle characteristics of participants (results shown are either in percentage of yes answer or Mean± SD, n = 328).
Univariate and multivariate GAM
The association between the demographic and lifestyle factors and semen parameters were first analyzed by univariate GAM model (Jee et al. Citation2017) (Supplementary Table 3) and 19 significant associations were identified by the model ().
Table 3. The significant associations between semen parameters and demographic lifestyle variables in both univariable and multivariable generalized additive model.
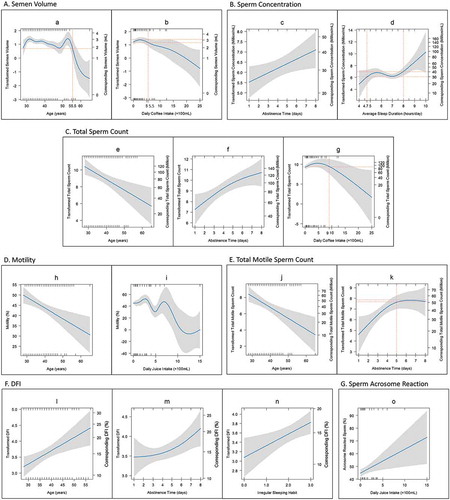
To adjust for the interaction between various independent variables, multivariate GAM was subsequently employed (Supplementary Table 4). There are only 15 associations were still significant in multivariate GAM models (); the pattern of these 15 associations are shown in . The significant associations were described as followed: (1) Semen volume had a non-linear relationship with age, with no apparent change in volume before 55.5 years of age, but a noticeable drop afterwards; (2) Semen volume also had a non-linear association with daily coffee intake. It had no obvious changes when coffee intake less than 550 ml/day, but it declined steadily when more than 550 ml coffee/day was consumed; (3) Sperm concentration had a positive and linear association with abstinence time; (4) Sperm concentration had a non-linear relationship with average sleep hours. The sperm concentration had no apparent changes when sleep was 4.7–8 h/day, however, it dropped when sleep was less than 4.7 h/day and increased when sleep was more than 8 h/day; (5) Total sperm count was positively and linearly associated with age; (6) Total sperm count had a non-linear association with abstinence time, the total sperm count increased with the increase of abstinence time; (7) Total sperm count also had non-linear association with daily coffee intake. It did not change obviously with less than 900 ml coffee/day, however, it dropped steadily with more than 900 ml coffee/day; (8) Sperm motility had a negative and linear association with age; (9) Sperm motility had non-linearly association with daily juice intake; (10) Total motile sperm count was negatively and linearly associated with age; (11) Total motile sperm count had non-linear association with abstinence time. It increased with an increase in abstinence time up to 5 days but there was no noticeable change beyond abstinence time of 5 days; (12) Sperm DFI had positive and linear association with age; (13) Sperm DFI had a non-linear association with abstinence time, the slope of change being steeper after an abstinence time of 4 days; (14) It also had linear association with irregular sleep habits. The more irregular sleep habits the participants had, the higher sperm DFI; (15) The percentage of acrosome reacted sperm had linear and positive association with daily juice intake.
Figure 2. Variation in semen parameters with increased intensity of demographic and lifestyle factors. The blue lines in the figure show the regression lines of semen parameters based on the GAM model. The grey shadows represent the 95% confidence interval. Both transformed semen parameters and the corresponding original semen parameters were shown in the left Y axis and right Y axis respectively in A, B, C, E and F for data which underwent Box-Cox transformation, such as semen volume, sperm concentration, total sperm count, total motile sperm count and DFI. (A) The variation of semen volume with increased age (a) and daily coffee intake (b). (B) The variation of sperm concentration with increased abstinence time (c) and average sleep duration (d). (C) The trend of total sperm count with increased age (e), abstinence time (f) and daily coffee intake (g). (D) The association of sperm motility and age (h) and daily juice intake (i). (E) The changes of total motile sperm count with increased age (j) and abstinence time (k). (F) The relationship between DFI and age (l), abstinence time (m) and irregular sleeping habits (n). The irregular sleeping habit included night shift, insomnia and staying up. Irregular sleeping habits, including night shift, insomnia and stay up. X = 0 represent participants without irregular sleeping habits. X = 1–3 represent participants with one to three irregular sleeping habits respectively. (G) The increase of acrosome reacted sperm with increased daily juice intake (o).
