ABSTRACT
Despite the increasing use of computer-aided sperm analysis (CASA) in clinical practice, there is still no golden standard for the type of slide to be used with these systems. Counting chamber depth and loading method, can profoundly influence motility and concentration estimates, thereby undermining the validity and accuracy of CASA. To contribute toward standardized sperm analysis, this study compared different commercially available capillary-filled slides including 10 and 20 µm deep Leja slides (Leja10 and Leja20); 10, 16 and 20 µm deep CellVision slides (CV10, CV16 and CV20); and drop-loaded slides including slide and coverslip (SCS) with a depth of 20.1 µm and the Makler chamber with a depth of 10 µm for sperm analysis when using CASA. The Sperm Class Analyzer (SCA) CASA system was used to assess concentration, motility, and detailed kinematic parameters of 20 normozoospermic human samples using the different chamber slides. Results were evaluated by the repeated measures ANOVA and Intraclass correlation coefficients. The Makler chamber showed significantly (P < 0.05) higher concentrations than other slides. However, there was no significant difference in the percentage of sperm in different motility groups among the slides. CV10, Leja10 and Makler showed significantly higher curvilinear-, average path- and straight-line velocity (VCL, VAP, VSL) values than other slides. In conclusion, despite the objectiveness of the assessments by CASA systems, there are still some discrepancies in the results of sperm concentration, motility and other kinematic parameters when using different commercially available slides. The possible negative influence of the sperm quality misdiagnosis on the selection of treatment strategy in a clinical setting, emphasizes the need for further standardization and quality control of the commercially available chamber slides for use with CASA. Furthermore, this study found more consistent results for capillary-filled chambers compared to drop-loaded slides, suggesting a superior method when using CASA.
Abbreviations: DNA: Deoxyribonucleic acid; CASA: Computer-aided semen analysis; SCA: Sperm Class Analyzer; WHO: World Health Organization; CV: CellVision; SCS: Slide and Coverslip; SD: Standard deviation; ANOVA: Analysis of Variance; PR: Progressive; NP: Non-progressive; IMT: Immotile; VCL: Curvilinear velocity; VAP: Average path velocity; VSL: Straight-line velocity; Lin: Linearity; STR: Straightness; WOB: Wobble; ALH: Amplitude of lateral head displacement; BCF: Beat cross frequency
Introduction
Infertility rates have been increasing since the early 1990s and affect approximately 10% of all couples (Cho et al. Citation2003; Wasilewski et al. Citation2020), reaching up to more than 50 million couples globally (Nosrati et al. Citation2017). About half of the infertility cases are associated with male-related factors (McCormack et al. Citation2006; Nosrati et al. Citation2016). As the first step in the investigation of male fertility potential, sperm quality assessment is commonly based on the evaluation of sperm with normal morphology, concentration, motility, vitality and DNA fragmentation (Kathiravan et al. Citation2011).
Before the introduction of the computer-aided semen analysis (CASA), semen quality assessment was performed manually by a technician visually assessing a semen sample using a microscope. As the mean value for motility of the entire sperm population in a sample is not a reliable and definitive indicator of sperm quality, the percentages of different sperm subpopulations, categorized based on velocity and progression, are also used for a more precise determination of sperm quality and fertilization potential (World Health Organization Citation2010; Maree and van der Horst Citation2013). However, the manual assessment method has demonstrated significant subjectivity, including intra-observer and inter-laboratory variations, especially in the estimated sperm counts and motility categorizations (Rivera-Montes et al. Citation2013).
The use of microscopy combined with photography was introduced in the 1970s to eliminate such variations, which led to the manufacturing of the first commercially available CASA systems following the advancement of computers and digital video imaging in the 1980s (Mortimer et al. Citation2015).
CASA has significantly advanced since, and the improved tail detection in the newer generations of CASA systems has even further enhanced sperm detection and identification, allowing for a better analysis of counts and motility by viewing the flagellum as opposed to the head (Mortimer et al. Citation2015). Thus, the modern CASA systems are now considered as an inexpensive yet superior method to the manual assessment, which can rapidly provide accurate results to be used for diagnosis and possibly predicting of fertility potential and pregnancy outcome (Hirano et al. Citation2001; Dearing et al. Citation2014; Lammers et al. Citation2014; Talarczyk-Desole et al. Citation2017). Furthermore, the detailed sperm kinematic parameters provided by CASA have also been shown to be of clinical relevance in the prediction of fertility and pregnancy in both animals and humans (Paston et al. Citation1994; Youn et al. Citation2011; Nagy et al. Citation2015).
