ABSTRACT
Standard protocols for clinical in vitro fertilization (IVF) laboratories recommend incubating semen at 37°C in 5% CO2 without strictly specifying which medium should be used or for how long. This study aimed to test the most common different incubation media used in Latin American andrology and micromanipulation laboratories and verify which, if any, is the most appropriate medium to improve asthenozoospermic semen samples’ motility in the infertile male population. Ejaculates (136) collected from asthenozoospermic men were divided into two cohorts with similar characteristics (cohort 1; n = 28 and cohort 2; n = 108). Cohort 1 was used to evaluate the optimal incubation time with regard to unprepared asthenozoospermic sample sperm motility. After defining an optimal incubation period of 2 h, cohort 2 was used to evaluate which of the four media commonly used in IVF clinics (continuous single culture medium = CSCM®; SpermRinse medium = SR®; in vitro fertilization medium = G-IVF® and human tubal fluid medium = HTF®) was preferred for semen samples from asthenozoospermic patients. Overall, it was determined that a 2-h incubation in CSCM® medium led to the highest asthenozoospermic sperm motility. Thus, this simple, cost-effective, easily reproducible protocol could prove extremely useful for andrology laboratories working with IVF clinics dealing with asthenozoospermic semen specimens. This is particularly relevant since the incidence of the latter is on the rise as semen quality decreases around the globe.
Abbreviations: ANOVA: Analysis of variance; ARTs: Assisted reproductive techniques; BWW: Biggers, Whitten, and Whittingham; CO2: Carbon dioxide; CPM: counted per minute; CSCM: Continuous Single Culture Medium; DAB: 3.3′- diaminobenzidine; DFI: DNA Fragmentation Index; DMSO: Dimethyl sulfoxide; G-IVF: In Vitro Fertilization Medium; GSH: Glutathione; GPx: glutathione peroxidase; HDS: High DNA Stainability; HSA: Human Serum Albumin; HTF: Human Tubal Fluid; HYP: Hyperactivity; ICSI: Intracytoplasmic sperm injection; IUI: Intrauterine insemination; IVF: in vitro fertilization; LIN: Linearity; ROS: Reactive Oxygen Species-level; SC: Sperm concentration; SCA: Sperm Computer Analysis; SCSA: Sperm Chromatin Structural Assay; SR: SpermRinse medium; SSS: Synthetic Serum Substitute; STR: Straightness; SOD: superoxide dismutase; TNE: Tris-Borate-EDTA; TSC: Total sperm count; VAP: Mean velocity; VCL: Curvilinear velocity; VSL: Linear velocity; WHO: World Health Organization; WOB: Wobble; spz: spermatozoa; AO: antioxidant
Introduction
The controversial concept of deteriorating human sperm quality has been under considerable debate for the past 30 years (Carlsen et al. Citation1992). However, a recently conducted meta-analysis with rigorous inclusion criteria, using 185 studies involving nearly 43,000 men from all continents, has given credence to this idea by reporting a significant steady decrease in sperm concentration (SC) and total sperm count (TSC) of the order of minus 0.7 million spermatozoa/ml/year between 1973 and 2011 (Levine et al. Citation2017). Consistent with these findings, an increasing number of men with poor sperm quality often associated with increased oxidative stress damage seek help from andrology clinics worldwide (Ahmed Citation2019; Quinn Citation2004; Wright et al. Citation2014). Intrinsic and extrinsic factors such as individual genetics, chronic diseases, lifestyle, and environmental exposures are the major causative factors affecting sperm structures and functions. These result in the inability of spermatozoa to reach and fertilize the oocyte (Edwards et al. Citation1980; Pasqualotto et al. Citation2006; Lichtenfels et al. Citation2007; Hallak et al. Citation2018; Ciccone et al. Citation2020). Such instances lead couples to pursue clinical assistance via assisted reproductive techniques (ART), which include intrauterine insemination (IUI), conventional in vitro fertilization (IVF), and IVF after intracytoplasmic sperm injection (IVF-ICSI) (Hallak Citation2017a; Pariz et al. Citation2020). This is particularly true in cases of moderate-to-severe male component infertility as these techniques bypass most sperm functional deficiencies. As a result, infertile couples increasingly turn to ART, including the most invasive IVF-ICSI technology (Hallak Citation2017a, Citation2017b). Although the efficacy of these techniques is still suboptimal, culminating with a mere 25 to 30% take-home baby rate, they offer a welcome solution for couples experiencing difficulty conceiving. Despite this low effectiveness, the relative success of ART and the high demand have obscured the need for basic research into the underlying causes and treatment of male infertility. The somewhat low success rate of ART could be attributed to two factors: reproductive specialists are consulted by an increasing number of couples plagued with severe infertility and/or suboptimal gamete handling during ART.