Discussion
In this study, we examined the association of 18 demographic and lifestyle factors with nine classic and functional semen parameters in men who underwent investigation for infertility assessment. We have come across several interesting findings.
The results of our study showed that among all traditional and additional semen parameters measured, total sperm count and DFI appeared to more sensitive to demographic and lifestyle factors, as they were significantly associated with three demographic and lifestyle features (). In comparison, other semen parameters were only significantly associated with up to two demographic and lifestyle features (). Among the various demographic and lifestyle features examined, age appeared to be the most important feature as it was significantly associated with five semen parameters, followed by abstinence period which was significantly correlated to four semen parameters. The average sleep duration, irregular sleep habit, daily coffee intake and daily juice intake also seems to affect semen quality but to a lesser extent (associated with 1–3 parameters).
Regarding age, we found that it was positively associated with sperm DFI (P < 0.05); negatively associated with total sperm count, total motile sperm count and sperm motility (P < 0.05); and non-linearly associated with the volume (P < 0.05). Our findings are consistent with previous observations of Sartorius and Freour (Sartorius and Nieschlag Citation2010; Freour et al. Citation2012). However, given that age was not significantly correlated with percentage of acrosome-reacted sperm (P>0.05), it seems that age, across the age range examined (28–65 years), did not appear to affect sperm fertilization ability. This is consistent with the results of a recent study, which showed that paternal age had no impact on fertilization rate in an IVF program (Wu et al. Citation2015). However, since sperm DFI has been reported to be a good predictor for embryo quality (Tandara et al. Citation2014; Zheng et al. Citation2018), our result further indicated that sperm of aged men may still retain the ability to fertilize the oocyte but are diminished in ability to support the subsequent embryo development.
Abstinence time was significantly associated with four sperm parameters, namely sperm concentration, total sperm count, total motile count and DFI. This finding was in line with the previous studies (Gosalvez et al. Citation2011; Sanchez-Martin et al. Citation2013). Nevertheless, we found that abstinence time was not associated with sperm vitality and AR, which suggests that it, like age, does not appear to affect sperm viability and fertilization ability. Our study also illustrated that the total motile sperm count increased with abstinence time only up to 5 days but not beyond. Since a higher motile sperm count may improve the pregnancy rate of intrauterine insemination (IUI) (Dong et al. Citation2011), our findings are of relevance in the counseling of IUI patients.
Regarding sleep habits, our study found that sperm concentration dropped remarkably if participants slept less than 4.7 h/day, remained stable if they slept between 4.7 and 8 h/day and increased noticeably when slept more than 8 h/day. We also found that irregular sleep was positively and linearly associated with DFI. The underlying mechanism of such an association is unclear. A previous study has shown that melatonin, which plays a major role in the regulation of the circadian wake-sleep cycle (Ortiz et al. Citation2011), was lower in night shift workers than that of workers without night shift (Bhatti et al. Citation2017). It has also been reported that the urinary melatonin level was positively associated with sperm concentration (Ortiz et al. Citation2011) and the cellular oxidative DNA damage repair capacity (Bhatti et al. Citation2017). The findings of our study and previous studies together suggest a significant association between sleep quality and sperm quality, probably both occurring as a result of disturbed melatonin secretion.
As for daily juice intake, we found that it was associated with sperm motility in a non-linear fashion, and positively associated with sperm AR. However, we were unable to confirm the finding of a previous study that it was significantly associated with sperm concentration (Turk et al. Citation2008), which may be due to the different type of juice investigated. Regarding daily coffee intake, we found that it was significantly and non-linearly associated with semen volume and total sperm count, which was not previously observed; although a number of studies reported inconsistent results on the association between coffee intake and semen parameters (Eslamian et al. Citation2012; Jurewicz et al. Citation2014a; Xia et al. Citation2015).
