ABSTRACT
Preimplantation genetic testing for aneuploidy is associated with increased pregnancy success and reduced miscarriage in women 35 years and older when embryos are available for transfer. In this retrospective cohort study our objective was to evaluate if this holds true in good prognosis patients and across all age groups. Data were obtained from the Society for Assisted Reproductive Technology between 2014–2015. We included only the first single frozen embryo transfer where indication for corresponding ‘stimulation/freeze-all cycle’ was for reducing risk of ovarian hyperstimulation syndrome and performance of PGT-A for selecting euploid embryos. Our main outcomes were live birth and miscarriage rates. Among <35 age group, no difference in LBR was observed between cycles who underwent single embryo FET using non-PGT-A tested vs. tested embryos (51.7% vs. 50.9%, aOR 1.03, 95% CI 0.87–1.21). Additionally, the miscarriage rates (8.7% vs. 8.8%, aOR 0.97, 95% CI 0.72–1.30) were not different. Among 35–37 years old, no difference was observed between non-PGT-A tested and tested groups in LBR (50.4% vs. 54.7%, aOR 1.26, 95% CI 0.96–1.67) or miscarriage rates (8.3% vs. 10%; aOR 1.11, 95% CI 0.68–1.82). Similarly, among > 37 year old, no difference was observed between non-PGT-A tested and tested groups in LBR (48.1% vs. 53.2%, aOR 1.27, 95% CI 0.8–2.02) and miscarriage rates (6.2% vs. 8.5%, aOR1.34, 95% CI 0.52–3.43). To conclude, PGT-A tested embryos did not improve LBR and miscarriage rates in a good prognosis IVF population across all age groups.
Abbreviations: PGT-A: preimplantation genetic testing for aneuploidy; FET: frozen embryo transfer; LBR: live birth rate; OHSS: ovarian hyperstimulation syndrome; SART: society for assisted reproductive technology
Introduction
The ultimate goal of assisted reproductive technology (ART) is to achieve a healthy birth, ideally from a single transferred embryo. The purpose of preimplantation genetic testing for aneuploidy (PGT-A) is to distinguish chromosomally euploid embryos, considered to have better development potential, from other compromised aneuploid embryos. Since its inception many years ago, PGT-A usage has undergone tremendous evolution, however defining the appropriate patient population that will benefit the most from PGT-A, has been controversial.
PGT-A has been shown to reduce the practice of transferring multiple embryos and to confer a higher live birth rate per transfer (Forman et al. Citation2012, Citation2013, Citation2014). A multi-center randomized control trial (RCT) demonstrated that in patients of advanced maternal age, PGT-A significantly improves the chances of a live birth after the first attempt (Munne et al. Citation2019). While it also may reduce the time to a successful pregnancy and eliminate unnecessary transfers, a large proportion of patients (39%) might not develop any euploid embryos (Rubio et al. Citation2017). The results of a recent large-scale multicenter RCT, the STAR trial (Single embryo transfer of euploid embryos) found higher ongoing pregnancy and live birth rates in women aged 35–40 years after PGT-A, compared with embryo selection by morphologic criteria alone, however this was not the case in women aged 25–34 years. A relatively high aneuploidy rate was found in younger patients, however no improvement in pregnancy outcome was detected (Munne et al. Citation2019). In addition, reanalysis of the results of the STAR study by other groups revealed that even in the 35–40 year old group, the previously reported improvement in ongoing pregnancy rate was not seen (Orvieto and Gleicher Citation2020). In the opinion paper by Pagliardini et al., they suggest that transferring only blastocysts classified as ‘euploid’ after PGT-A leads to a reduction from 82.2% to 50% of the live birth rate for competent embryos, thus supporting the idea that PGT-A is associated with some embryo wastage (Pagliardini et al. Citation2020).
