ABSTRACT
Administration of platelet-rich plasma (PRP) is one of the well-recommended strategies for the treatment of endometrium- and ovary-associated infertility. Due to the autologous source of PRP, minimal risks for disease transmission and immunogenic and allergic responses are expected in this method. Despite the extensive use of PRP in medicine, its precise mechanism of action in endometrial and ovarian tissues is still unknown. Nevertheless, the induction of cell proliferation, chemotaxis, regeneration, extracellular matrix synthesis, remodeling, angiogenesis, and epithelialization are the main pathways for PRP to affect female reproductive organs. Given the promising results of previous studies, it is necessary to standardize PRP preparation protocols for different therapeutic purposes and also clearly determine appropriate inclusion and exclusion criteria for recruiting patients. In the current review, we presented a summary of studies on PRP therapy for endometrium- and ovary-associated infertility with a focus on the possible mechanisms by which PRP enhances endometrial receptivity and regenerates ovarian function.
Abbreviations: PRP: platelet-rich plasma; ART: assisted reproductive technology; POF: premature ovarian failure; TGF: transforming growth factors; PDGF: platelet-derived growth factors; IGF-I: insulin-like growth factor-1; HGF: hepatocyte growth factor; EGF: epidermal growth factor; FGF: fibroblast growth factor; VEGF: vascular endothelial growth factor; ADP: adenosine diphosphate, ATP: adenosine triphosphate; PDGF: platelet-derived growth factor; COX2: cyclooxygenase-2; TP53: tumor protein 53; ER-α: estrogen receptors alpha; ER-β: estrogen receptors beta; PR: progesterone receptor; RIF: recurrent implantation failure; G-CSF: granulocyte colony-stimulating factor; iNOS: inducible nitric oxide synthase; NF-kβ: nuclear factor kappa beta; MMPs: matrix metalloproteinases; Col1a1: collagen type I alpha 1; IL: interleukin; FSH: follicle-stimulating hormone; AMH: anti-Mullerian hormone; GDF-9: growth differentiation factor 9.
Introduction
Endometrium- and ovary-associated infertilities are the main issues among women in reproductive age that need new therapeutic strategies (Unuane et al. Citation2011). Despite the recent progress made in the field of assisted reproductive technology (ART), statistics show that around 70% to 80% of transferred embryos are not able to implant (Lédée et al. Citation2018). Although embryo quality and immunological factors are involved in the implantation outcome, the quality of endometrium is at the first line of this process (Hajipour et al. Citation2018). Ovarian disorders such as lack of mature follicles or inability in response to ovarian stimulation are the other obstacles in ART (Shelling Citation2010). In this regard, it has been documented that about 1% of women under the age of 40 suffer from premature ovarian failure (POF) (Maclaran and Panay Citation2011).
Given the importance of inappropriate endometrium and ovarian failure in reproductive medicine, novel and effective therapeutic strategies are required to combat these issues. In this respect, intra-uterine and intra-ovarian administration of platelet-rich plasma (PRP) has been recently introduced to improve ART outcomes. PRP is prepared from fresh blood and consists of plasma supplemented with platelets (Amable et al. Citation2013). This fraction of blood is rich in growth factors with anti-inflammatory and mitogenic potentials that introduce an evolving therapeutic application in tissue engineering and regeneration (Gentile et al. Citation2012). PRP contains 3- to 5-fold greater levels of platelets than plasma. Interestingly, the α-granules of platelets encompass numerous vital factors for regeneration, such as transforming growth factors (TGF-β1, TGF-β2), platelet-derived growth factors (PDGFAA, PDGF-BB, PDGF-AB), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-I), epidermal growth factor (EGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) (Amable et al. Citation2013). Indeed, these factors synergistically act to enhance the diffusion of macrophages and neutrophils and consequently promote angiogenesis, fibroplasia, and re-epithelialization that finally cause tissue regeneration (Opneja et al. Citation2019). Moreover, the presence of anti-inflammatory molecules in PRP such as HGF can prevent the inflammatory process (Bendinelli et al. Citation2010). The dense granules in platelets also contain calcium ions, serotonin, histamine, dopamine, adenosine diphosphate (ADP), and adenosine triphosphate (ATP) that are required for tissue homeostasis (Bos-Mikich et al. Citation2018). In addition to the growth factors, platelets contain other substances such as fibronectin, vitronectin, and sphingosine 1-phosphate that are involved in wound healing (Jo et al. Citation2013; Dawood and Salem Citation2018). The ability of PRP in triggering the abovementioned phenomenon makes this fraction an effective factor for tissue regeneration. In this review, we aimed to highlight the importance of PRP therapy in the improvement of ovarian reserve and also preparation of the endometrium for embryo implantation.
For the identification and selection of the articles, we conducted a literature search from electronic databases such as the Cochrane Library, PubMed, and Google Scholar without publication date limitation. The following keywords were used individually or in combination to find the related articles: ‘platelet-rich plasma’, PRP, ‘thin endometrium’, ‘endometrial receptivity’, ‘endometrial thickness’, ‘recurrent implantation failure’, RIF, endometritis, ‘endometrial inflammation’, ‘Asherman’s syndrome’, ‘ovarian reserve’, ‘ovarian function’, ‘premature ovarian failure’, ‘ovary rejuvenation’, ‘ovarian torsion’. Then, the title and abstract of the articles were screened to find the relevant literature, and non-eligible papers were excluded.