Some negative findings in this study were also of interest. We found that BMI, smoking and alcohol drinking index, daily tea intake, daily carbonated drink intake, water intake, meat intake habits, sports frequency and trouser cell phone use were not significantly associated with any of the sperm parameters examed. Regarding BMI, a previous meta-analysis has demonstrated a J-shaped link between BMI categories and risk of oligozoospermia or azoospermia (Sermondade et al. Citation2013). However, two recent multicenter, large sample size studies showed no correlation between BMI and sperm quality (Povey et al. Citation2012; Cherry et al. Citation2014; Eisenberg et al. Citation2014). Our result was consistent with the finding of latter studies. When considering cigarette smoking, our result was in line with the CHAPS study (Cherry et al. Citation2014; Pacey et al. Citation2014), but different to a meta-analysis, which showed that smoking has a detrimental effect on sperm motility and concentration (Sharma et al. Citation2016b). Also, we did not find any association between alcohol consumption and semen quality which was again in line with the CHAPS study (Povey et al. Citation2012; Pacey et al. Citation2014). Discordance with a recent review reported a positive association between excess alcohol intake and semen volume and morphology was noted (Ricci et al. Citation2017). When considering tea intake, our result was in line with a recent meta-analysis which showed that caffeine intake (including tea and caffeinated beverages) did not show a clear association with semen parameters (Salas-Huetos et al. Citation2017). Regarding carbonated drink intake habits, our result was different to previous studies, which found a negative association between carbonated drink and semen volume, total sperm count and sperm motility (Yang et al. Citation2015). We also found that meat intake was not significantly associated with semen parameters; in previous studies, no clear association was found between meat (full-fat dairy products) and semen quality (Afeiche et al. Citation2014b; Salas-Huetos et al. Citation2017). However, we were unable to confirm the finding of previous studies which showed that increased fish intake was related to higher sperm count and sperm morphology (Afeiche et al. Citation2014a). Finally, we did not find any significant association between trouser cell phone use and semen quality which was in line with one previous study (Zhang et al. Citation2016), but different from others (Jurewicz et al. Citation2014a; Zilberlicht et al. Citation2015).
There are several strengths in our study. Firstly, we employed a GAM model which can detect both linear and non-linear association between demographic lifestyle factors and semen parameters, whereas many previous studies used logistic or linear regression analysis that can only detect linear association (Braga et al. Citation2012; Povey et al. Citation2012; Pacey et al. Citation2014). Secondly, our study examined not only the effect of demographic and lifestyle factors on the results of conventional SA but also sperm vitality, DFI and sperm AR. Although the traditional semen parameters were helpful to assess the ability of spermatogenesis and sperm maturation, the discriminative power of traditional SA is often considered to be insufficient (Oehninger et al. Citation2014; Zhu et al. Citation2016). DFI were considered to be a good predictor for pregnancy and miscarriage (Simon et al. Citation2017; Zheng et al. Citation2018); The AR test is helpful to estimate the sperm fertilization ability; and sperm vitality is a good test to exam the sperm viability (World Health Organization Citation2010).
One possible weakness of our study, which is common to cross-sectional studies of its kind, is that our study only described association but not causation. Moreover, the subjects included in the study were all drawn from the infertile population, and so the findings may not be extrapolated to the general population.
In conclusion, the results of our study showed that DFI and total sperm count appeared to be the most sensitive semen parameters to demographic and lifestyle factors, whereas age was the most influential factors that significantly influenced five sperm parameters. Our analysis using the GAM model provided a better understanding of the type of association between semen parameters and lifestyle and demographic factors and should help with the counseling of subjects regarding lifestyle modification and how it can be monitored. In future studies examining the relationship between life style and demographic features and sperm parameters, we would recommend (1) the use of GAM model as it can analyze not only the linear but also non-linear associations; (2) the inclusion of subjects not only from those attending the infertility service but also from the general population, such as sperm donors.
Material and methods
Study population
In this study, men under 65 years of age who underwent SA in the andrology lab of Prince of Wales Hospital, Chinese University of Hong Kong, between November 2015 and November 2016, were initially recruited in this study. Written informed consents were obtained from all of the recruited men. Participants with known andrological and systematical disorders that are known causes of poor semen quality were excluded. The exclusion criteria were applied to the following exclusion criteria present at the time: (1) azoospermia; (2) andrological conditions known to affect semen parameters including genetic conditions involving the sex chromosome (such as AZFc microdeletion), history of mumps orchitis, severe varicocele (bilateral or unilateral), undescended testis, history of testicular torsion or scrotal injury, congenital bilateral absence of vas deferens (CBAVD) and urogenital infection; (3) taking medication known to affect semen parameters, including steroid, finasteride, calcium channel blockers; (4) history of malignant disease; (5) known mental disorders; (6) drug abuse; and (7) failure to complete the lifestyle questionnaire.