These results consistently indicate the lack of benefit of PGT-A in young or good prognosis patients. This observation has led to question whether the process of PGT-A is counterproductive to certain IVF populations and causes embryo wastage, ultimately leading to suboptimal pregnancy outcomes. The findings of a recent retrospective study indicate that PGT-A may be detrimental for those < 38 years old undergoing their first IVF cycle (Murphy et al. Citation2019). Current evidence has not been in favor of universal utilization of PGT-A. Nonetheless, in the United States based on Society for Assisted Reproductive Technology (SART) data, the number of PGT cycles being performed is increasing. In the years 2011–2012, 4.5% of autologous IVF cycles underwent PGT-A (Chang et al. Citation2016). From 2014 to 2016, the number of PGT-A cycles increased from 13% to 27% and the majority of women undergoing PGT-A cycles were under 35 years of age (Theobald et al. Citation2020). The increasing utilization of PGT-A without robust evidence of benefits in clinical practice is concerning and it is important to ensure we are not compromising IVF cycle pregnancy outcomes. Therefore, the goal of this study was to evaluate whether the utilization of PGT-A in good prognosis women undergoing IVF impacts pregnancy outcomes across all age groups. To address this issue, we selected women undergoing ‘freeze all’ autologous IVF cycles with subsequent first single frozen embryo transfer (FET) with or without PGT-A and compared their pregnancy outcomes.
Results
Patient demographics and cycle characteristics
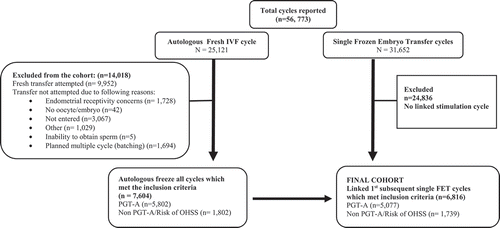
In the years 2014 and 2015, a total of 56,773 autologous IVF cycles were reported to the SART database. The final cohort of this study includes the first single embryo FET cycle after undergoing freeze-all to reduce the risk of developing OHSS during the linked stimulation cycle or for performance of PGT-A (). There were 1,739 cycles in the OHSS/non PGT-A group and 5,077 cycles in the PGT-A group.
The mean age of patients in the non PGT-A group was younger (31.5 ± 3.7 years) than those in the PGT-A group (36.1 ± 3.9 years). The majority of patients had normal BMI: 50.7% in non PGT-A group and 49.4% in the PGT-A group. The majority of the study population was nulligravida (43.2%). The most common infertility diagnosis in the non PGT-A group was male factor (40%) followed by polycystic ovarian syndrome (39.6%) and in the PGT-A group was also male factor (27%) followed by diminished ovarian reserve (22.4%). The median number of oocytes retrieved from non PGT-A group was 24 (range 18–32) and 16 (range 11–22) in the PGT-A cycles. The median number of 2 pronuclei (2 PN) embryos was higher in the non PGT-A (15 [11–20]) compared to the PGT- A (10 [7–14]) group. The median number of embryos available for cryopreservation in the non PGT-A group was 7 (5–11) compared to 5 (3–7) in the PGT-A group. The gonadotropin dose required for the stimulation was lower in the non PGT-A group compared to the PGT-A group (2000 IU vs. 3,150 IU; p < 0.001) ().