Effects of PRP therapy on thin endometrium
For human embryo implantation, the endometrial thickness should be at least 7 mm (El-Toukhy et al. Citation2008). Therefore, a thin endometrium in the ART cycle can lead to cycle cancellation and unexpected embryo cryopreservation (Chang et al. Citation2015). Due to the strong effect of PRP on the up-regulation of endometrial receptivity- and proliferation-related genes, it has been recently administrated to enhance the endometrial thickness (Marini et al. Citation2016). It has been reported that PRP treatment before embryo transfer could significantly increase the implantation rate; such a finding encouraged researchers to apply PRP for implantation-related issues (Farimani et al. Citation2016). Jang et al. (Citation2017) found that intrauterine administration of autologous PRP could accelerate the regeneration of damaged endometrium and decrease fibrosis in a murine model. The application of PRP to improve human embryo implantation and pregnancy has been well investigated in recent years (summarized in ). For example, Chang et al. (Chang et al. Citation2015) showed that intrauterine administration of 0.5–1 ml autologous PRP on the 10th day of the hormone replacement therapy cycle could increase endometrial thickness to 7 mm in five infertile women with inadequate endometrial growth and poor response to the conventional therapy; more interestingly, four patients got pregnant following embryo transfer. In a similar study, ten patients with thin endometrium who underwent a frozen embryo transfer cycle received PRP and the results showed that endometrial thickness reached more than 7 mm in all cases and five patients (50%) achieved pregnancy (Zadehmodarres et al. Citation2017). Colombo et al. (Colombo et al. Citation2017) also investigated the effects of PRP on eight women with poor endometrial growth (<6 mm) who had more than three canceled cryo-transfers with negative hysteroscopic and bacteriologic screening for endometrial pathology. They reported that the endometrial three-layer pattern with thickness greater than 6.5 mm was achieved in all of the patients except one; of these, six women showed positive beta-HCG, two women had normal pregnancy progress, and one had an early miscarriage. In a review article, all relevant articles for PRP therapy in thin endometrium, published from 2000 to 2018, were studied and it has been concluded that local administration of PRP is significantly effective in increasing pregnancy rates of infertile women with refractory endometrium with ET less than 6 mm (Samy et al. Citation2020).
Table 1. Clinical application of platelet-rich plasma (PRP) in endometrium related infertilities
The mechanisms by which PRP enhances endometrial receptivity are not yet clear, however, it is believed that the growth factors have a crucial role (Marini et al. Citation2016). Moreover, the growth factors, chemokines, cytokines, and active metabolites of platelets can have paracrine effects on endometrial cells, including fibroblasts (Anitua et al. Citation2009), myocytes (Mazzocca et al. Citation2012), and mesenchymal stem cells (Cho et al. Citation2011). It has also been reported that multiple implantation failures are a result of the inefficient expression of adhesion molecules that could potentially be improved by PRP (Colombo et al. Citation2017). The well-known factors of PRP and their possible roles in the endometrium are presented in and .
Table 2. The main factors of platelet-rich plasma (PRP) and their possible effects on endometrium and ovaries
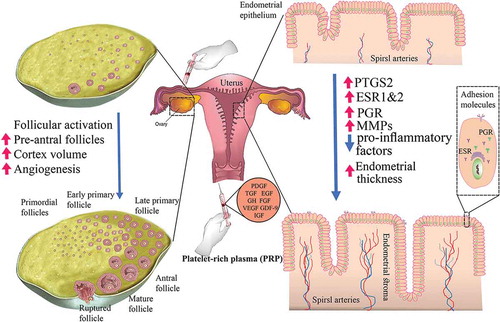
Figure 1. The putative role of platelet-rich plasma (PRP) on endometrial receptivity and ovarian function. Intraperitoneal infusion of PRP may support ovarian tissue regeneration and reactivation via 1) activation of dormant follicles, 2) increase in cortex volume, and 3) induction of neoangiogenesis in the dysfunctional ovarian tissue. Intrauterine administration of PRP may pave the way for embryo implantation via 1) upregulation of COX2, a converter of arachidonic acid to prostaglandins, 2) increase expression of ER and PR which are involved in endometrium response to ovarian hormones and endometrium thickness, 3) enhancement of MMPs expression and so regulation of the tissue remodeling, and 4) reduction of pro-inflammatory factors in abnormal conditions such as endometritis. COX2: cyclooxygenase-2; EGF: epidermal growth factor; ER: estrogen receptors; FGF: fibroblast growth factors; GDF-9: growth differentiation factor 9; GH: growth hormone; IGF: insulin-like growth factor; MMPs: matrix metalloproteinases; PR: progesterone receptor; PDGF: platelet-derived growth factor; TGF: transforming growth factor; VGEF: vascular endothelial growth factor
Although the growth factors of PRP have beneficial effects on the endometrium, Marini et al. (Citation2016) showed that low concentration (5%) of PRP could increase the proliferation of bovine endometrial cells, while the high concentration (10%) prevented the proliferation rate and led to negative side effects. Lange-Consiglio et al. (Citation2015) also reported that a 5% platelet concentration increased the rate of blastocyst production and the cell number of a bovine embryo, while a 10% of platelet concentration decreased the number of embryos. Therefore, it can be postulated that excessive amounts of growth factors have an inhibitory effect on the proliferation of endometrial and embryonic cells.
It has been demonstrated that PRP administration could induce expression of several genes that are involved in implantation, including cyclooxygenase-2 (COX2), tumor protein 53 (TP53), estrogen receptors alpha (ER-α), estrogen receptors beta (ER-β), and progesterone receptor (PR) (Marini et al. Citation2016). For example, COX2 plays an important role in the synthesis of prostaglandins which are involved in several reproductive events, such as implantation, pregnancy preservation, and parturition (Sheldon et al. Citation2009). PRP therapy also enhances c-Myc expression, which has a role in cell proliferation; this effect can be due to the existence of EGF in PRP (Anitua et al. Citation2004). Given the previous studies, PRP can be considered as a novel and effective strategy for improvement of the endometrial thickness in women with a thin endometrium (Kim et al. Citation2019). However, further studies are needed to find an approved dosage, number, and time of PRP administration for clinical uses and make PRP therapy as a standard and recommended-therapeutic strategy.