Routine SA was performed for all participants. Additional sperm tests, such as sperm vitality assessments, AR assay and sperm DNA fragmentation was performed for research purpose for a subset of subjects who had sufficient semen sample remaining after routine diagnostic evaluation. This study was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (REC No.: 2015.491).
Semen analysis
All semen samples were obtained in the hospital by masturbation after 3–7 days of sexual abstinence. SA was performed manually according to World Health Organization guidelines (version V) (World Health Organization Citation2010), and the SA performer has fulfilled the external quality assessment system from United Kingdom National External Quality Assessment Service (UK NEQAS). Briefly, after semen collection, samples were liquefied at 37°C and analyzed within 1 h after ejaculation. After liquefaction, semen volume was measured by a wide-bore graduated pipette with a graduation of 0.1 ml. Sperm concentration was measured, and motility was assessed under a phase contrast microscope (OLYMPUS BX43, Japan) at a magnification of × 200 or 400. When measuring the sperm concentration, standard dilutions were used when necessary, and counting was done after 10–15 min sedimentation by hemocytometers with improved Neubauer ruling. When assessing sperm motility, a wet preparation was made with a drop of 10 μl semen sample and a 22 mm× 22 mm coverslip to give a depth of 20 μm. Duplicate assessments were performed, and at least 200 spermatozoa were assessed on each occasion. To evaluate sperm morphology, Tygerberg Strict Criteria were used after staining the slides with a Diff-Quik staining kit (Dade Behring AG, Switzerland), and assessments were performed under a microscope with an oil immersion of × 100 objective (OLYMPUS BX43, Japan). All analyses were performed by one experienced technician who was blinded to the study. The quality control of the andrology lab at Prince of Wales Hospital is conducted by performing routinely internal quality assessments and participating in the external quality control scheme of UK NEQAS.
Sperm vitality assessments
Sperm vitality was assessed within 30 min of ejaculation according to the requirement of WHO (Moskovtsev and Librach Citation2013) by eosin-nigrosin staining (Vita Eosine, RAL Instruments) on sperm smears. The percentage of sperm membrane integrity (sperm head unstained indicating sperm with intact membrane) was assessed by counting a minimum of 100 spermatozoa. For each sample, two replicate counts were performed. These were then repeated if >5% difference was found.
In vitro sperm capacitation and acrosome reaction assay
Fresh semen samples were processed by two-layer density gradient (80–40%) separation using SpermGrad (Vitrolife, San Diego, CA, SA). Highly motile sperms pelleted at the bottom of the tube were collected after density gradient. Then, the sperm pellet was resuspended to a concentration of 5 × 106 using human tubal fluid with 5% human serum albumin (HTF and HSA, Irvine Scientific, Santa Ana, CA, USA). The sperm suspension was subsequently incubated at 37°C in an atmosphere of 5% CO2 for 3 h to induce sperm capacitation. After that, 10 µM calcium ionophore A23187 (Sigma-Aldrich, MO, USA) was added to the sperm suspension and incubated at 37°C and 5% CO2 for 15 min to induce AR. Next, 10 μl of the sperm suspension was transferred onto a microscope slide, air-dried and fixed for 20 min in 4% paraformaldehyde at room temperature. Then, 5 μg/ml peanut agglutinin (PNA)-FITC (Sigma-Aldrich, MO, USA) was added to the sperm smear and incubated in the dark to stain the acrosome. Finally, the slides were analyzed by counting 200 cells per sample by high-magnification at × 400 using a Leica fluorescence microscope (Leica Microsystem DFC450, Germany).