Despite the recommendations of the WHO task force ((WHO) World Health Organization Citation2010) with regard to gamete manipulation in IVF clinics, it is clear that there is still room for improvement. The search for better basic and advanced laboratory procedures could be a valuable approach to increase pregnancy success rates (Edwards et al. Citation1980; Palermo et al. Citation1992; Quinn Citation2004). The existence of a handful of factors that may negatively influence gamete quality during semen sample handling and spermatozoa preparation for IVF is acknowledged, yet not all have been adequately addressed. Factors that may impact sperm quality include: media composition in which spermatozoa are resuspended after collection and liquefaction; incubation time in preparation media; temperature and mechanical constraints during centrifugation steps; and the presence of leucocytes even at concentrations considered normal according to WHO standards (Yavas and Selub Citation2004; Athayde et al. Citation2007; Boomsma et al. Citation2007; Marchesi et al. Citation2010; Franken et al. Citation2011; Monteiro et al. Citation2016). Of note is that laboratory protocols for IVF/ICSI recommend sperm incubation at 37°C in 5% carbon dioxide (CO2) in various commercial culture media (Biggers, Whitten, and Whittingham [BWW®]; Earle®, Ham-F-10®, Human Tubal Fluid [HTF®]) that were primarily optimized for oocyte/embryo culture (Calamera et al. Citation2001; Cicaré et al. Citation2014; Thijssen et al. Citation2014; Hosseini and Khalili Citation2017; Ahmed et al. Citation2018).
Considering these observations, we investigated the extent to which spermatozoa structure and function are preserved in commercial IVF media, especially when dealing with asthenozoospermic samples with compromised motility. This is particularly important in most South American infertility clinics, which, for cost-efficiency and regulatory issues, often use a single media for spermatozoa preparation and fertilized embryo culture following IVF-ICSI. We used a Sperm Computer Analysis (SCA®, Microptic, Barcelona, Spain) system to evaluate sperm hyperactivation, a critical parameter for successful fertilization. In parallel, nuclear integrity was evaluated using the sperm chromatin structure assay (SCSA®) to determine spermatozoa quality. As sperm motility is dependent on mitochondria function, we also examined sperm mitochondrial activity using the 3.3 diaminobenzidine (DAB) staining method. Finally, since mitochondria-derived reactive oxygen species (ROS) may impair motility by affecting mitochondria efficiency and sperm membrane fluidity, ROS levels were monitored by luminol-mediated chemiluminescence.
Results
The overall study design is presented in . Before comparing the potential advantages conferred by incubation in the different IVF media under evaluation, we determined the ideal incubation period for the acquisition of optimal motility in asthenozoospermic semen samples. To this end, sperm motility was evaluated in a cohort of 28 asthenozoospermic semen samples before (T0) or after a 1 to 4 h incubation (T1 to T4) under mineral oil at 37°C in a 5% CO2 controlled atmosphere. We show that the percentage of progressively motile spermatozoa reached a significant peak (p = 0.003) after a 2-h incubation (T2; ). After the same incubation time (2 h), the percentage of total motile spermatozoa showed a clear tendency to increase () that did not reach statistical significance. Furthermore, the lowest percentage of immotile spermatozoa was observed at this time point (T2; ). Incubation time was negatively correlated with total progressive spermatozoa (R = −0.328; p < 0.001) and with non-progressive spermatozoa (R = −0.181; p = 0.36).
Table 1. Motility of asthenozoospermic sperm samples before (T0) and after different incubation periods (T1 to T4).
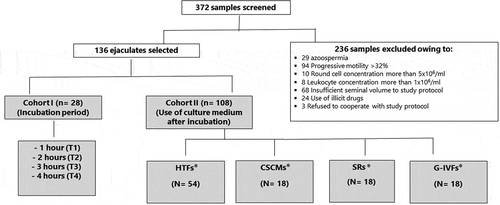
Figure 1. Study design. Out of a total of 372 screened samples, 236 were excluded for the reasons indicated leaving 136 samples for inclusion in the study. A first cohort of 28 samples was assigned to protocol 1 to determine the optimum incubation period to support the best motility parameters of asthenozoospermic samples. In protocol 2, a total of 108 samples were analyzed in different media (HTF®, CSCM®, SR® and GIVF®) using HTF® medium as a reference (3 sub-cohorts of 18 samples each = 54 samples).