Table 1. Demographics and stimulation characteristics of patients undergoing IVF stimulation cycles (n = 6, 816)
Clinical outcomes
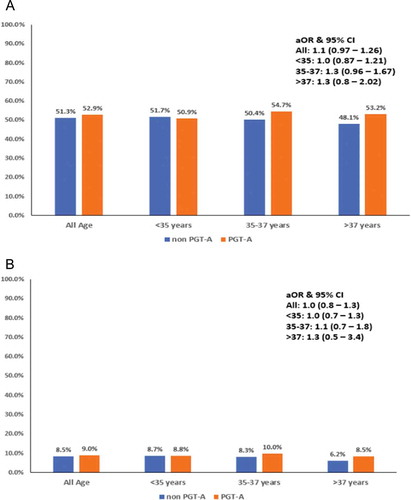
There was no difference in LBR (51.3% vs. 52.9%, p = 0.23) and pregnancy loss (8.5% vs. 9%, p = 0.50) when compared between the two good prognosis groups of non-PGT-A vs PGT-A cycles, respectively. Data was further stratified based on age groups, among women < 35 years old (n = 2,931), LBR and pregnancy loss remained comparable between non PGT-A and PGT-A cycles [(51.7% vs. 50.9%, p = 0.69; aOR 1.03, 95% CI: 0.87–1.21) and (8.7% vs. 8.8%, p = 0.88; aOR 0.97, 95% CI: 0.72–1.30), respectively] . Similarly, among women who were 35–37 years old (n = 1,752), LBR and pregnancy loss were similar [(50.4% vs. 54.7%, p = 0.19, aOR: 1.26, 95% CI 0.96–1.67) and (8.3% vs. 10%, p = 0.40, aOR 1.11, 95% CI 0.68–1.82), respectively]. Moreover, among women > 37 years old (n = 2,133), LBR and pregnancy loss was not significantly different [(48.1% vs. 53.2%, p = 0.37, aOR: 1.27, 95% CI: 0.8–2.02) and (6.2% vs. 8.5%, p = 0.45, aOR: 1.34, 95% CI: 0.52–3.43), respectively] .
Figure 2. Bar diagram showing the live birth rate (LBR) (A) and miscarriage rate (B) among IVF population without PGT-A (represented in blue) and with PGT-A (represented in orange) across age groups <35 years, 35–37 years, > 37 years. Odds ratios and 95% confidence intervals for the association between live birth and PGT status across all age groups is mentioned on the right box. aOR, adjusted odds ratio; CI, confidence interval
In women > 37 years old, increasing numbers of 2 PN were associated with the odds of live birth (OR: 1.02; 95% CI 1.00–1.06), as was the total number of cryopreserved embryos (OR: 1.03; 95% CI 1.01–1.06). However, these results were not significant after controlling for potential confounders. Overweight and obese women were less likely to have a live birth compared to normal weight women (). In women < 35 years old, overweight women were more likely to have a pregnancy loss compared to women of normal weight (aOR 1.58, 95% CI 1.08–2.31)().
Table 2. Odds ratios and 95% confidence intervals for the association between pregnancy loss, and live birth and PGT-A status in all patients and different age groups
Discussion
In this retrospective cohort analysis of a large national database, we found that a trophectoderm biopsy for PGT-A to select euploid embryos did not improve live birth and miscarriage rates per transfer, compared to good prognosis IVF population where embryos were selected by morphology alone, following single FET. Further stratification by age groups did not demonstrate any significant benefit of PGT-A for pregnancy outcomes in patients who were <35, 35–37 and > 37 years old.
The findings of our study are in contrast to prior studies that demonstrated higher LBR and decreased pregnancy loss per single euploid embryo transfer (Yang et al. Citation2012; Forman et al. Citation2013, Citation2014) and congruent with other studies that found that PGT-A did not improve LBR in all women (Chang et al. Citation2016; Munne et al. Citation2019). Our results echo the recent STAR trial by Munne et al., which showed that PGT-A did not improve overall pregnancy outcomes in all women when analyzed per embryo transfer as well as per intention to treat. However, further subgroup analysis of the STAR trial showed that women aged 35–40 years had a greater ongoing pregnancy rate when analyzed per embryo transfer (Munne et al. Citation2019). In contrast to the results of the subgroup analysis, our findings showed no improvement in live birth or pregnancy loss rates per embryo transfer in the 35–37 and > 37 year old women. Moreover, our results support the findings by Orvieto et al., who reanalyzed the results of the STAR study and suggested that, even in the age group 35–40 years, the previously reported improvement in ongoing pregnancy rate by Munne et al. was not seen (Orvieto and Gleicher Citation2020).