Effects of PRP therapy on recurrent implantation failure (RIF)
Recurrent implantation failure (RIF) refers to the failure to attain a clinical pregnancy after transfer of at least four good-quality embryos in a minimum of three fresh or frozen cycles in the woman at the age of <40 years (Coughlan et al. Citation2014). It has been demonstrated that mid-secretory growth factors in RIF patients are lower than fertile women (Sak et al. Citation2013). Therefore, it can be hypothesized that growth factor-enriched PRP might compensate for this deficiency. In this regard, several studies have been conducted on RIF patients and the results demonstrate the effectiveness of PRP therapy in increasing rates of implantation and pregnancy. In a study by Nazari et al. (Citation2016) on 20 women suffering RIF, it has been reported that 18 women got pregnant (16 continuing pregnancies, one miscarriage, and one molar pregnancy) following autologous PRP therapy. They concluded that PRP with a large number of cytokines and growth factors could motivate the proliferation and regeneration of endometrium in RIF patients. This group, in another study on 49 RIF patients, has shown that intrauterine infusion of 0.5 ml PRP, 48 h before blastocyst transfer could significantly increase the chemical and clinical pregnancy rates (Nazari et al. Citation2020). Farimani et al. have been also demonstrated that administration of 0.5–1 mL PRP into the uterine cavity of RIF patients about 36 h before embryo transfer resulted in six clinical pregnancies out of nine patients (66%) (Farimani et al. Citation2016). Furthermore, in a case report study, the pregnancy of a 45 years-old woman with RIF disease has been reported following the intrauterine administration of PRP 24 h before embryo transfer and it has been mentioned that the pregnancy gave birth to a healthy boy (Farimani et al. Citation2017). Given the simplicity and lack of serious side effects and also the obtained promising results, this therapeutic approach can be suggested for RIF.
The mechanisms by which PRP provides an appropriate condition for pregnancy remain to be determined. However, based on the previous findings, the following mechanisms can be suggested: 1) Fibroblast growth factors-1 (FGF-1) levels are decreased in patients with RIF (Sak et al. Citation2013), and the FGF-1 of PRP can compensate this deficiency, 2) Most of the growth factors in PRP such as insulin-like growth factors, growth hormone, PDGF, EGF, and TGF-β have corresponding receptors on the endometrial tissue and thus they can promote endometrial remodeling and consequently endometrial receptivity and embryo implantation (Anitua et al. Citation2016; Yu et al. Citation2016), and, p. 3) EGF of PRP can regulate expression of uterine VEGF which locally contributes to the decidual vascularization, placenta angiogenesis, and endometrium proliferation (Furukawa et al. Citation2009).
Effects of PRP therapy on endometritis and endometrial inflammation
Endometritis, the inflammation of endometrium without systemic signs, is associated with abnormal uterine bleeding, recurrent abortion, and infertility (Kasius et al. Citation2011). Endometritis could be diagnosed in about 30% of the patients with RIF and it is generally associated with the low chance of ART success (Cicinelli et al. Citation2014). PRP administration can be one of the potential therapies for endometritis due to its anti-inflammatory properties and ability in the reduction of uterine neutrophils (Reghini et al. Citation2016). In a case report study, it has been indicated that the intrauterine administration of autologous PRP resulted in no signs of endometritis and a twin pregnancy and birth in a woman with chronic endometritis, POF, and RIF history (Sfakianoudis et al. Citation2019). It has also been shown that PRP could reduce the release of pro-inflammatory proteins such as IL-1β, I-L8, and inducible nitric oxide synthase (iNOS) in lipopolysaccharide-induced endometrial inflammation (Marini et al. Citation2016). Furthermore, PRP can act as an immunomodulator to reduce inflammatory responses. In this regard, Reghini et al. (Citation2016) have reported that PRP modulated the uterine inflammatory responses in mares with chronic degenerative endometritis. Nuclear Factor Kappa B (NF-kB) is one of the possible targets for PRP through which PRP regulates expression of genes that are involved in inflammatory and immune responses (Cundell and Mickle Citation2018). NF-kB is present in an inactive form in the cytoplasm of endometrial cells and the inflammatory signals can induce its phosphorylation and translocation into the nucleus where it can trigger expression of the inflammatory factors (Cronin et al. Citation2012). Therefore, prevention of NF-kB phosphorylation could be a possible mechanism by which PRP exerts its anti-inflammatory effects (Andia and Maffulli Citation2013). Interestingly, hepatocyte growth factor (HGF) present in PRP can disrupt the activity of NF-kB via its retention in the cytosol (Bendinelli et al. Citation2010).
It has been shown that PRP could increase expression of matrix metalloproteinases (MMPs) including MMP1, MMP3, MMP7, and MMP26 in endometrial stromal fibroblasts and endometrial mesenchymal stem cells (Aghajanova et al. Citation2018b). On the other hand, it is well known that MMPs are involved in the degradation of the extracellular matrix (ECM), a step in tissue regeneration and wound healing, that is essential for endometritis treatment (Caley et al. Citation2015). Furthermore, platelet-derived factors such as platelet-derived growth factor (PDGF) could activate endometrial progenitor cells (Gargett et al. Citation2008) and induce proliferation, migration, and contractility of the endometrial stromal cells (Matsumoto et al. Citation2005). Besides, PRP contains proteins with the anti-microbial activity, which might positively affect endometrial growth and receptivity (Bos-Mikich et al. Citation2018). Given the anti-inflammatory, wound healing, and regenerative potentials of PRP, its intrauterine administration can combat endometritis-associated issues. However, a randomized controlled clinical trial with a larger sample size is required to confirm the beneficial effect of PRP on chronic endometritis (Sfakianoudis et al. Citation2019).