Animal production laboratories currently use CASA routinely with excellent results in both domestic and wildlife species (Mortimer et al. Citation2015). However, despite the clear limitations and weaknesses in manual semen analysis, the human ‘fertility industry’ is still skeptical toward the use of CASA (Tomlinson and Naeem Citation2018).
On one hand, CASA has always worked well for analyzing processed (washed) human sperm which usually have a higher motility and contain less artifacts and debris. On the other hand, some biological (Mortimer et al. Citation2015) and technical (Mortimer Citation1994; Mortimer et al. Citation1995; Talarczyk-Desole et al. Citation2017) limitations can affect CASAs ability to provide as accurate results for sperm counts and motility in unprocessed semen. These limitations, and lack of knowledge and consensus on the value of detailed sperm motility and kinematic parameters could be an underlying cause of the skepticism toward the clinical use of CASA in the field of human fertility (Tomlinson and Naeem Citation2018).
Sperm quality assessment can be performed by analyzing the liquefied semen sample under a coverslip on a standard glass slide (slide-coverslip), in unique chamber slides designed for sperm analysis such as the Makler chamber (Makler Citation1978), or disposable chamber slides such as Leja and CellVision slides (Peng et al. Citation2015). Depending on the type of slide, chambers are either loaded by capillary refill action when semen is placed on the chamber entry port (disposable chamber slides) or by drop-loading when the sample is loaded directly onto a glass surface and covered with a coverslip (Makler and slide-coverslip).
Slides of different loading types have been reported to provide different results when comparing sperm quality. A comparative study by Lenz et al. in 2011, reported no significant difference in the percentage of total motile, or progressively motile sperm between slide-coverslip and the Makler chamber, which both use the drop loading method. The same study, however, demonstrated significantly higher progressive percentage and total motility determined by the Makler chamber and slide-coverslip compared to results from chambered Leja slides (Lenz et al. Citation2011). More recent studies have also reported a significantly higher sperm motility and concentration in chambers using the drop loading method compared to those loaded by capillary action in both human and animal semen (Lannou et al. Citation1992; Massányi et al. Citation2008; Hoogewijs et al. Citation2012; Peng et al. Citation2015; Del Gallego et al. Citation2017).
Slides with different loading types have been reported to provide different results when comparing sperm quality. A comparative study by Lenz et al. in 2011, reported no significant difference in the percentage of total motile, or progressively motile sperm between slide-coverslip and the Makler chamber, which both drop-loaded and chambers. The same study, however, demonstrated significantly higher progressive percentage and total motility determined by the Makler chamber and slide-coverslip compared to results from chambered Leja slides (Lenz et al. Citation2011).
Overall, there seems to be no golden standard for which type of slide to use for sperm analysis when using a CASA system, but what is known is that the counting chamber can, however, profoundly influence the motility and concentration estimates, thereby undermining the validity and accuracy of results provided even by CASA. Therefore, this study aimed to comparatively assess the differences in human sperm concentration, motility, and detailed kinematic parameters, when assessing the same sample using seven different commercially available slides by CASA. The results of this comparative study, considering the loading method and chamber depth begin to allow for a more standardized sperm analysis.
Results
The results of concentration assessment using different methods (slides) have been presented in . The Makler chamber displayed significantly higher concentrations compared to all other slides except the slide-coverslip (SCS). No significant difference was observed between the other slides.
Figure 1. Boxplot of sperm concentration (106/ml) assessed using CellVision chamber slides with depths of 10, 16 and 20 µm (CV10, CV16 and CV20), Leja chamber slides with a depth of 10 and 20 µm (Leja10, Leja20), Makler chamber (10 µm depth), and slide-coverslip (SCS; 20.1 µm depth). The number of asterisks demonstrates the significance value: *p < 0.05; **p < 0.01; ***p < 0.001
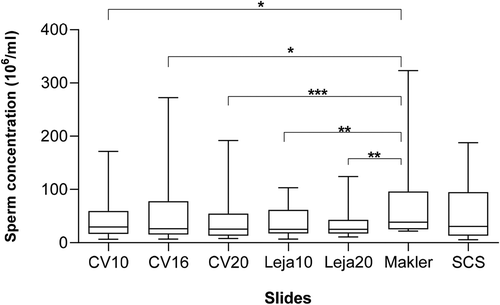
Ten of the twenty samples initially considered as normozoospermic by the Makler chamber had a concentration less than 40 × 106/ml. These samples demonstrated a concentration below the reference value (15 × 106/ml) recommended by the WHO guidelines (World Health Organization Citation2010), when assessed by at least one of the other slides in the study. The percentage of progressive (PR), non-progressive (NP) and immotile (IMT) spermatozoa did not show any significant difference among any of the groups ().