The results of the STAR trial, which was the largest multicenter randomized clinical trial to date, challenge the relevance of continued offering and utilization of PGT-A to good prognosis patients and women <35 years old. It has been speculated that PGT-A failed to show an improvement in pregnancy outcomes in young women secondary to embryo loss due to sub-optimal biopsy or transfer techniques in different clinics, wrong diagnosis, or biopsy-related damage to embryo (Munne et al. Citation2019). In addition, it was estimated that by transferring only euploid blastocysts after PGT-A, leads to a drop in the LBR from 82.2% to 50% for competent embryos, suggesting embryo wastage (Pagliardini et al. Citation2020). In a recent single-center retrospective study, PGT-A was not found to improve cumulative live birth per oocyte retrieval as compared to controls for patients undergoing their first ART cycle (8). They reported significantly lower cumulative LBR per oocyte retrieval in the age group < 38 years old, raising concerns about safety of continuing PGT-A (Murphy et al. Citation2019). Therefore, it is important to ensure that by offering PGT-A to good prognosis women, we are not compromising their IVF outcome. The current study demonstrated that in young women and good prognosis patients, the LBR is not higher or pregnancy loss is not lower when PGT-A is performed as compared to those without PGT-A. Therefore, the continued use of PGT-A in these groups of patients is not clinically justified, especially knowing the additional cost burden to patients (Neal et al. Citation2018).
A recent retrospective study by Haviland et al. proposed that PGT-A is associated with greater probability of live birth in women 35 years and older per embryo transfer. In this regard, our study contradicts this recent literature. They also stratified the age per SART standard and reported that in women >37 years old there is substantially higher chance of live birth when PGT-A is utilized. It is important to note that this study population included fresh as well as frozen embryo transfer. They also included multiple FETs from the same patient (Haviland et al. Citation2020). The main premise of our study was to consider first-order single embryo FET to avoid the confounding effect of different hormonal milieu, endometrial receptivity and alteration in endometrial preparation protocols in subsequent cycles. .
The strengths of this study include the unique design of only including the first single FET as a way to evaluate the embryo quality/competence as the sole factor. This design eliminates any effect of cumulative FET outcomes such as possible changes in endometrial preparation and laboratory protocols. This national database provides a large sample size and has excellent representation of the IVF practice in various clinics throughout the country, which make the results generalizable. Nevertheless, the limitations of the study other than being a retrospective analysis involves the fact that it was not a true intention to treat analysis. Since the database relies on reports provided by member clinics, we did not have the information on how many cycles were started with the intention of PGT-A and were canceled due to unavailability of oocytes/ embryos. Although this study uses a large nationally representative database, these databases are not immune to data entry error by the individual clinic members of SART and missing information on a number of factors, including indication for freeze-all. Because of this, a number of cycles were excluded from the study. Furthermore, we are limited to the available data in regard to potential confounders.
In conclusion, trophectoderm biopsy for PGT-A to obtain euploid embryos does not lead to additional improvement in live birth or decrease in pregnancy loss during IVF as compared to good prognosis women across all age groups. The observation that PGT-A was not associated with suboptimal pregnancy outcome as compared to good prognosis patients might seem reassuring however will need further investigation through analysis by intention to treat or initiated cycles. Based on the current evidence available and given the added cost of PGT-A, its utilization and recommendation in good prognosis IVF population may not be clinically justified.
Materials and methods
Study data and oversight
SART collects, validates and reports clinic-specific IVF outcome data from their member clinics (representing 94% of all IVF cycles). SART performs annual random site visits to validate the data reported and the validation visits are based on American Society for Reproductive Medicine (ASRM)/ SART guidelines (Jain et al. Citation2019). For the years 2014 and 2015, there were 458 and 464 reporting clinics, respectively.
Study population and linking cycles to individual patient
All women whose initial ART stimulation cycle was reported to the SART database from January, 2014 through December, 2015 were included in the study. We captured all the ‘freeze-all’ autologous IVF cycles which did not have a fresh embryo transfer and ultimately resulted in at least one FET during the study period. 2014 was the first year that FET cycles were linked to their source retrieval cycles in SART. Due to this initiative, it was possible for the first time to obtain the details of fresh and corresponding FET cycle information.
Following identification of all single-embryo FET cycles, we excluded any FET cycle which did not have a preceding linked fresh cycle. Only the first-order FET cycles were included in the analysis and all subsequent FET cycles (second-order or more) for those women were excluded. Few women underwent more than one fresh cycle. In such cases, only the first IVF stimulation cycle was included as we tried to avoid any bias introduced with repeated IVF cycles and the associated gonadotropin dose modification.