Sperm DNA fragmentation
To avoid the generation of oxygen species that would cause DNA fragmentation, no extra centrifugation was performed on sperm samples before sperm DNA fragmentation testing (Shi et al. Citation2016). Sperm DNA fragmentation was measured according to conventional sperm chromatin structure assay (SCSA) as described by Evenson et al. (Citation2002). In brief, stored semen samples were thawed on ice and 2 × 106 sperm cells were aliquoted into the flow tube. Then, samples were treated for 30 s with 400 μl of a solution containing 0.1% Triton X-100, 0.15 m NaCl, and 0.08 N HCl (pH 1.2). After 30 s, 1.2 ml of staining buffer (6 g/mL acridine orange (AO), 37 ml citric acid, 126 ml Na2HPO4, 1 ml disodium EDTA, 0.15 M NaCl (pH 6.0)) was mixed into the flow tube. The sample was then placed in an FC500 flow cytometer (Becton Dickinson, San Jose, CA, USA) for testing. A minimum of 20,000 cells from two aliquots of each sample was analyzed. The DFI was calculated as the ratio of denatured DNA (red fluorescence) to total DNA (red + green fluorescence).
Demographic and lifestyle factors examined in relation to sperm quality
All demographic and lifestyle related information was collected by a self-report questionnaire immediately before or after the semen collection. Events believed to be a risk factor were included in the lifestyle questionnaire, and the followed items were included: (1) BMI; (2) cigarette smoking frequency (pieces/week) and alcohol drinking frequency (units/week) within 3 months; (3) sleeping habit: (a) irregular sleeping habit: including night shift, insomnia and stay up, and (b) average sleep duration per day; (4) daily fluid intake (including coffee, tea, carbonated drinks, juice and water); (5) weekly meat intake frequency (including livestock, poultry, fish and seafood); (6) sports frequency; (7) trouser cell phone use; (8) age; and (9) abstinence time. The items in the questionnaire were explained by one investigator before reporting by participants.
Statistical analysis
All data were analyzed by using the R software (version 3.4.2 for Windows) (Development Core and Team Citation2004). Continuous variables with normal distribution were presented as the mean ± standard deviation (SD); whereas data with skewed distribution were presented as a median and interquartile range (IQR). Categorical data were presented as percentages and numbers. The Mann-Whitney U test was used to compare the differences in demographic and lifestyle factors and semen parameters between participants with or without additional sperm functional testing.
To examine the relationship between various semen parameters and demographic and lifestyle factors, the generalized additive model (GAM) was employed. GAM could handle the complex linear and nonlinear relationships at the same time among outcome variables and numerous explanatory variables, and is therefore suitable for investigating the relationship between lifestyle factors and semen quality (Chen et al. Citation2016). A Box-Cox Transform (Box and Cox Citation1964) was applied to skewed variable including semen volume, sperm concentration, TSC, TMC, sperm vitality and percentage of acrosome-reacted sperm. The initial step of GAM consisted of univariate analysis to identify significant associations, followed by the use of multivariate analysis to examine if there was still any significant association when adjusted for other demographic and lifestyle factors. The analysis of the GAM was performed using the ‘mgcv’ package, and the visualization of GAM model was conducted by package ‘visreg’. Values of P < 0.05 were considered statistically significant.
Supplemental Material
Download Zip (75 KB)Acknowledgments
We are grateful to all subjects who agreed to participate in this study.
Supplemental data
Supplemental data for this paper can be accessed on the publisher’s website.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Tin-Chiu Li
Collected questionnaire, carried out the experiments, analyzed the data, and drafted the manuscript: XS; Semen analysis: CPS-C; Assisted with manuscript writing: TW; Assisted with experiment: LC; Advised on the presentation of data and participated in drafting the manuscript: DYL-C; Conceived the idea and provided overall supervision of the project: TC-L.
References
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE. 2014a. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 144(7):1091–1098.
- Afeiche MC, Williams PL, Gaskins AJ, Mendiola J, Jorgensen N, Swan SH, Chavarro JE. 2014b. Meat intake and reproductive parameters among young men. Epidemiology. 25(3):323–330.
- Bandel I, Bungum M, Richtoff J, Malm J, Axelsson J, Pedersen HS, Ludwicki JK, Czaja K, Hernik A, Toft G, et al. 2015. No association between body mass index and sperm DNA integrity. Hum Reprod. 30(7):1704–1713.