Table 1. The table illustrates the means ± standard deviations for the percentage of progressive (PR) non-progressive (NP) and immotile (IMT) spermatozoa assessed by CellVision chamber slides with depths of 10, 16 and 20 µm (CV10, CV16 and CV20), Leja chamber slides with a depth of 10 and 20 µm (Leja10, Leja20), Makler chamber (10 µm depth), and slide-coverslip (SCS; 20.1 µm depth)
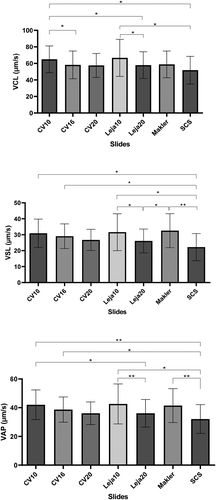
The average curvilinear velocity (VCL) value for different slides has been illustrated in . CV10 showed significantly higher VCL (p < 0.05) than Leja20, CV16 and SCS, while Leja10 showed significantly higher VCL (p < 0.05) than SCS and Leja20. SCS also demonstrated significantly lower straight-line velocity (VSL) and average path velocity (VAP) compared to CV10, CV16, Leja10 and the Makler chamber. Leja 20 showed significantly lower VSL than Leja10 and the Makler chamber, and lower VAP than Leja10 and CV10.
Figure 2. Average means (SD) of curvilinear velocity (VCL, upper panel), average path velocity (VAP, lower panel), and straight-line velocity (VSL, middle panel) assessed using CellVision chamber slides with depths of 10, 16 and 20 µm (CV10, CV16 and CV20), Leja chamber slides with a depth of 10 and 20 µm (Leja10, Leja20), Makler chamber (10 µm depth), and slide-coverslip (SCS; 20.1 µm depth). The horizontal lines and asterisks demonstrate a pairwise significance: *p < 0.05; **p < 0.01; ***p < 0.001
The data for other kinematic values including Linearity (LIN), straightness (STR), wobble (WOB), the amplitude of lateral head displacement (ALH) and beat cross frequency (BCF) are displayed in . Compared to other slides, Makler and SCS demonstrated the highest and lowest values of LIN, STR and WOB, respectively. Additionally, Makler and SCS displayed the lowest values for ALH and BCF compared to other slides, respectively ().
Table 2. The table illustrates the means ± standard deviations of the kinematic parameters LIN, STR, WOB, ALH and BCF assessed using CellVision chamber slides with depths of 10, 16 and 20 µm (CV10, CV16 and CV20), Leja chamber slides with a depth of 10 and 20 µm (Leja10, Leja20), Makler chamber (10 µm depth), and slide-coverslip (SCS; 20.1 µm depth)
Intraclass correlation coefficient (ICC) estimates and their 95% confident intervals, based on single measures, absolute-agreement, 2-way mixed-effects model, for all the assessed parameters have been presented in . Overall, PM and IM demonstrated good agreement; while concentration, VCL, VAP, LIN, ALH and WOB presented moderate agreement; and NP, VSL, STR and BCF showed a poor agreement among the slides.
Table 3. Intraclass correlation coefficient (ICC) estimates and their 95% confident intervals, based on single measures, absolute-agreement, 2-way mixed-effects model, for concentration, percentage of progressive (PR) non-progressive (NP) and immotile (IMT) spermatozoa, curvilinear velocity (VCL), average path velocity (VAP), straight-line velocity (VSL), and the kinematic parameters LIN, STR, WOB, ALH and BCF
Discussion
Recent developments of advanced hardware and software technologies have resulted in more reliable and precise computer-aided sperm analysis (CASA) systems. Most of these systems can be used to assess sperm concentration and motility using any of the many commercially available slides. However, there seems to be no standardized recommendation for the type of slide (with different loading methods, and chamber depths) to be used for the evaluation of sperm concentration, motility and kinematic parameters by CASA.