Subsequently, all included FET cycles were separated into two groups based on whether the embryo used for transfer was selected based on morphologic criteria versus embryos selected after PGT-A testing. As per SART data, the reason for ‘freeze-all’ cycles in the non PGT-A group was either secondary to risk of developing ovarian hyperstimulation syndrome (OHSS), endometrial receptivity concern, no availability of sperm, no oocyte or embryo availability and ‘reason not mentioned’. Among the PGT cycles, only cycles specifically done for PGT-A (aneuploidy screening) were selected as study group. PGT done for single gene disorder, gender selection, HLA matching, chromosomal translocation, or unknown reason were excluded. We assumed that during our study period, the vast majority, if not all of the ART laboratories were already performing PGT-A using trophectoderm biopsy on day 5/6. It is known that only better prognosis patients have embryos that successfully develop to blastocyst. Hence, the embryos transferred post PGT-A are automatically favorably selected. To avoid bias related to the favorability of selected embryos for transfer in the PGT-A group, the non PGT-A (untested) group in this study exclusively included ‘freeze-all’ cycles secondary to risk of developing OHSS. These represent the cycles with the best possible prognosis for pregnancy during IVF. The other reason to select hyper-responders as our comparison group was secondary to the findings of recent meta-analysis where significant increase in live birth rate was reported with elective FET in hyper-responders and in patients undergoing PGT-A (Roque et al. Citation2019).
To present age-specific data and outcomes, the final study cohort was stratified by age (< 35, 35–37 and >37 years old, based on SART standards) [].
Outcomes
The primary outcome was live birth rate (LBR), which is defined as delivery of any viable infant at 24 weeks or more of gestation. To study the role of PGT-A in different age groups, we divided the study cohort based on maternal age at egg retrieval to < 35, 35–37 and >37 years old. This study only included the first IVF stimulation cycle and the first subsequent FET to avoid any bias secondary to gonadotropin dose modification during ovarian stimulation or use of different protocols for endometrial preparation prior to subsequent FET. The secondary outcome was pregnancy loss (loss of pregnancy prior to 20 weeks) and treatment outcomes were analyzed per transfer basis.
Statistical analysis
Demographic information available included age and body mass index (BMI) at the start of IVF stimulation, race-ethnicity, smoking status, gravidity, and etiology of infertility (male factor, endometriosis, polycystic ovarian syndrome, diminished ovarian reserve, tubal factor, uterine, or unexplained). Stimulation cycle characteristics included dose of follicle stimulation hormone (FSH), total oocyte retrieved, number of 2 pronuclei (2 PN) embryos on day 1, number of embryos available for biopsy, total embryos vitrified, and the stimulation protocol used (agonist suppression, agonist flare, or antagonist suppression).
The descriptive characteristics of freeze-all IVF cycles were compared between cycles who were at risk of OHSS (non PGT-A or untested group) and cycles which had PGT-A (tested group). For PGT-A cycles, we assumed that a euploid embryo was transferred, as this was not specifically indicated in the SART CORS database. Results were reported as mean with standard deviation (or median with interquartile range) for continuous variables and differences were compared using independent samples t-test or Mann-Whitney test, as appropriate. Categorical variables were compared using chi-square or Fisher exact tests. Multivariable logistic regression was used to estimate adjusted odds ratios (aOR) and 95% confidence intervals (CI) representing the association between PGT-A and selected outcomes (LBR and pregnancy loss rate). Morphologically selected non-PGT-A tested embryos were considered as the reference group. Potential confounders selected for adjustment in regression models were selected a priori based on previous literature and clinical knowledge: maternal age and BMI at IVF stimulation, gravidity, gonadotropin dose, total 2 PN, number of oocytes retrieved, and total number of embryos vitrified from the cycle. All statistical tests were two-sided and considered significant at a p-value < 0.05. Statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
Ethics approval
This study was approved by the Institutional Review Board at the University of South Florida (Pro00030426). Our research proposal was approved by the SART committee permitting access to the national SART dataset. Informed consent was waived by IRB for this project.
