ABSTRACT
The purpose of our study was to use a time-lapse monitoring (TLM) system to determine if day 3 blastomere biopsy for preimplantation genetic testing (PGT) had an impact on subsequent morphokinetic parameters at the morula and blastocyst stages. In this retrospective monocentric study conducted between May 2013 and August 2017, we compared late morphokinetic parameters in embryos undergoing day 3 blastomere biopsy for PGT and in control non-biopsied embryos obtained in intracytoplasmic sperm injection (ICSI) cycles for male infertility. All embryos in both groups were cultured in a TLM system. The biopsy group was composed of 1691 embryos (386 PGT cycles). The control group was composed of 2578 embryos (786 ICSI cycles). Early morphokinetic parameters up to day 3 were similar in both groups. Concerning late morphokinetic parameters, the onset of compaction (tSC), fully-compacted morula stage (tM), onset of cavitation/early blastulation (tSB), and blastocyst stages (tB and tEB) appeared significantly earlier in the biopsy group than in the control group. We found that late morphokinetic events at the morula and the blastocyst stages occurred significantly earlier in biopsied embryos than in control non-biopsied-embryos. The mechanisms underlying these modifications of embryo development after biopsy should be investigated in order to determine precisely, and this phenomenon could be associated with embryo, fetal, and offspring development.
Abbreviations: TLM: time-lapse monitoring; PGT: preimplantation genetic testing; ICSI: intracytoplasmic sperm injection; tSC: the onset of compaction; tM: fully-compacted morula stage; tSB: onset of cavitation/early blastulation; tB and tEB: blastocyst stages; OHSS: ovarian hyperstimulation syndrome
Introduction
Pre-implantation genetic testing (PGT) is a procedure developed in the early 90s for couples with a high risk of transmitting a genetic abnormality or with a high risk of miscarriage because of chromosomal translocation (Harton et al. Citation2011a). Cleavage-stage blastomere biopsy has been the most commonly used method during many years. However, it is an invasive procedure in which a zona pellucida breaching, a cell adhesion disruption and an aspiration of one or 2 blastomeres are needed (Harton et al. Citation2011b). Trophectoderm biopsy has recently gained interest in PGT cycles. Indeed, this approach allows a better assessment of embryo quality and the analysis of more embryonic cells, leading to improved clinical outcome in PGT cycles (Scott et al. Citation2013). However, day 3 blastomere biopsy also has a few advantages, as it allows fresh embryo transfer and requires less time and material for embryo culture. As such, day 3 embryo biopsy is used in a significant number of PGT centers.
Some studies have explored the potential impact of the day 3 biopsy procedure on subsequent embryo development. Most authors found that removing two cells on day 3 could reduce the blastulation rate and subsequently implantation rate as compared to one cell [2, 3]. However, few authors studied in detail the impact of day 3 embryo biopsy on subsequent embryo developmental stages, i.e., morula and blastocyst. Studies on human embryos evocated delayed compaction and blastulation as compared to non-biopsied embryos (Kirkegaard et al. Citation2012, Bar-El et al. Citation2016).
Since the release of the first commercial time-lapse monitoring (TLM) system in 2009, several laboratories around the world have implemented this technology in order to improve embryo culture conditions and embryo quality evaluation. As it allows continuous monitoring of embryo development, this technology is a useful tool to observe embryo development after blastomere biopsy. The studies of Kirkegaard et al. Citation2012 and Bar-El et al. Citation2016, reported the use of TLM system to evaluate post-biopsy embryo development in PGT cycles. Both found that embryonic development was significantly delayed after blastomere biopsy on day 3, as compared to non-biopsied embryos. However, these studies were conducted in relatively limited population (109 and 751, respectively), thus highlighting the need for confirmation in larger studies. Therefore, this study aimed at evaluating the potential impact of blastomere biopsy on subsequent embryo development by comparing late morphokinetic parameters in embryos with or without blastomere biopsy on day 3.
Results
A total of 1691 embryos obtained in 386 PGT cycles and undergoing blastomere biopsy on day 3, were compared to 2578 non-biopsied embryos obtained in 786 control ICSI cycles.
Demographic and cycle characteristics are presented in . Control group and biopsy group were not different concerning female characteristics, except for serum AMH level and smoking status. The proportion of couples with secondary infertility and fertilization rate were significantly higher in the PGT group than in the control ICSI group. There was no significant difference in terms of mean number of day 3 embryos available for biopsy (or theoretically compatible with biopsy), i.e., good or medium quality embryos. As expected in the context of PGT, the proportion of clinically usable embryos, i.e., transferred or frozen, was significantly lower in the biopsy group than in the control ICSI group.