Depth of the chamber slide is one of its most critical characteristics. In order to achieve reliable concentration results, the depth should not exceed ~20 µm (Mortimer et al. Citation2015). Since the depth of none of the slides used in this study exceeded 20 µm, it is reasonable to assume that the results displayed no variations in concentration due to larger depth, except for SCS which had a depth of 20.1 µm, which was not considered as a significant factor as the SCS showed similar results to the other slides. On the other hand, the Makler chamber, showed the highest concentration. This finding is in line with the results from previous studies which have reported the reusable Makler to show significantly higher values for latex bead, and sperm concentrations, compared with SCS and several capillary-filled slides in human and boar sperm (Peng et al. Citation2015; Basioura et al. Citation2019). An overestimation in concentration may result in the clinical misdiagnosis of the semen quality, and thereby negatively affect the infertility treatment strategy selection in a clinical setting. The misinterpretation of the grids in the Makler with sperm by CASA has been suggested as a possible cause (Peng et al. Citation2015). Thus, fields assessed by CASA were selected from the areas just outside the grid when using the Makler in this study. However, as previously mentioned, sperm cells could be forced to the perimeter due to the flow created upon placement of the cover (Lenz et al. Citation2011). As a result, a higher concentration might be seen at the perimeter since the motile sperm cells move freely while the immotile would stay at the perimeter (Lenz et al. Citation2011). It could be that Makler did not overestimate, but the capillary loaded chambers underestimated concentration. This could be explained by the Segre-Silberberg effect causing lower sperm counts in the center of the chamber (Douglas-Hamilton et al. Citation2005; Dearing et al. Citation2014). The loading method itself might also have a considerable impact on concentration measurements since Makler and SCS both showed higher concentration levels than all of the capillary loaded slides, which could indicate a tendency for the drop loaded chambers to display higher concentrations compared with capillary loaded chambers when using CASA.
It has also been suggested that factors including variable volumes of specimen used in any loading and the time elapsed for placing the coverslip may introduce errors in the results of drop-loaded or reusable chambers (Bompart et al. Citation2018). The Segre-Silberberg effect in capillary-loaded chambers could also result in an inappropriate distribution of specimen and consequently, unreal analysis (Douglas-Hamilton et al. Citation2005; Dearing et al. Citation2014). A previous study reported that the concentration of human sperm assessed using capillary-filled chambers by both the manual and CASA methods, was similar to that obtained by hemocytometer. In contrast, the concentration in the drop filled chambers such as Makler was significantly higher (Johnson et al. Citation1996). Altogether, this study found more coherent results for capillary-filling chambers than drop-loading chambers. This difference may well be due to the results of drop-loading slides being more affected by the flow created when placing the coverslip, which may itself be influenced by the slightest differences in the properties of the coverslip (size, weight, etc.) as well as the mounting technique; while the capillary loaded slides are not prone to as much variation during the loading procedure.
As presented in , Leja10 and SCS showed the maximum and minimum percentages of progressively motile sperm respectively, although insignificant. Overall, no significant difference in terms of motility groups was found among any of the slides in this study, which is in line with some previous studies reporting no significant difference in sperm motility parameters of some species evaluated in different chambers (Gallego et al. Citation2013; Gączarzewicz Citation2015). It has been reported that factors such as depth and design of chamber have little impact on sperm motility results in capillary loaded chambers (Del Gallego et al. Citation2017; Yániz et al. Citation2019). Contrary, some studies found significant differences for Makler compared to Leja20 and SCS regarding progressive motility (Massányi et al. Citation2008; Contri et al. Citation2010). The capillary flow itself, affecting the sperm count in the different areas of capillary-filled chambers (Lenz et al. Citation2011; Dearing et al. Citation2014), the flow through the small orifice openings of the capillary loaded chambers, and the toxic effects caused by adhesives, such as glue, or other components in the wall of the chambers which can damage the sperm membrane, have been suggested as possible causes of lower apparent sperm motility (Lenz et al. Citation2011; Gloria et al. Citation2013; Peng et al. Citation2015). There is a controversy regarding sperm motility as well as velocity parameters in different species when the cells are loaded in different slides, which may well be due to the various patterns of sperm motility in different species (Bompart et al. Citation2018).