- Barratt CL, Mansell S, Beaton C, Tardif S, Oxenham SK. 2011. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl. 13(1):53–58.
- Bhatti P, Mirick DK, Randolph TW, Gong J, Buchanan DT, Zhang JJ, Davis S. 2017. Oxidative DNA damage during night shift work. Occup Environ Med. 74(9):680–683.
- Box GEP, Cox DR. 1964. An analysis of transformations (with discussion). J R Statist Soc. 26:211–252.
- Braga DP, Halpern G, Figueira Rde C, Setti AS, Iaconelli A Jr., Borges E Jr. 2012. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 97(1):53–59.
- Chen J, Peng L, He S, Li Y, Mu Z. 2016. Association between environmental factors and hospital visits among allergic patients: A retrospective study. Asian Pacific J Allergy Immunol. 34(1):21–29.
- Cherry N, Povey AC, McNamee R, Moore H, Baillie H, Clyma JA, Dippnall M, Pacey AA; Participating Centres of C-U. 2014. Occupation exposures and sperm morphology: a case-referent analysis of a multi-centre study. Occup Environ Med. 71(9):598–604.
- Collodel G, Moretti E, Del Vecchio MT, Biagi M, Cardinali R, Mazzi L, Brecchia G, Maranesi M, Manca D, Castellini C. 2014. Effect of chocolate and propolfenol on rabbit spermatogenesis and sperm quality following bacterial lipopolysaccharide treatment. Syst Biol Reprod Med. 60(4):217–226.
- R Development Core Team RDC. 2004. R: A language and environment for statistical computing R. Vienne: Austria Foundation for Statistical Computing.
- Dong F, Sun Y, Su Y, Guo Y, Hu L, Wang F. 2011. Relationship between processed total motile sperm count of husband or donor semen and pregnancy outcome following intrauterine insemination. Syst Biol Reprod Med. 57(5):251–255.
- Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. 2014. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 29(2):193–200.
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. 2012. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 27(11):3328–3336.
- Evenson DP, Larson KL, Jost LK. 2002. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 23(1):25–43.
- Freour T, Jean M, Mirallie S, Barriere P. 2012. Computer-assisted sperm analysis parameters in young fertile sperm donors and relationship with age. Syst Biol Reprod Med. 58(2):102–106.
- Gaskins AJ, Mendiola J, Afeiche M, Jorgensen N, Swan SH, Chavarro JE. 2015. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med. 49(4):265–270.
- Gosalvez J, Gonzalez-Martinez M, Lopez-Fernandez C, Fernandez JL, Sanchez-Martin P. 2011. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril. 96(5):1083–1086.
- Jee HJ, Cho CH, Lee YJ, Choi N, An H, Lee HJ. 2017. Solar radiation increases suicide rate after adjusting for other climate factors in South Korea. Acta Psychiatrica Scandinavica. 135(3):219–227.
- Jurewicz J, Radwan M, Sobala W, Ligocka D, Radwan P, Bochenek M, Hanke W. 2014a. Lifestyle and semen quality: role of modifiable risk factors. Syst Biol Reprod Med. 60(1):43–51.
- Jurewicz J, Radwan M, Sobala W, Radwan P, Jakubowski L, Hawula W, Ulanska A, Hanke W. 2014b. Lifestyle factors and sperm aneuploidy. Reprod Biol. 14(3):190–199.
- Merzenich H, Zeeb H, Blettner M. 2010. Decreasing sperm quality: a global problem? BMC Public Health. 10:24.
- Mikkelsen EM, Riis AH, Wise LA, Hatch EE, Rothman KJ, Cueto HT, Sorensen HT. 2016. Alcohol consumption and fecundability: prospective Danish cohort study. BMJ. 354:i4262.
- Moskovtsev SI, Librach CL. 2013. Methods of sperm vitality assessment. Methods Mol Biol. 927:13–19.
- Oehninger S, Franken DR, Ombelet W. 2014. Sperm functional tests. Fertil Steril. 102(6):1528–1533.
- Ortiz A, Espino J, Bejarano I, Lozano GM, Monllor F, Garcia JF, Pariente JA, Rodriguez AB. 2011. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J Pineal Res. 50(2):132–139.