A selection of IVF media was then compared on a second equivalent cohort () of 108 semen samples (, )
Figure 2. Representative photographs of mitochondrial activity, viability, and morphological defects of spermatozoa. A) Photograph of defective spermatozoa staining by deposition of DAB (3,3 diaminobenzidine) in the mitochondrial sheath along the middle part of the spermatozoon (black arrows). B) Photographs of spermatozoa viability assessed by the hypo-osmotic (HOS) test. In a hypo-osmotic environment viable spermatozoa with an intact membrane exhibit swelling of the tail curvature (right photograph) or a coiling of the tail (left photograph) due to water influx as shown for the cells circled. C) Example of abnormal human spermatozoa morphology according to WHO methodology, showing the presence of 2 vacuoles in the nucleus. All images courtesy of the Androscience laboratory (São Paulo, Brazil).
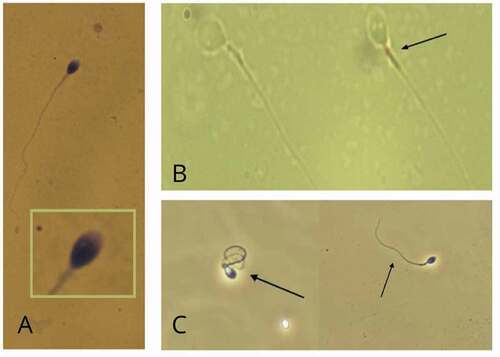
Table 2. Characteristics of semen samples included in media comparison groups.
Table 3. Seminal and sperm parameters assessed in the four media before and after a 2-h incubation period (Mean ± SE, n = 18 each group except for HTF, n = 54).
The 2-h incubation did not result in a statistically significant improvement in the other parameters tested. On the contrary, the sperm DNA fragmentation index (DFI) tended to increase (HTF®, CSCM®, SR®) or was significantly greater (GIVF® medium), in the media under test. This suggested that while the 2-h incubation significantly improved sperm motility in asthenozoospermic semen samples, it also slightly increased sperm DNA damage.
Discussion
Culture media optimization has played an important role in improving ART outcomes during the past decade (Quinn Citation2004). There has been a shift to more complex culture media from simple media designed primarily for somatic cell culture (Biggers Citation1987; Pool Citation2004; CitationLane and Gardner ; Meintjes et al. Citation2009). Although most of these media were initially optimized for oocyte and embryo culture, they are also currently used as incubation media for spermatozoa prior to ART, particularly in most Latin America IVF centers (Palini et al. Citation2016; Fácio et al. Citation2016). For decades, spermatozoa incubation at 37°C under 5% CO2 has been widely recommended in andrology laboratories and IVF clinics. However, there is no consensus to date on the optimal duration of this incubation, nor on the most appropriate type of medium to use (Calamera et al. Citation2001; Yavas and Selub Citation2004; Athayde et al. Citation2007; (WHO) World Health Organization Citation2010; Liu et al. Citation2011; Pasha et al. Citation2012; Cicaré et al. Citation2014; Nabi et al. Citation2014; Thijssen et al. Citation2014; Hosseini and Khalili Citation2017; Ahmed et al. Citation2018). It is also unclear whether these incubation conditions are appropriate for all semen samples, particularly for samples from moderate-to-severe asthenozoospermic patients.