It has been shown that sperm motility can be affected by chamber depth, in the way that a lower depth may limit the natural sperm motility (Hoogewijs et al. Citation2012; Gloria et al. Citation2013). A study by Soler et al. (Citation2018) on 3D sperm motility patterns demonstrated that the sperm intrinsic movement was significantly affected by chamber depth. This study demonstrated significantly different motility patterns in 100 µm deep chambers compared to 10 and 20 µm chambers. The values for most of the kinematic parameters were similar in a 3D setting as they move in a natural fashion without interference from glass surfaces when classical chambers with the depth of 10 and 20 µm were used(Soler et al. Citation2018). This is in accordance with the findings of our study where no significant difference in the overall percentage of progressive, non-progressive, and immotile spermatozoa was observed among the chambers, which were all between a 10 and 20 µm.
When assessing the kinematic parameters separately, the, Leja10, CV10 and Makler displayed higher values f VCL, VSL and VAP, compared to the other slides (). In other words, the lower the chamber depth, the higher the velocity. Lannou et al., reported that human sperm velocity was inversely related to the depth of the chambers (Lannou et al. Citation1992). Hence, chamber depth seems to be considered the main factor. Thishas been overlooked in the previous studies assessing the effect of different counting chambers on sperm characteristics, in which the different results have been generally associated with the loading method (Del Gallego et al. Citation2017; Bompart et al. Citation2018).
For LIN, STR and WOB; the Makler chamber and SCS showed the highest and lowest values respectively. The Makler chamber and SCS also showed minimum values for ALH and BCF (). In this regard, capillary-filled chambers seem to show more consistent results compared to the drop-filled chambers, as Makler and SCS showed in several values. Del Gallego et al. found that drop loaded chambers displayed higher mean values for most kinematic parameters (Del Gallego et al. Citation2017).
Since each sample is usually assessed using only one type of)slide at the fertility clinic, the Intra-class Correlation Coefficient was calculated based on the ‘single measures’ type. Despite the observed significant differences in velocity parameters (VCL, VSL and VAP) among the different slides, the overall ICC results showed a good method agreement among the slides in PM and IM, which could indicate that all the slides would provide a rather similar assessment of motility using CASA. However, the moderate to poor method agreement for all other parameters among the slides still highlights the need for the standardization of the chambers used for CASA assessment of sperm both in clinical practice, and in research when data are collected or compared between different laboratories.
In conclusion, despite the objectiveness of the assessments by CASA systems, there are still some discrepancies in the results of sperm concentration, motility and other kinematic parameters when using different commercially available slides. The possible negative influence of the sperm quality misdiagnosis on the selection of treatment strategy in a clinical setting, emphasizes the need for further standardization and quality control of the commercially available chamber slides for use with CASA. In this study, chamber depth (10 or 20) did not show any significant effect on concentration and motility categorizations, while more consistent results for capillary-filled chambers compared to the drop-loaded slides, suggest them as a superior method when using CASA.
Materials and methods
Study design and samples
This repeated cross-sectional quality assurance study was performed on 20 normozoospermic samples (assessed using the Makler chamber) from healthy donors between the ages of 20–26 years. The sperm samples were delivered by masturbation into a collection cup in a private room close to the laboratory, to limit exposure to changes in temperature and to improve control of time from collection to analysis (World Health Organization Citation2010). All samples were allowed to liquefy at 37°C for approximately 45 (±5) minutes (World Health Organization Citation2010) before the start of the study. To eliminate bias, each participant was identified with a random number blinding the technician performing the tests, and the different slides were analyzed in a random order for each sample.
The Motility/Concentration module of the Sperm Class Analyzer (SCA® Ver. 6.1; Microptic, Spain) CASA system was used to assess and compare the concentration and motility parameters (VCL cutoff values: rapid > 35 < medium > 15 < slow > 10 > immotile; progressivity: STR >80%) of each semen sample using different commercially available slides according to WHO (World Health Organization Citation2010) criteria as previously described by Alipour et al. (Alipour et al. Citation2017). The slides included Leja (Leja, Netherlands) chamber slides with a depth of 20 µm (Leja20, four-chamber, LOT: 481815B1) and 10 µm (Leja10, four-chamber, LOT#: 191611C3), CellVision (CellVision, Netherlands) chamber slides with depths of 10, 16 and 20 µm (CV10, LOT: NC 17 506; CV16, LOT:NC 16 412-B; and CV20, LOT:NC 17–498 respectively), a Makler (Sefi medical, Israel) chamber with a 10 µm depth (Makler), and a glass Slide loaded with 6.5 µl semen covered with an 18 mm × 18 mm coverslip (area 324 mm2) providing a depth of 20.1 µm (SCS; Menzel-Gläser, Thermo scientific, USA) (World Health Organization Citation2010).