Table 1. Patients’ and IVF cycle characteristics: demographic, cycle, and embryologic characteristics in control and biopsy groups
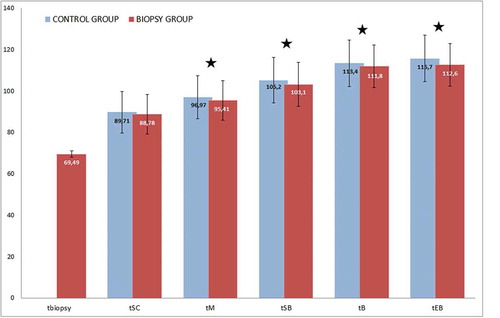
Next, we compared embryo morphokinetic parameters between the two groups. Early morphokinetic events up to day 3 were similar in both groups (data not shown). Late embryo development events (day 4 onwards) were significantly different between the two groups. Indeed, the onset of compaction (tSC), fully-compacted morula stage (tM), onset of cavitation/early blastulation (tSB) and blastocyst stages (tB and tEB) occurred significantly earlier in the biopsy group than in the control group ().
Figure 1. Average timing of late morphokinetic parameters in control and biopsy groups. Results are represented in blue for control group and red for biopsy group. Time is expressed in hours post injection, time when embryos are biopsied (tbiopsy), the 9-cell stage (t9+), the onset of compaction (tSC), the fully-compacted morula stage (tM), the onset of cavitation (tSB) and blastocyst stages (tB) were compared. Parameters with star are significantly different between the 2 groups (*p < 0.05). Late morphokinetic events at the morula and the blastocyst stages, tM, tSB, tB and tEB, occurred significantly earlier in biopsied embryos than in control non biopsied-embryos
Discussion
In the present study, we found that late morphokinetic events at the morula and the blastocyst stages occurred significantly earlier in biopsied day 3 embryos than in non-biopsied control embryos, suggesting that blastomere biopsy on day 3 affects subsequent embryo development.
Apart from embryo development in vitro, the impact of day 3 embryo biopsy on implantation potential has already been reported. Scott et al. (Citation2013) demonstrated that day 3 embryo biopsy-impaired embryo implantation compared to a control sibling embryo. Indeed, the transfer of two embryos on day 3, one biopsied and one sibling control, resulted in significantly higher live birth rate in control embryos as compared to the biopsied embryos (50% vs 30%, respectively).
Our results are in contradiction with the only two previous studies performed with a quite similar method. In a series of 56 biopsied and 53 non-biopsied embryos cultured in a time-lapse system, Kirkegaard et al. (Citation2012) reported that the duration of compaction, morula, and early blastocyst stages were identical in the two groups, while the duration of the expanded blastocyst stage was significantly shorter in the biopsied group than in the control group. In addition to the very limited number of embryos included in this study, it should be noted that embryo culture was performed under atmospheric O2 pressure (20%) in this study, whereas we cultured embryos under low O2 pressure (5%). This might account, at least in part, for these conflicting results, as oxygen concentration has been shown to influence embryo developmental rate (Kirkegaard et al. Citation2013). Although this remains speculative in the absence of specific trial, the different method used for blastomere biopsy (i.e., blastomere aspiration versus pressure/extrusion) might also account for these differences. Another similar recent study compared morphokinetic parameters in 366 biopsied embryos and 385 non-biopsied embryos (Bar-El et al. Citation2016). Unlike our study, the authors reported that post-biopsy morphokinetic parameters were significantly delayed in the biopsy group as compared to the control group. The main difference between this study and ours lies within its design. Indeed, control embryos were not selected according to their morphology on day 3, but rather to their late developmental ability (blastulation).
An older study based on conventional static microscopy (without time-lapse) also evaluated the effect of blastomere biopsy on day 3 on subsequent embryo development (Tarín et al. Citation1992). Tarin et al. studied 129 human embryos randomized in the day 2 biopsy group on or non-biopsy group. Although the blastulation rate was similar in both groups, they observed a significantly higher proportion of embryos reaching the morula stage after day 4 in the biopsied-group as compared to the control group, suggesting acceleration to late embryo developmental stages in the biopsy group, in agreement with our findings.
In parallel to morphologic/morphokinetic events occurring after blastomere biopsy, the issue of genetic modifications potentially induced by the biopsy procedure is of utmost importance. All experiments conducted in mouse lead to reassuring conclusions, with no significant modification of the global gene expression pattern (Duncan et al. Citation2009) while maintaining the expression of a critical micro RNA and its target genes (Naseri et al. Citation2019) after blastomere biopsy.