As a prerequisite for comparing the different sperm culture media commonly used in South American infertility clinics for the preparation and incubation of sperm prior to IVF, we show that untreated asthenozoospermic samples show higher progressive motility following a 2-h incubation at 37°C in 5% CO2. The latter is a critical parameter for reproductive success (Fitzpatrick and Lüpold Citation2014). It is difficult to make robust comparisons with existing data in the literature because of differences in sample etiologies, media, and semen sample preparation methods. However, similar incubation times have been reported to improve sperm function. For example, a 2-h incubation has previously been shown to promote optimal human sperm motility from unprepared semen samples (Matson and Tardif Citation2012). In another report, a 3-h incubation was identified as the best condition to achieve higher fertilization rates prior to IVF-ICSI with prepared sperm samples from male factor infertile patients (Mansour et al. Citation2008). These spermatozoa incubation periods are consistent with the rationale that spermatozoa functional parameters were improved by incubating spermatozoa for 2–3 h regardless of the sperm preparation method or the inclusion of suboptimal sperm samples. This is consistent with the ‘activation theory,’ which suggests that non-motile spermatozoa stored in the epididymis undergo an activation phase that facilitates the acquisition of motility, thereby imparting fertilizing ability (Si Citation1997; Okamura et al. Citation1985; Lane and Gardner Marin-Briggiler et al. Citation2007; Gallup et al. Citation2009). This activation step is presumed to be a consequence of spermatozoa exposure to seminal, prostatic, and bulbourethral glandular secretions during ejaculation (Biggers Citation1987). Another contributor to this activation is the increase in spermatozoa temperature when they reach the female genital tract (Pool Citation2004; Meintjes et al. Citation2009). Incubation of sperm samples at 36.6°C–37°C in 5–6% CO2, as is standard in IVF clinics and micromanipulation laboratories, is believed to mimic this physiological context in part. However, there are reports that prolonged incubation of more than 3 h is associated with increased oxidative damage to spermatozoa (Aitken et al. Citation2012; Sposito et al. Citation2017). This is in agreement with the original observation by Tosic and Walton (Citation1946) that spermatozoa per se are efficient producers of reactive oxygen species, leading to rapid depletion of the protective antioxidant (AO) capacity of seminal fluid or any AO-deficient medium. This situation is particularly pertinent to asthenozoospermic semen samples, where it has long been demonstrated that oxidative stress is often a critical component of sperm structural and functional defects (Petrella et al. Citation2003, Citation2005; Hossain et al. Citation2010; Matsuura et al. Citation2010; Sposito et al. Citation2017). This could account for the increase in DFI recorded after a 2-h incubation in the different media tested in this study.
Using this 2-h incubation time as a reference, we compared four different incubation media in widely used in IVF clinics to determine which might be the most beneficial for the motility of asthenozoospermic samples. The classical HTF® medium was taken as a reference. Although it was not necessary to compare unprepared semen samples with prepared semen samples, it is notable that spermatozoa preparation decreased the values of each of the parameters monitored. This suggests that seminal fluid remains the incubation medium of choice even for asthenozoospermic specimens, despite the common belief that an unbalanced seminal fluid may be partly responsible for asthenozoospermic sperm defects. Although the removal of seminal plasma is common practice in IVF laboratories, it could be argued that it might be preferable to conduct sperm incubation in the original seminal fluid prior to IVF, barring obvious limitations such as abnormal pH, high ROS content, or excessive leukocytospermia. A similar observation was recently made with regard to cryopreservation of semen samples. It was shown that at the time of thawing, the recovery of sperm parameters was improved when the cryoprotective medium was added directly to the raw semen (Ben et al. Citation1997; MacKenna et al. Citation2017; Szczykutowicz et al. Citation2019). This is consistent with the widespread view that seminal plasma is a highly engineered fluid necessary for many aspects of sperm protection, nutrition, and survival (Clark and Schust Citation2013; Schjenken and Robertson Citation2014; Szczykutowicz et al. Citation2019).
A statistically significant improvement in sperm motility (progressive and total), a reduction in immobile spermatozoa, and a higher proportion of spermatozoa with functional mitochondria were only observed following a 2-h incubation in CSCM® (supplemented with 5% HSA®). None of the other media led to a significant improvement in any of the parameters analyzed, except for incubation in G-IVF® (supplemented with fructose and SSS®), which was accompanied by a significant improvement in progressive sperm motility. A limitation of our study is that we omitted to assess the percentage of spermatozoa that prematurely engaged in acrosome reaction during the 2-h incubation. This would have been an interesting parameter to investigate.