To ensure homogeneity and the sample being representable of the entire ejaculate, following liquefaction, the samples were well-mixed by pipetting before loading each slide. The Leja and CellVision chamber slides were loaded by placing a drop of the sperm sample on the entry port, and the chamber was allowed to fill by capillary action (Kuster Citation2005). The Makler and SCS were loaded by placing a drop of the sample (10 µl and 6.5 µl respectively) directly on the slide and subsequently covering with the ‘Makler’s glass cover’ or an 18 mm ×18 mm coverslip, respectively.
The remaining semen was wiped off the entry port of the chamber-slides by gently touching it with a clean tissue as the slightest movement of the surplus semen at the entry port (by for instance the airflow in the room), could cause the semen in the chamber to move, thus resulting in an unnatural or incorrect recording of the movement of sperm. To avoid misreading due to drifting, slides were allowed to rest for one-two minutes after loading of the sample to ensure the drifting of the sample had come to a complete stop. During this waiting time, the slides were placed on the heated stage of the microscope to maintain a temperature of 37°C (World Health Organization Citation2010).
The assessment performed by the SCA CASA system was controlled and examined manually by a skilled technician in order to correct any possible error (e.g. misreading due to air bubbles or dust in the chambers). The fields containing errors were identified and deleted or manually corrected.
Statistical analysis
The data are presented as mean ± standard deviations (SD). All dependent variables were checked for normal distribution using the Shapiro-Wilk test. To determine levels of significance, repeated measures one-way Analysis of Variance (ANOVA) was applied to the data with normal distribution, while the non-normally distributed data were compared by the non-parametric Friedman test using the GraphPad Prism (Ver. 8.6, USA) software. A P-value of < 0.05 was considered significant.
The SPSS statistical package version 27 (SPSS Inc, Chicago, IL) was used to calculate the Intraclass correlation coefficient (ICC) estimates and their 95% confident intervals, based on single measures, absolute-agreement, 2-way mixed-effects model (Koo and Li Citation2016). ICC values were used to calculate poor (<0.5), moderate (0.5 to 0.75) good (0.75 to 0.9), and excellent (>0.90) (Watson and Petrie Citation2010) total method agreement for each of the different parameters.
Ethics approval
This ‘quality assurance study’ was approved to be performed by the North Jutland Region scientific ethics committee, according to the committee law (§ 14, stk. 1, cf. § 2, nr. 1–3). All data were collected and handled anonymously following written informed consent from the donors. Subjects cannot be identified via this paper and all biological specimen was discarded as biological waste after performing the required assessments.
Author contributions
All authors contributed significantly to this study, are in agreement with the content of the manuscript, and have approved the revisions and final manuscript. Conceiving and design: HA, FD; Data collection and assessment: HA, MH, FD; Drafting of the manuscript: HA, MH, FD; Supervision: HA.
Disclosure statement
The authors report no conflict of interest. This study was financially supported by internal funds from Aalborg University (Aalborg, Denmark).
Additional information
Funding
References
- Alipour H, Van Der Horst G, Christiansen OB, Dardmeh F, Jørgensen N, Nielsen HI, Hnida C. 2017. Improved sperm kinematics in semen samples collected after 2 h versus 4–7 days of ejaculation abstinence. Hum Reprod. 32(7):1364–1372. doi:10.1093/humrep/dex101.
- Basioura A, Tsousis G, Boscos C, Lymberopoulos A, Tsakmakidis I. 2019. Method agreement between three different chambers for comparative boar semen computer-assisted sperm analysis. Reprod Domest Anim. 54(S4):41–45.
- Bompart D, García-Molina A, Valverde A, Caldeira C, Yániz J, Núñez De Murga M, Soler C. 2018. CASA-Mot technology: how results are affected by the frame rate and counting chamber. Reprod Fertil Dev. 30(6):810–819. doi:10.1071/RD17551.
- Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S. 2003. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem. 75(7):1671–1675. doi:10.1021/ac020579e.