Biophysical mechanisms and forces regulating actively participate in embryonic cell adhesion, which in turn sets the stage for blastocyst formation and cell fate (Maître Citation2017); (White et al. Citation2018). In this context, it can be speculated that the use of calcium and magnesium-free medium followed by an invasive procedure such as blastomere biopsy on day 3 might disturb spatial organization and emerging cell-to-cell interactions, even though they are theoretically put in place slightly later at the morula stage (on day 4 in humans and on day 3 in mouse). However, the majority of the existing literature on cell fate and cell adhesion in embryos was obtained in mouse, thus questioning its generalizability in humans. Whether embryo biopsy procedure significantly impacts cell fate and embryo organization in human embryos is not known yet, and the need for research focused on blastomere biopsy has been recently highlighted in a review (Maître Citation2017). In any case, the increasing use of trophectoderm biopsy will probably soon make these concerns less relevant.
Although statistical power calculation could not be performed according to the retrospective design of this study, the large number of embryos included here might represent a significant strength over the existing literature. Time-lapse systems are objective and accurate tools to observe cell division kinetics. In this respect, this technology is relevant for the study of fine differences in embryo developmental speed after biopsy. However, our results should be interpreted with care, as the biopsy procedure was not strictly homogeneous in all cases, with either 1 or 2 blastomeres extracted. Moreover, this study was not designed to evaluate the impact of embryo biopsy on implantation, and the statistical differences identified might not be representative of altered implantation potential. The significant differences observed between the 2 groups in terms of ovarian reserve and smoking status might also be considered as potential confounding factors, as some studies reported an association between these factors and morphokinetic parameters (Salvarci et al. Citation2017) (Bourdon et al. Citation2020).In conclusion, the present study demonstrates that late morphokinetic events at the morula and the blastocyst stages occurred significantly earlier in biopsied embryos than in control non-biopsied-embryos. The mechanisms underlying these modifications of embryo development after biopsy should be investigated in order to determine precisely is this phenomenon could be associated with embryo, fetal and offspring development.
Materials and methods
Patients
This monocentric retrospective cohort study was conducted in couples referred for PGT because of a structural chromosomal rearrangement (PGT-SR) or for monogenic/single gene defect (PGT-M). PGT cycles for aneuploidy screening (PGT-A) are not allowed in our country. We analyzed the clinical and biological data of all consecutive patients who had undergone a PGT cycle with autologous oocyte and embryo culture performed using the Embryoscope® (Vitrolife®) between May 2013 and August 2017 in our University Fertility Center.
All morphokinetic parameters were compared in PGT cycles (pre- and post-biopsy) and in the control group. The control group consisted in intracytoplasmic sperm injection (ICSI) cycles performed in patients with male factor during the same time period and whose embryos (cultured in the Embryoscope® up to blastocyst stage) had medium to good morphology on day 3, i.e., theoretically compatible with embryo biopsy.
Ovarian stimulation
All patients underwent controlled ovarian stimulation with the antagonist protocol. Gonadotropin starting dose was chosen according to female age, ovarian reserve and previous PGT cycles, if any. Cycle monitoring consisted of regular hormonal assays and ultrasonography, and ovulation was triggered with recombinant hCG when at least three follicles reached 18 mm in diameter, or with GnRH agonist in case of ovarian hyperstimulation syndrome (OHSS).
Oocyte retrieval, ICSI and embryo culture
Oocyte retrieval was performed 34–36 h later. Control and biopsied embryos were treated exactly the same way for embryo culture. After denudation with hyaluronidase (SynVitro® hyadase, Origio), all mature oocytes were microinjected and immediately placed in individual microwells within a specific culture dish (Embryoslide®, Vitrolife®) before being loaded into the Embryoscope®. Embryo culture was performed at 37°C under a controlled atmosphere with low oxygen pressure (5% O2, 6% CO2). Sequential media were used for embryo culture, (G1plus® up to day 3 and G2plus® up to day 6, Vitrolife®).
Time lapse analysis
Each embryo was investigated by detailed time-lapse analysis measuring the exact timing of the developmental events in hours after ICSI procedure, as described by (Ciray et al. Citation2014). The terms t2, t3, t4, t5, t6, t7, and t8 were used for the exact timings of appearance of embryos with 2, 3, 4, 5, 6, 7, and 8 well-defined blastomeres, respectively. Concerning late embryonic events, the onset of compaction (tSC), the fully-compacted morula stage (tM), the early cavitation/onset of blastulation (tSB), the onset of expansion (tB) and the fully expanded stage (tEB) were recorded. The annotation of morphokinetic parameters was performed by two trained senior embryologists. Quality control where regularly processed in the laboratory to ensure the reproducibility and the precision of the results.
Embryo biopsy and embryo transfer
Embryo biopsy was performed on day 3 for all embryos with at least 6 blastomeres, <25% fragmentation and fair evenness. Briefly, embryos were first placed in Ca/Mg-free medium (G-PGD, Vitrolife®) for a few minutes, before laser-assisted zona pellucida hatching (ZilosTK, Hamilton Thorn®). One to 2 cells were then extracted for subsequent genetic analysis depending on the number of blastomeres (1 cell in 6 to 7-cell embryos, 2 cells in ≥8-cell embryos). Cell extrusion was obtained by gently pressing on the zona pellucida with the biopsy pipette close to the breach. In our experience, this allows faster procedure and less cell damage than with conventional blastomere aspiration.