PRP therapy in Asherman’s syndrome
Asherman’s syndrome defined by the presence of intrauterine adhesions is one of the endometrial-associated infertility causes. It can lead to implantation difficulties, abnormal placentation, miscarriage, and other psychological distress (Santamaria et al. Citation2018). In Asherman’s syndrome, the endometrial basal layer is damaged that causes endometrial regeneration deficiency and intrauterine adhesion (Zhu et al. Citation2019). It has been reported that the intrauterine administration of PRP could improve endometrial function and cause clinical pregnancies in patients with Asherman’s syndrome (CitationGonzalo et al.; Aghajanova et al. Citation2018a). Such beneficial effects of PRP are possibly due to its ability to eliminate scar tissue and provide regeneration-related growth factors. Kim et al. (Kim et al. Citation2018) showed that intrauterine administration of PRP significantly enhanced the number of implantation sites (2.1 vs. 4.6, p < 0.01) and live birth rate (83.3% vs 0%) by affecting the expression of fibrosis-related factors including collagen type I alpha 1 (Col1a1), transforming growth factor-beta 1 (TGFβ1), and TIMP metallopeptidase inhibitor 1 (Timp1) in a murine model of Asherman’s syndrome. Zhang et al. (Zhang et al. Citation2017) also indicated that uterine co-injection of PRP with menstrual stromal cells could magnify the therapeutic effect of PRP in rats with Asherman’s syndrome. They found that the co-treatment method down-regulated collagen 1 and an up-regulated IL1b, IL4, and IL10 levels. Another animal study showed that human PRP improved endometrial morphology, reduced the degree of fibrosis, and down-regulated expression of fibrosis-related factors in the murine model of Asherman’s syndrome (Kim et al. Citation2020). Although the reported findings for PRP therapy in animal models of Asherman’s syndrome are promising, clinical studies are required to confirm its application potential.
PRP therapy in ovarian disorders
The decline or loss of ovarian reserve has attracted the focus of a considerable volume of ongoing researches to find an effective solution. Ovarian failure is characterized by ovarian atrophy, reduction of follicles, and menopausal-level serum gonadotropins (Ranjbaran et al. Citation2019). The potential of PRP in improving the ovarian microenvironment and providing growth factors for ovarian germline stem cells makes PRP a suitable candidate for the treatment of ovarian reserve loss (). Recently, Dehghani et al. (Dehghani et al. Citation2018) demonstrated that injection of PRP into the peritoneum of rats with cyclophosphamide-induced ovarian failure could enhance the pre-antral follicles number, ovarian cortex volume, and diameter of antral follicles and their oocytes. PRP administration for the regeneration of the human ovary has also been investigated in several studies (). In this regard, Sills et al. (Sills et al. Citation2018) showed that intra-ovarian injection of calcium gluconate-activated autologous PRP could improve ovarian function after 2 months in women with age of 42 ± 4 years. In a case series report, ovarian infusion of autologous PRP in poor responders resulted in a 67.33% reduction of FSH and 75.18% enhancing of AMH after 3 months (Sfakianoudis et al. Citation2019). Two hypotheses regarding how PRP can improve oocyte retrieval from women with ovarian failure have been suggested; First, growth factors of PRP can awaken latent oocytes of the ovary, and second PRP provides the proper condition for differentiation and development of uncommitted ovarian stem cells into de novo oocytes (Sills et al. Citation2018).
Table 3. Clinical application of platelet-rich plasma (PRP) in ovary-related infertilities
The ovary is an extremely angiogenic organ, therefore it can be expected that PRP-derived angiogenic factors provoke neoangiogenesis in ovarian tissue and pave the way for tissue regeneration and reactivation (Farimani et al. Citation2019). This potential of PRP has also been used to induce angiogenesis in the autologous transplantation of human ovary (Callejo et al. Citation2013). Besides, it has been revealed that growth and survival rates of follicles from PRP supplemented culture media were significantly higher than those without PRP; this proves that PRP can support the early stage of follicle development (Hosseini et al. Citation2017). Growth differentiation factor 9 (GDF-9), a TGF-β superfamily member is one of the factors that can be found in PRP, and its role in oocyte maturation has previously been demonstrated (Krüger et al. Citation2013). More interestingly, mutation of the GDF-9 gene can result in premature ovarian failure (Otsuka et al. Citation2011). Furthermore, the establishment of a balance between cell apoptosis and survival can be another possible mechanism by which PRP affects follicular growth and development. This feature of PRP is related to the presence of both apoptotic (Fas-L, CD40L, TRAIL, and TWEAK) and anti-apoptotic (HGF, SDF-1, serotonin, adenosine diphosphate, and sphingosine-1 phosphate) molecules in this fraction (Hu et al. Citation2012).
The beneficial effects of PRP on the preservation of ovarian function in the cases with ovarian torsion have also been documented. In this regard, Bakacak et al. (Bakacak et al. Citation2015) reported that intraperitoneal administration of PRP could reduce the ovarian damages caused by torsion-related ischemia. Free radical-induced damage of the gonads is one of the adverse effects of the torsion, which can be diminished following local administration of PRP (Varoga et al. Citation2008). PRP prevents oxidative stress-induced injuries by increasing VEGF and other nuclear factors that are involved in angiogenesis (Tohidnezhad et al. Citation2014). Besides, PRP contains EGF, TGFα, and heparin-binding EGF, which stimulate ovarian tissue survival against ischemia through activating EGFRs and Akt signaling pathways (Cao et al. Citation2009). Despite findings of the beneficial effects of PRP therapy on ovarian disorders, additional investigations are required to clarify the dose and type of PRP as well as the inclusion criteria for PRP therapy candidacy.