Authors’ contributions
Study conceptualization and writing original draft: PS, EN, RS, SJ and AI. Data curation: PS, AI. Data analysis: JPT. All authors reviewed, edited and approved the final manuscript.
Acknowledgments
We wish to thank SART and all of its members for providing clinical information to the SART CORS database for use by researchers. Without the efforts of SART members, this research would not have been possible.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. 2016. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States assisted reproductive technology surveillance data, 2011-2012. Fertil Steril. 105(2):394–400. doi:https://doi.org/10.1016/j.fertnstert.2015.10.018.
- Forman EJ, Hong KH, Franasiak JM, Scott RT Jr. 2014. Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 210(2):157 e151–156. doi:https://doi.org/10.1016/j.ajog.2013.10.016.
- Forman EJ, Hong KH, Treff NR, Scott RT. 2012. Comprehensive chromosome screening and embryo selection: moving toward single euploid blastocyst transfer. Semin Reprod Med. 30(3):236–242. doi:https://doi.org/10.1055/s-0032-1311526.
- Forman EJ, Upham KM, Cheng M, Zhao T, Hong KH, Treff NR, Scott RT Jr. 2013. Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil Steril. 100(3):718–724. doi:https://doi.org/10.1016/j.fertnstert.2013.04.043.
- Haviland MJ, Murphy LA, Modest AM, Fox MP, Wise LA, Nillni YI, Sakkas D, Hacker MR. 2020. Comparison of pregnancy outcomes following preimplantation genetic testing for aneuploidy using a matched propensity score design. Hum Reprod. 35(10):2356–2364. doi:https://doi.org/10.1093/humrep/deaa161.
- Jain T, Grainger DA, Ball GD, Gibbons WE, Rebar RW, Robins JC, Leach RE. 2019. 30 years of data: impact of the United States in vitro fertilization data registry on advancing fertility care. Fertil Steril. 111(3):477–488. doi:https://doi.org/10.1016/j.fertnstert.2018.11.015.
- Munne S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M, et al. 2019. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 112(6):1071–1079 e1077. doi:https://doi.org/10.1016/j.fertnstert.2019.07.1346.
- Murphy LA, Seidler EA, Vaughan DA, Resetkova N, Penzias AS, Toth TL, Thornton KL, Sakkas D. 2019. To test or not to test? A framework for counselling patients on preimplantation genetic testing for aneuploidy (PGT-A). Hum Reprod. 34(2):268–275. doi:https://doi.org/10.1093/humrep/dey346.
- Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT Jr. 2018. Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 110(5):896–904. doi:https://doi.org/10.1016/j.fertnstert.2018.06.021.
- Orvieto R, Gleicher N. 2020. Preimplantation genetic testing for aneuploidy (PGT-A)-finally revealed. J Assist Reprod Genet. 37(3):669–672. doi:https://doi.org/10.1007/s10815-020-01705-w.
- Pagliardini L, Vigano P, Alteri A, Corti L, Somigliana E, Papaleo E. 2020. Shooting STAR: reinterpreting the data from the ‘Single Embryo TrAnsfeR of Euploid Embryo’ randomized clinical trial. Reprod Biomed Online. 40(4):475–478. doi:https://doi.org/10.1016/j.rbmo.2020.01.015.
- Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. 2019. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 25(1):2–14. doi:https://doi.org/10.1093/humupd/dmy033.
- Rubio C, Bellver J, Rodrigo L, Castillon G, Guillen A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohi J, et al. 2017. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 107(5):1122–1129. doi:https://doi.org/10.1016/j.fertnstert.2017.03.011.
- Theobald R, SenGupta S, Harper J. 2020. The status of preimplantation genetic testing in the UK and USA. Hum Reprod. 35(4):986–998. doi:https://doi.org/10.1093/humrep/deaa034.
- Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. 2012. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 5(1):24. doi:https://doi.org/10.1186/1755-8166-5-24.