Balanced or unaffected embryos were selected for transfer on day 4 or 5 according to post-biopsy development for practical and organizational reasons. Single or double embryo transfer was chosen jointly by medical staff and the couple. A pregnancy test was carried out 11 or 12 days after embryo transfer, and if positive, clinical pregnancy was confirmed ultrasonographically 4–5 weeks later by the detection of a gestational sac and fetal heart activity.
Statistics
Student’s or Wilcoxon’s tests were used for continuous variables and χ2 or Fischer’s tests for categorical variables. The non-parametric Mann–Whitney test was used for non-normally distributed variables. Statistical analysis was performed using GraphPad Prism® software. P values <0.05 were considered to denote a statistically significant difference.
Ethics approval
All patients gave consent for the anonymous use of their data registered in this database. This protocol was approved by the local ethics committee (GNEDS).
Author contributions
Analyzed the data, wrote and revised the manuscript: JL, AR; expert knowledge, critically revised the manuscript: SL, SC, TL, PB; designed and supervised the study: TF.
Disclosure statement
The authors have nothing to disclose.
References
- Bar-El L, Kalma Y, Malcov M, Schwartz T, Raviv S, Cohen T, Amir H, Cohen Y, Reches A, Amit A, et al. 2016. Blastomere biopsy for PGD delays embryo compaction and blastulation: a time-lapse microscopic analysis. J Assist Reprod Genet. 33(11):1449–1457. doi:10.1007/s10815-016-0813-2
- Bourdon M, Ferreux L, Maignien C, Patrat C, Marcellin L, Pocate-Cheriet K, Chapron C, Santulli P. 2020. Tobacco consumption is associated with slow-growing day-6 blastocysts. F&S Rep. 1(1):30–36. doi:10.1016/j.xfre.2020.04.006.
- Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, Sayed S. 2014. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 29(12):2650–2660. doi:10.1093/humrep/deu278.
- Duncan FE, Stein P, Williams CJ, Schultz RM. 2009. The effect of blastomere biopsy on preimplantation mouse embryo development and global gene expression. Fertil Steril. 91(4):1462–1465. doi:10.1016/j.fertnstert.2008.07.1710.
- Harton GL, Harper JC, Coonen E, Pehlivan T, Vesela K, Wilton L. 2011a. ESHRE PGD consortium best practice guidelines for fluorescence in situ hybridization-based PGD. Hum Reprod (Oxford, England). 26(1):25–32. doi:10.1093/humrep/deq230.
- Harton GL, Magli MC, Lundin K,Montag M, Lemmen J, Harper JC. 2011b. ESHRE PGD consortium/embryology special interest group–best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum Reprod (Oxford, England). 26(1):41–46.
- Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. 2013. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril. 99(3):738–744.e4. doi:10.1016/j.fertnstert.2012.11.028.
- Kirkegaard K, Juhl Hindkjaer J, Ingerslev HJ. 2012. Human embryonic development after blastomere removal: a time-lapse analysis. Hum Reprod. 27(1):97–105. doi:10.1093/humrep/der382.
- Maître J-L. 2017. Mechanics of blastocyst morphogenesis: mechanics of blastocyst morphogenesis. Biol Cell. 109(9):323–338. doi:10.1111/boc.201700029.
- Naseri F, Hosseini S, Ghaffari Novin M, Hosseini A, Heidari MH, Salehi M. 2019. Does blastomere removal alter the expression level of MiR‐Let7a and its target genes following mouse embryo biopsy? J Cell Biochem. 120(6):9430–9436. doi:10.1002/jcb.28218.
- Salvarci A, Gurbuz AS, Uzman S, Kaya M, Gorkemli H. 2017. Comparison of embryo morphokinetics following intracytoplasmic sperm injection in smoker and non-smoker couples: are the results different? J Pak Med Assoc. 67(10):6.
- Scott RT, Upham KM, Forman EJ, Zhao T, Treff NR. 2013. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 100(3):624–630. doi:10.1016/j.fertnstert.2013.04.039.
- Tarín JJ, Conaghan J, Winston RM, Handyside AH. 1992. Human embryo biopsy on the 2nd day after insemination for preimplantation diagnosis: removal of a quarter of embryo retards cleavage. Fertil Steril. 58(5):970–976. doi:10.1016/S0015-0282(16)55444-2.
- White MD, Zenker J, Bissiere S, Plachta N. 2018. Instructions for assembling the early mammalian embryo. Dev Cell. 45(6):667–679. doi:10.1016/j.devcel.2018.05.013.