- Contri A, Valorz C, Faustini M, Wegher L, Carluccio A. 2010. Effect of semen preparation on casa motility results in cryopreserved bull spermatozoa. Theriogenology. 74(3):424–435. doi:10.1016/j.theriogenology.2010.02.025.
- Dearing CG, Kilburn S, Lindsay KS. 2014. Validation of the sperm class analyser CASA system for sperm counting in a busy diagnostic semen analysis laboratory. Hum Fertil. 17(1):37–44. doi:10.3109/14647273.2013.865843.
- Del Gallego R, Sadeghi S, Blasco E, Soler C, Yániz JL, Silvestre MA. 2017. Effect of chamber characteristics, loading and analysis time on motility and kinetic variables analysed with the CASA-mot system in goat sperm. Anim Reprod Sci. 177:97–104. doi:10.1016/j.anireprosci.2016.12.010.
- Douglas-Hamilton DH, Smith NG, Kuster CE, Vermeiden JPW, Althouse GC. 2005. Capillary-loaded particle fluid dynamics: effect on estimation of sperm concentration. J Androl. 26(1):115–122.
- Gączarzewicz D. 2015. Influence of chamber type integrated with computer-assisted semen analysis (CASA) system on the results of boar semen evaluation. Pol J Vet Sci. 18(4):817–824. doi:10.1515/pjvs-2015-0106.
- Gallego V, Carneiro PCF, Mazzeo I, Vílchez MC, Peñaranda DS, Soler C, Pérez L, Asturiano JF. 2013. Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software. Theriogenology. 79(7):1034–1040. doi:10.1016/j.theriogenology.2013.01.019.
- Gloria A, Carluccio A, Contri A, Wegher L, Valorz C, Robbe D. 2013. The effect of the chamber on kinetic results in cryopreserved bull spermatozoa. Andrology. 1(6):879–885. doi:10.1111/j.2047-2927.2013.00121.x.
- Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, Tsunoda H, Sato I. 2001. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 18(4):213–218. doi:10.1023/A:1009420432234.
- Hoogewijs MK, De Vliegher SP, Govaere JL, De Schauwer C, de Kruif A, Van Soom A. 2012. Influence of counting chamber type on CASA outcomes of equine semen analysis. Equine Vet J. 44(5):542–549. doi:10.1111/j.2042-3306.2011.00523.x.
- Johnson JE, Boone WR, Blackhurst DW. 1996. Manual versus computer-automated semen analyses. Part I. Comparison of counting chambers. Fertil Steril. 65(1):150–155. doi:10.1016/S0015-0282(16)58043-1.
- Kathiravan P, Kalatharan J, Karthikeya G, Rengarajan K, Kadirvel G. 2011. Objective Sperm Motion Analysis to Assess Dairy Bull Fertility Using Computer-Aided System - A Review. Reprod Domest Anim. 46(1):165–172. doi:10.1111/j.1439-0531.2010.01603.x.
- Koo TK, Li MY. 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 15(2):155–163. doi:10.1016/j.jcm.2016.02.012.
- Kuster C. 2005. Sperm concentration determination between hemacytometric and CASA systems: why they can be different. Theriogenology. 64(3):614–617. doi:10.1016/j.theriogenology.2005.05.047.
- Lammers J, Splingart C, Barrière P, Jean M, Fréour T. 2014. Double-blind prospective study comparing two automated sperm analyzers versus manual semen assessment. J Assist Reprod Genet. 31(1):35–43. doi:10.1007/s10815-013-0139-2.
- Lannou D, Le Griveau JF, Le Pichon JP, Quero JC. 1992. Effects of chamber depth on the motion pattern of human spermatozoa in semen or in capacitating medium. Hum Reprod. 7(10):1417–1421. doi:10.1093/oxfordjournals.humrep.a137585.
- Lenz RW, Kjelland ME, Vonderhaar K, Swannack TM, Moreno JF. 2011. A comparison of bovine seminal quality assessments using different viewing chambers with a computer-assisted semen analyzer. J Anim Sci. 89(2):383–388. doi:10.2527/jas.2010-3056.
- Makler A. 1978. A new chamber for rapid sperm count and motility estimation. Fertil Steril. 30(3):313–318. doi:10.1016/S0015-0282(16)43518-1.
- Maree L, van der Horst G. 2013. Quantification and identification of sperm subpopulations using computer-aided sperm analysis and species-specific cut-off values for swimming speed. Biotech Histochem. 88(3–4):181–193. doi:10.3109/10520295.2012.757366.