Side effects of PRP therapy
There are noticeable reports about the application of PRP on more than thousands of patients for a long time without any side effects. Therefore, it could be claimed that PRP is fully safe and has minimal risk for disease spread and immunogenic or allergic responses due to its autologous source (Everts et al. Citation2006). Dehghani et al. (Dehghani et al. Citation2018) showed that PRP administration had no negative effect on the structures and function of rat ovaries. Besides, PRP therapy is done in an aseptic condition, and also plasma has antimicrobial effects to prevent infection (Ulbin-Figlewicz et al. Citation2015). However, face skin hyperpigmentation (Uysal and Ertas Citation2017) and blindness in one eye have been reported following PRP injection into the glabellar lines (Kalyam et al. Citation2017). Up to now, no serious side effects following administration of PRP into reproductive organs have been observed and just simple complications such as local pain, irritation, erythema, and swelling around the injection sites have been seen (Chang et al. Citation2018). However, given the lack of a unified PRP preparation method and certain protocol for PRP administration, clinicians should apply this treatment with caution.
Conclusion
Administration of PRP for endometrium- and ovary-associated infertilities has attracted a lot of attention due to its potential in the promotion of endometrial regeneration and ovarian reserve. Furthermore, simple and easy preparation, the minimal possibility for disease transmission and infection, no immunologic responses due to its autologous source, promising results, and no side effects make the PRP administration as one of the popular therapies for endometrium- and ovary-related infertilities (Anitua and Orive Citation2010). For the first time, the current review brings together the studies using PRP therapy in the treatment of female infertility. In addition to the clinical outcome, this review discussed the underlying mechanisms involved in the therapeutic effect of PRP on female infertility. However, the significant portion of the reviewed studies was experimental rather than clinical which can be considered as the main limitation of the current study.
Despite significant signs of progress in the application of PRP in most medical cases, there is no standard preparation method and also a clear guideline to candidate patients for PRP therapy. Besides, the exact mechanism of PRP therapy in endometrial and ovarian regeneration has not completely been elucidated. However, induction of cell proliferation and migration, chemotaxis, cell regeneration, extracellular matrix synthesis, remodeling, angiogenesis, and epithelialization are the main pathways for the PRP effects (Dawood and Salem Citation2018). Given the promising results obtained from previous studies described in this review, it is necessary to standardize PRP preparation protocols for different therapeutic purposes and also clearly determine appropriate patient inclusion and exclusion criteria.
Authors contributions
Design and critical revisions: AF, MN, RD; clinical advice: LF, NN; literature review and manuscript drafting: HH, ZL, NK. All authors have made substantial contributions to the final approval of the version to be submitted.
Acknowledgments
We thank the academic staff of the Department of Reproductive Biology, Tabriz University of Medical Sciences for their valuable advice. This study has been extracted from the thesis registered in the Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences (Thesis No. 61208, IR.TBZMED.REC.1397.951).
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Aghajanova L, Cedars M, Huddleston H. 2018a. Platelet-rich plasma in the management of Asherman syndrome: case report. J Assist Reprod Genet. 35(5):771–775. doi:10.1007/s10815-018-1135-3.
- Aghajanova L, Houshdaran S, Balayan S, Manvelyan E, Irwin JC, Huddleston HG, Giudice LC. 2018b. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J Assist Reprod Genet. 35(5):757–770. doi:10.1007/s10815-018-1130-8.
- Allahveisi A, Seyedoshohadaei F, Rezaei M, Bazrafshan N, Rahimi K. 2020. The effect of platelet-rich plasma on the achievement of pregnancy during frozen embryo transfer in women with a history of failed implantation. Heliyon. 6(3):e03577
- Amable PR, Carias RBV, Teixeira MVT, da Cruz Pacheco Í, Do Amaral RJFC, Granjeiro JM, Borojevic R. 2013. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 4(3):67. doi:10.1186/scrt218.
- Andia I, Maffulli N. 2013. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 9(12):721. doi:10.1038/nrrheum.2013.141.
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. 2004. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 91(1):4–15. doi:10.1160/TH03-07-0440.
- Anitua E, de la Fuente M, Ferrando M, Quintana F, Larreategui Z, Matorras R, Orive G. 2016. Biological effects of plasma rich in growth factors (PRGF) on human endometrial fibroblasts. Eur J Obstet Gynecol Reprod Biol. 206:125–130. doi:10.1016/j.ejogrb.2016.09.024.
- Anitua E, Orive G. 2010. Short implants in maxillae and mandibles: a retrospective study with 1 to 8 years of follow-up. J Periodontol. 81(6):819–826. doi:10.1902/jop.2010.090637.
- Anitua E, Sánchez M, Zalduendo M, De La Fuente M, Prado R, Orive G, Andía I. 2009. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 42(2):162–170. doi:10.1111/j.1365-2184.2009.00583.x.
- Bakacak M, Bostanci MS, İnanc F, Yaylali A, Serin S, Attar R, Yildirim G, Yildirim OK. 2015. Protective effect of platelet rich plasma on experimental Ischemia/Reperfusion injury in rat ovary. Gynecol Obstet Invest. 81(3):225–231.
- Bendinelli P, Matteucci E, Dogliotti G, Corsi MM, Banfi G, Maroni P, Desiderio MA. 2010. Molecular basis of anti‐inflammatory action of platelet‐rich plasma on human chondrocytes: mechanisms of NF‐κB inhibition via HGF. J Cell Physiol. 225(3):757–766. doi:10.1002/jcp.22274.
- Bos-Mikich A, de Oliveira R, Frantz N. 2018. Platelet-rich plasma therapy and reproductive medicine. J Assist Reprod Genet. 35(5):753–756. doi:10.1007/s10815-018-1159-8.
- Caley MP, Martins VL, O’Toole EA. 2015. Metalloproteinases and wound healing. Adv Wound Care. 4(4):225–234. doi:10.1089/wound.2014.0581.
- Callejo J, Salvador C, González-Nuñez S, Almeida L, Rodriguez L, Marqués L, Valls A, Lailla JM. 2013. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 6(1):33. doi:10.1186/1757-2215-6-33.