- Pacey AA, Povey AC, Clyma JA, McNamee R, Moore HD, Baillie H, Cherry NM; Participating Centres of Chaps UK. 2014. Modifiable and non-modifiable risk factors for poor sperm morphology. Hum Reprod. 29(8):1629–1636.
- Povey AC, Clyma JA, McNamee R, Moore HD, Baillie H, Pacey AA, Cherry NM; Participating Centres of C-u. 2012. Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Hum Reprod. 27(9):2799–2806.
- Ricci E, Al Beitawi S, Cipriani S, Candiani M, Chiaffarino F, Vigano P, Noli S, Parazzini F. 2017. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online. 34(1):38–47.
- Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. 2013. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 28(2):462–470.
- Salas-Huetos A, Bullo M, Salas-Salvado J. 2017. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 23(4):371–389.
- Sanchez-Martin P, Sanchez-Martin F, Gonzalez-Martinez M, Gosalvez J. 2013. Increased pregnancy after reduced male abstinence. Syst Biol Reprod Med. 59(5):256–260.
- Sartorius GA, Nieschlag E. 2010. Paternal age and reproduction. Hum Reprod Update. 16(1):65–79.
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M, et al. 2013. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 19(3):221–231.
- Sharma R, Ahmad G, Esteves SC, Agarwal A. 2016a. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet. 33(2):291–300.
- Sharma R, Harlev A, Agarwal A, Esteves SC. 2016b. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 70(4):635–645.
- Shi X, Wang T, Qiu ZL, Li K, Li L, Chan CP, Chan SM, Li TC, Quan S. 2016. Effects of mechanical stresses on sperm function and fertilization rate in mice. Syst Biol Reprod Med. 62(2):152–159.
- Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. 2017. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 19(1):80–90.
- Tandara M, Bajic A, Tandara L, Bilic-Zulle L, Sunj M, Kozina V, Goluza T, Jukic M. 2014. Sperm DNA integrity testing: big halo is a good predictor of embryo quality and pregnancy after conventional IVF. Andrology. 2(5):678–686.
- Turk G, Sonmez M, Aydin M, Yuce A, Gur S, Yuksel M, Aksu EH, Aksoy H. 2008. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr. 27(2):289–296.
- World Health Organization. 2010. WHO laboratory manual for the Examination and processing of human semen. Fifth Edition. Geneva: WHO Press.
- Wu Y, Kang X, Zheng H, Liu H, Liu J. 2015. Effect of paternal age on reproductive outcomes of in vitro fertilization. PLoS One. 10(9):e0135734.
- Xia W, Chiu YH, Williams PL, Gaskins AJ, Toth TL, Tanrikut C, Hauser R, Chavarro JE. 2015. Men’s meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil Steril. 104(4):972–979.
- Yang H, Chen Q, Zhou N, Sun L, Bao H, Tan L, Chen H, Zhang G, Ling X, Huang L, et al. 2015. Lifestyles associated with human semen quality: results from MARHCS Cohort Study in Chongqing, China. Medicine (Baltimore). 94(28):e1166.
- Zhang G, Yan H, Chen Q, Liu K, Ling X, Sun L, Zhou N, Wang Z, Zou P, Wang X, et al. 2016. Effects of cell phone use on semen parameters: results from the MARHCS cohort study in Chongqing, China. Environ Int. 91:116–121.
- Zheng WW, Song G, Wang QL, Liu SW, Zhu XL, Deng SM, Zhong A, Tan YM, Tan Y. 2018. Sperm DNA damage has a negative effect on early embryonic development following in vitro fertilization. Asian J Androl. 20(1):75–79.
- Zhu QX, Gao ES, Pathak N, Wu JQ, Zhou WJ. 2016. Single or double semen samples: the dilemma in epidemiological studies on semen quality. Hum Reprod. 31(3):511–517.
- Zilberlicht A, Wiener-Megnazi Z, Sheinfeld Y, Grach B, Lahav-Baratz S, Dirnfeld M. 2015. Habits of cell phone usage and sperm quality - does it warrant attention? Reprod Biomed Online. 31(3):421–426.