- Massányi P, Chrenek P, Lukác N, Makarevich AV, Ostro A, Zivcak J, Bulla J. 2008. Comparison of different evaluation chambers for analysis of rabbit spermatozoa motility parameters using casa system. Slovak J Anim Sci. 41(2):60–66.
- McCormack MC, McCallum S, Behr B. 2006. A novel microfluidic device for male subfertility screening. J Urol. 175(6):2223–2227. doi:10.1016/S0022-5347(06)00276-X.
- Mortimer D. 1994. Practical Laboratory Andrology. New York: Oxford University Press.
- Mortimer D, Aitken RJ, Mortimer ST, Pacey AA. 1995. Workshop report: clinical casa—the quest for consensus. Reprod Fertil Dev. 7(4):951–959. doi:10.1071/RD9950951.
- Mortimer ST, van der Horst G, Mortimer D. 2015. The future of computer-aided sperm analysis. Asian J Androl. 17(4):545–553. doi:10.4103/1008-682X.154312.
- Nagy Á, Polichronopoulos T, Gáspárdy A, Solti L, Cseh S. 2015. Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis. Acta Vet Hung. 63(3):370–381. doi:10.1556/004.2015.035.
- Nosrati R, Gong MM, San Gabriel MC, Pedraza CE, Zini A, Sinton D. 2016. Paper-based quantification of male fertility potential. Clin Chem. 62(3):458–465. doi:10.1373/clinchem.2015.250282.
- Nosrati R, Graham PJ, Zhang B, Riordon J, Lagunov A, Hannam TG, Escobedo C, Jarvi K, Sinton D. 2017. Microfluidics for sperm analysis and selection. Nat Rev Urol. 14:707–730.
- Paston MJ, Sarkar S, Oates RP, Badawy SZA. 1994. Computer-aided semen analysis variables as predictors of Male fertility potential. Syst Biol Reprod Med. 33(2):93–99.
- Peng N, Zou X, Li L. 2015. Comparison of different counting chambers using a computer-assisted semen analyzer. Syst Biol Reprod Med. 61(5):307–313.
- Rivera-Montes AM, Rivera-Gallegos A, Rodríguez-Villasana E, Juárez-Bengoa A, Díaz-Pérez M, de Los A, Hernández-Valencia M. 2013. Estimate of the variability in the evaluation of semen analysis. Ginecol Obstet Mex. 81(11):639–644.
- Soler C, Picazo-Bueno J, Micó V, Valverde A, Bompart D, Blasco FJ, Álvarez JG, García-Molina A. 2018. Effect of counting chamber depth on the accuracy of lensless microscopy for the assessment of boar sperm motility. Reprod Fertil Dev. 30(6):924–934. doi:10.1071/RD17467.
- Talarczyk-Desole J, Berger A, Taszarek-Hauke G, Hauke J, Pawelczyk L, Jedrzejczak P. 2017. Manual vs. computer-assisted sperm analysis: can CASA replace manual assessment of human semen in clinical practice? Ginekol Pol. 88(2):56–60. doi:10.5603/GP.a2017.0012.
- Tomlinson MJ, Naeem A. 2018. CASA in the medical laboratory: CASA in diagnostic andrology and assisted conception. Reprod Fertil Dev. 30(6):850. doi:10.1071/RD17520.
- Wasilewski TLM, Wasilewska J, Mroczko B. 2020. Biochemistry of infertility. 508(April):185–190.
- Watson PF, Petrie A. 2010. Method agreement analysis: A review of correct methodology. Theriogenology. 73(9):1167–1179. doi:10.1016/j.theriogenology.2010.01.003.
- World Health Organization. 2010. WHO laboratory manual for the Examination and processing of human semen. Geneva: World Health Organization.
- Yániz J, Palacín I, Santolaria P. 2019. Effect of chamber characteristics, incubation, and diluent on motility of honey bee (Apis mellifera) drone sperm. Apidologie. 50(4):472–481. doi:10.1007/s13592-019-00659-y.
- Youn JS, Cha SH, Park CW, Yang KM, Kim JY, Koong MK, Kang IS, Song IO, Han SC. 2011. Predictive value of sperm motility characteristics assessed by computer-assisted sperm analysis in intrauterine insemination with superovulation in couples with unexplained infertility. Clin Exp Reprod Med. 38(1):47–52. doi:10.5653/cerm.2011.38.1.47.