- Canipari R, Cellini V, Cecconi S. 2012. The ovary feels fine when paracrine and autocrine networks cooperate with gonadotropins in the regulation of folliculogenesis. Current pharmaceutical design. 18(3):245–255
- Cao C, Huang X, Han Y, Wan Y, Birnbaumer L, Feng G-S, Marshall J, Jiang M, Chu W-M. 2009. Gαi1 and Gαi3 are required for epidermal growth factor–mediated activation of the Akt-mTORC1 pathway. Sci Signal. 2(68):ra17–ra17. doi:10.1126/scisignal.2000118.
- Carlson MJ. 2012. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discovery medicine. 14(76):215
- Chang BL, Beer J, Percec I. 2018. Platelet-rich plasma: fact or fantasy? Adv Cosmet Surg. 1(1):193–209. doi:10.1016/j.yacs.2018.02.004.
- Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, Liang X. 2015. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 8(1):1286.
- Cho HS, Song IH, Park S-Y, Sung MC, Ahn M-W, Song KE. 2011. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 31(3):212–218.
- Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, Marrocchella S, Greco P, Resta L. 2014. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 30(2):323–330. doi:10.1093/humrep/deu292.
- Coksuer H, Akdemir Y, Ulas Barut M. 2019. Improved in vitro fertilization success and pregnancy outcome with autologous platelet-rich plasma treatment in unexplained infertility patients that had repeated implantation failure history. Gynecological Endocrinology. 35(9):815–818
- Colombo G, Fanton V, Sosa D, Criado ES, Lotti J, Aragona S, Lotti T. 2017. Use of platelet rich plasma in human infertility. J Biol Regul Homeost Agents. 31(2 Suppl. 2):179–182.
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K, Sallam H, Li T. 2014. Recurrent implantation failure: definition and management. Reprod Biomed Online. 28(1):14–38. doi:10.1016/j.rbmo.2013.08.011.
- Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM. 2012. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol Reprod. 86(2):51,51–59. doi:10.1095/biolreprod.111.092718.
- Cundell DR, Mickle KE. 2018. Osteoarthritis: potential for herbal medicines as therapies in the management of chronic inflammatory damage. Curr Immunol Rev. 14(2):68–80. doi:10.2174/1573395514666180530093702.
- Dawood AS, Salem HA. 2018. Current clinical applications of platelet-rich plasma in various gynecological disorders: an appraisal of theory and practice. Clin Exp Reprod Med. 45(2):67–74. doi:10.5653/cerm.2018.45.2.67.
- Dehghani F, Aboutalebi H, Esmaeilpour T, Panjehshahin MR, Bordbar H. 2018. Effect of platelet-rich plasma (PRP) on ovarian structures in cyclophosphamide-induced ovarian failure in female rats: a stereological study. Toxicol Mech Methods. 28(9):653–659. doi:10.1080/15376516.2018.1491662.
- Demir R, Yaba A, Huppertz B. 2010. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta histochemica. 112(3):203–214
- El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, Braude P. 2008. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 89(4):832–839. doi:10.1016/j.fertnstert.2007.04.031.
- Everts PA, Knape JT, Weibrich G, Schonberger J, Hoffmann J, Overdevest EP, Box HA, van Zundert A. 2006. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 38(2):174.
- Farimani M, Bahmanzadeh M, Poorolajal J. 2016. A new approach using autologous platelet-rich plasma (PRP) to treat infertility and to improve population replacement rate. J Res Health Sci. 16(3):172–173.
- Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M. 2019. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep. 46(2):1611–1616. doi:10.1007/s11033-019-04609-w.
- Farimani M, Poorolajal J, Rabiee S, Bahmanzadeh M. 2017. Successful pregnancy and live birth after intrauterine administration of autologous platelet-rich plasma in a woman with recurrent implantation failure: A case report. Int J Reprod BioMed. 15(12):803.
- Furukawa Y, Kawano Y, Fukuda J, Matsumoto H, Narahara H. 2009. The production of vascular endothelial growth factor and metalloproteinase via protease-activated receptor in human endometrial stromal cells. Fertil Steril. 91(2):535–541. doi:10.1016/j.fertnstert.2007.11.080.
- Gargett CE, Chan RW, Schwab KE. 2008. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 288(1–2):22–29. doi:10.1016/j.mce.2008.02.026.
- Gargett CE, Nguyen HP, Ye L. 2012. Endometrial regeneration and endometrial stem/progenitor cells. Reviews in Endocrine and Metabolic Disorders. 13(4):235–251
- Garor R, Abir R, Erman A, Felz C, Nitke S, Fisch B. 2009. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertility and sterility. 91(5):1967–1975
- Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. 2012. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 1(3):230–236. doi:10.5966/sctm.2011-0054.
- Gonzalo EP, Pacheco LA, Jiménez AV, Vitale SG, Raffone A, Laganà AS. Intrauterine infusion of platelet‑rich plasma for severe Asherman syndrome: a cutting‑edge approach. Pathophysiology. 6:7.
- Hajipour H, Nejabati HR, Latifi Z, Hamdi K, Bahrami‐asl Z, Fattahi A, Nouri M. 2018. Lymphocytes immunotherapy for preserving pregnancy: mechanisms and challenges. Am J Reprod Immunol. 80:e12853. doi:10.1111/aji.12853.
- Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Boroujeni SB, Sarvari A, Sadeghnia S, Behzadi B, Akhondi MM. 2017. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed Online. 35(4):343–350. doi:10.1016/j.rbmo.2017.04.007.
- Hu X, Wang C, Rui Y. 2012. An experimental study on effect of autologous platelet-rich plasma on treatment of early intervertebral disc degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi= Zhongguo Xiufu Chongjian Waike Zazhi= Chinese J Reparat Reconstructive Surg. 26(8):977–983.
- Jang H-Y, Myoung SM, Choe JM, Kim T, Cheon Y-P, Kim YM, Park H. 2017. Effects of autologous platelet-rich plasma on regeneration of damaged endometrium in female rats. Yonsei Med J. 58(6):1195–1203. doi:10.3349/ymj.2017.58.6.1195.
- Jo CH, Roh YH, Kim JE, Shin S, Yoon KS. 2013. Optimizing platelet-rich plasma gel formation by varying time and gravitational forces during centrifugation. J Oral Implantol. 39(5):525–532. doi:10.1563/AAID-JOI-D-10-00155.
- Kalyam K, Kavoussi SC, Ehrlich M, Teng CC, Chadha N, Khodadadeh S, Liu J. 2017. Irreversible blindness following periocular autologous platelet-rich plasma skin rejuvenation treatment. Ophthal Plast Reconstr Surg. 33(3):S12–S16. doi:10.1097/IOP.0000000000000680.
- Kasius J, Broekmans F, Sie-Go D, Bourgain C, Eijkemans M, Fauser B, Devroey P, Fatemi H. 2011. The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases: a multicenter observer study. Hum Reprod. 27(1):153–158. doi:10.1093/humrep/der341.
- Kim H, Shin JE, Koo HS, Kwon H, Choi DH, Kim JH. 2019. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: a pilot study. Front Endocrinol (Lausanne). 10:61. doi:10.3389/fendo.2019.00061.
- Kim J, Hwang J, Lyu S. 2018. Human platelet-rich plasma improves endometrial regeneration and pregnancy outcomes in a murine model of Asherman syndrome. Fertil Steril. 110(4):e73. doi:10.1016/j.fertnstert.2018.07.221.
- Kim JH, Park M, Paek JY, Lee W-S, Song H, Lyu SW. 2020. Intrauterine infusion of human platelet-rich plasma improves endometrial regeneration and pregnancy outcomes in a murine model of Asherman’s syndrome. Front Physiol. 11:105. doi:10.3389/fphys.2020.00105.
- Knight PG, Glister C. 2006. TGF-β superfamily members and ovarian follicle development. Reproduction. 132(2):191–206
- Kosaka N, Sudo N, Miyamoto A, Shimizu T. 2007. Vascular endothelial growth factor (VEGF) suppresses ovarian granulosa cell apoptosis in vitro. Biochemical and biophysical research communications. 363(3):733–737
- Krüger JP, Freymann U, Vetterlein S, Neumann K, Endres M, Kaps C. 2013. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus Med Hemother. 40(6):432–440. doi:10.1159/000356329.
- Lange-Consiglio A, Cazzaniga N, Garlappi R, Spelta C, Pollera C, Perrini C, Cremonesi F. 2015. Platelet concentrate in bovine reproduction: effects on in vitro embryo production and after intrauterine administration in repeat breeder cows. Reprod Biol Endocrinol. 13(1):65. doi:10.1186/s12958-015-0064-6.
- Lédée N, Prat-Ellenberg L, Petitbarat M, Chevrier L, Simon C, Irani EE, Vitoux D, Bensussan A, Chaouat G. 2018. Impact of prednisone in patients with repeated embryo implantation failures: beneficial or deleterious? J Reprod Immunol. 127:11–15. doi:10.1016/j.jri.2018.03.003.
- Maclaran K, Panay N. 2011. Premature ovarian failure. BMJ Sex Reprod Health. 37(1):35–42.
- Marini MG, Perrini C, Esposti P, Corradetti B, Bizzaro D, Riccaboni P, Fantinato E, Urbani G, Gelati G, Cremonesi F. 2016. Effects of platelet-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod Biol Endocrinol. 14(1):58. doi:10.1186/s12958-016-0195-4.
- Matsumoto H, Nasu K, Nishida M, Ito H, Bing S, Miyakawa I. 2005. Regulation of proliferation, motility, and contractility of human endometrial stromal cells by platelet-derived growth factor. J Clin Endocrinol Metab. 90(6):3560–3567. doi:10.1210/jc.2004-1918.
- Mazzocca AD, McCarthy MBR, Chowaniec DM, Dugdale EM, Hansen D, Cote MP, Bradley JP, Romeo AA, Arciero RA, Beitzel K. 2012. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 40(8):1742–1749. doi:10.1177/0363546512452713.
- Melo P, Navarro C, Jones C, Coward K, Coleman L. 2020. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. Journal of Assisted Reproduction and Genetics.1-9
- Molina A, Sánchez J, Sánchez W, Vielma V. 2018. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA assisted reproduction. 22(1):42
- Nazari L, Salehpour S, Hoseini S, Zadehmodarres S. 2016. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: A pilot study. Int J Reprod BioMed. 14(10):625–628.
- Nazari L, Salehpour S, Hosseini MS, Hashemi Moghanjoughi P. 2020. The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial. Human Fertility. 23(3):209–213.
- Nilsson EE, Detzel C, Skinner MK. 2006. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 131(6):1007–1015.
- Opneja A, Kapoor S, Stavrou EX. 2019. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. 179:56–63. doi:10.1016/j.thromres.2019.05.001.
- Otsuka F, McTavish KJ, Shimasaki S. 2011. Integral role of GDF‐9 and BMP‐15 in ovarian function. Mol Reprod Dev. 78(1):9–21.
- Ranjbaran A, Nejabati HR, Ghasemnejad T, Latifi Z, Hamdi K, Hajipour H, Raffel N, Bahrami-asl Z, Hakimi P, Mihanfar A. 2019. Follicular fluid levels of adrenomedullin 2, vascular endothelial growth factor and its soluble receptors are associated with ovarian response during ART cycles. Geburtshilfe Frauenheilkd. 79(1):86–93. doi:10.1055/a-0764-4765.
- Reghini MFS, Neto CR, Segabinazzi LG, Chaves MMBC, Camila de Paula F, Bussiere MCC, Dell’Aqua JA, Papa FO, Alvarenga MA. 2016. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology. 86(2):516–522. doi:10.1016/j.theriogenology.2016.01.029.
- Runels C, Melnick H, Debourbon E, Roy L. 2014. A pilot study of the effect of localized injections of autologous platelet rich plasma (PRP) for the treatment of female sexual dysfunction. J Womens Health Care. 3(169):2167–0420.10001
- Sak M, Gul T, Evsen M, Soydinc H, Sak S, Ozler A, Alabalik U. 2013. Fibroblast growth factor-1 expression in the endometrium of patients with repeated implantation failure after in vitro fertilization. Eur Rev Med Pharmacol Sci. 17(3):398–402.
- Samy A, Abbas AM, Elmoursi A, Elsayed M, Hussein RS. 2020. Effect of autologous platelet-rich plasma transfusion in the treatment of infertile women with thin endometrium and its implications in IVF cycles: a literature review. Middle East Fertil Soc J. 25(1):5. doi:10.1186/s43043-020-0019-5.
- Santamaria X, Isaacson K, Simón C. 2018. Asherman’s Syndrome: it may not be all our fault. Hum Reprod. 33(8):1374–1380. doi:10.1093/humrep/dey232.
- Sfakianoudis K, Simopoulou M, Nitsos N, Lazaros L, Rapani A, Pantou A, Koutsilieris M, Nikas Y, Pantos K. 2019. Successful implantation and live birth following autologous platelet-rich plasma treatment for a patient with recurrent implantation failure and chronic endometritis. In Vivo (Brooklyn). 33(2):515–521. doi:10.21873/invivo.11504.
- Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pantou A, Vaxevanoglou T, Kokkali G, Koutsilieris M, Pantos K. 2019. A case series on platelet-rich plasma revolutionary management of poor responder patients. Gynecologic and obstetric investigation. 84(1):99–106.
- Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth H-J. 2009. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle 1. Biol Reprod. 81(6):1025–1032. doi:10.1095/biolreprod.109.077370.
- Shelling AN. 2010. Premature ovarian failure. Reproduction. 140(5):633–641. doi:10.1530/REP-09-0567.
- Sills ES, Rickers NS, Li X, Palermo GD. 2018. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 34(9):756–760.
- Silva J, Figueiredo J, Van den Hurk R. 2009. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology. 71(8):1193–1208.
- Silva JR, van den Hurk R, de Matos MH, dos Santos RR, Pessoa C, de Moraes MO, de Figueiredo JR. 2004. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 61(9):1691–1704
- Stoikos CJ, Harrison CA, Salamonsen LA, Dimitriadis E. 2008. A distinct cohort of the TGFβ superfamily members expressed in human endometrium regulate decidualization. Human reproduction. 23(6):1447–1456
- Tohidnezhad M, Wruck C-J, Slowik A, Kweider N, Beckmann R, Bayer A, Houben A, Brandenburg L-O, Varoga D, Sönmez -T-T. 2014. Role of platelet-released growth factors in detoxification of reactive oxygen species in osteoblasts. Bone. 65:9–17. doi:10.1016/j.bone.2014.04.029.
- Ulbin-Figlewicz N, Jarmoluk A, Marycz K. 2015. Antimicrobial activity of low-pressure plasma treatment against selected foodborne bacteria and meat microbiota. Ann Microbiol. 65(3):1537–1546. doi:10.1007/s13213-014-0992-y.
- Unuane D, Tournaye H, Velkeniers B, Poppe K. 2011. Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab. 25(6):861–873. doi:10.1016/j.beem.2011.08.001.
- Uysal CA, Ertas NM. 2017. Platelet-rich plasma increases pigmentation. J Craniofac Surg. 28(8):e793. doi:10.1097/SCS.0000000000002893.
- Uzumcu M, Pan Z, Chu Y, Kuhn PE, Zachow R. 2006. Immunolocalization of the hepatocyte growth factor (HGF) system in the rat ovary and the anti-apoptotic effect of HGF in rat ovarian granulosa cells in vitro. Reproduction. 132(2):291–299
- Varoga D, Tohidnezhad M, Paulsen F, Wruck C, Brandenburg L, Mentlein R, Lippross S, Hassenpflug J, Besch L, Müller M. 2008. The role of human β‐defensin‐2 in bone. J Anat. 213(6):749–757. doi:10.1111/j.1469-7580.2008.00992.x.
- Yu H, Gao S, Tang H, Chen H, Deng Z, Yang L, Liu Z, Tang Q, Tang T. 2016. Growth hormone intrauterine perfusion combined with replacement cycle in the treatment of non-response thin endometrium: report of 5 cases. Int J Clin Exp Med. 9(6):11982–11989.
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. 2017. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assisted Reprod. 21(1):54. doi:10.5935/1518-0557.20170013.
- Zamaniyan M, Peyvandi S, Heidaryan Gorji H, Moradi S, Jamal J, Yahya Poor AghmashhadiF, Hossein Mohammadi M. 2020. Effect of platelet-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: a randomized controlled trial. Gynecological Endocrinology.1–5.
- Zhang S, Tan J, Li P. 2017. Co-transplantation of menstrual stromal cell and platelet-rich plasma improves Asherman’s syndrome in rat model. Fertil Steril. 108(3):e193. doi:10.1016/j.fertnstert.2017.07.570.
- Zhu R, Gan L, Wang S, Duan H. 2019. A cohort study comparing the severity and outcome of intrauterine adhesiolysis for Asherman syndrome after first-or second-trimester termination of pregnancy. Eur J Obstet Gynecol Reprod Biol. 238:49–53. doi:10.1016/j.ejogrb.2019.02.030.