To account for the advantage of the CSCM® medium over the other media tested, we compared the respective media compositions (see Supplementary ) and noted that all media tested contained, or were supplemented with, human serum albumin (HSA®) or serum substitute supplement (SSS®: which is HSA® + human globulins). The importance of albumin, one of the most abundant proteins in the female genital tract fluids, is well known and is associated with its role as a fluidifying agent, energy substrate, and powerful antioxidant (Ashwood-Smith et al. Citation1989; Bungum et al. Citation2002; Moncla et al. Citation2016; Leese et al. Citation1998; Parinaud et al. Citation1998; Palasz et al. Citation2006; Matson and Tardif Citation2012). As all the media tested contained albumin or a similar molecule, it is unlikely that this could be the key factor explaining the superiority of CSCM®. Similarly, all media tested contained equivalent concentrations of salts and ions, which again is unlikely to explain why incubation in CSCM® media is more beneficial for asthenozoospermic sperm sample motility. A more detailed composition analysis revealed that only CSCM® contained the full complement of amino acids (Gardner and Lane Citation1997; Morbeck et al. Citation2014; Kleijkers et al. Citation2016). G-IVF® contained only glutamine, while SR® and HTF® were devoid of any amino acids. However, while the absence of amino acid supplementation may explain the inability of the SR® medium to improve asthenozoospermic semen sample motility, it does not explain the superiority of CSCM® medium over the other two media tested, since HTF® and G-IVF® were supplemented with SSS, which contains high levels of non-essential and essential amino acids (Setchell et al. Citation1967; Hinton Citation1990; Gardner and Lane Citation1993; Gardner Citation2008). The functions of amino acids include the promotion of protein synthesis, in addition to pleiotropic effects such as serving as chelating agents, osmotic modulators, antioxidants, substrates and/or regulators of energy metabolism, and biosynthetic precursors (Molina et al. Citation1995; Gadea et al. Citation2004; Gonzalez-Cuevas et al. Citation2011; Sangeeta et al. 201). Amino acids used as additives in ram sperm handling media exert a beneficial effect in pre-freeze and post-thaw sperm motility, plasma membrane integrity and lipid peroxidation levels (Sangeeta et al. Citation2015). In particular, L-glutamine is the most abundant free amino acid in seminal plasma and spermatozoa (Molina et al. Citation1995; Gadea et al. Citation2004). Of particular interest is that glutamine is required to synthesize glutathione (GSH) and may function as an antioxidant in semen (Gadea et al. Citation2004). In addition to the antioxidant action of albumin and amino acids, CSCM® medium contains EDTA and sodium citrate, which preserve sperm motility and increase sperm survival (Kuo et al. Citation1998; Sallam Citation2007; Gonzalez-Cuevas et al. Citation2011; Rehman et al. Citation2013). The enhanced antioxidant coverage provided by the CSCM medium is supported by our observation that the lowest level of ROS was recorded after incubation in this medium ().
In conclusion, under our experimental conditions, which largely mimic the daily procedures in the vast majority of andrology and micromanipulation laboratories in dozens of countries, the incubation of semen samples for 2 h in CSCM® supplemented with HSA® proved to be better than any of the other classical medium tested in terms of stimulating the motility of semen samples from asthenozoospermic patients. This simple, inexpensive, and easily reproducible protocol could prove useful for andrology laboratories working alone or in conjunction with micromanipulation laboratories and IVF clinics for improved management of infertile patients with asthenozoospermia. Further research will determine whether this improved motility translates into higher pregnancy rates following ART procedures. Indeed, we expect this to be the case because sperm motility is positively correlated with higher pregnancy rates (Shulman et al. Citation1998; Björndahl Citation2010; Lumley et al. Citation2015).
Materials & methods
Study design
Data were collected from September 2017 to August 2019 from the medical records of men aged 21–45 years old, following evaluation by a qualified andrologist at Androscience, Science and Innovation Center in Andrology; and High-Complex Clinical and Research Andrology Laboratory, a referral center for male infertility, hypogonadism, male reproductive and sexual health in São Paulo, Brazil. All patients included in the study signed an informed consent form. The main reasons that prompted these patients to consult a specialized physician were infertility, erectile dysfunction, loss of libido, premature ejaculation, other sexual-related problems, pre-vasectomy, or a general checkup. The data collected included self-reported physical exercise, tobacco, alcohol and any other addictive or non-addictive drug use (antidepressants, corticosteroids, etc.). This study was approved by the Research Ethics Committee, University of São Paulo Medical School (CAAE No. 42,790,915.9.0000.0065). The total cohort of 136 subjects was analyzed according to the WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edition 2010 ((WHO) World Health Organization Citation2010). The cohort included patients with progressive motility < 32% (classified as asthenozoospermia). Exclusion criteria included any medical, genetic or lifestyle condition that could interfere with spermatozoa quality or testicular function, such as high progressive motility > 32% (classified as normozoospermic) and round cell concentration greater than 5 × 106/ml. A white blood cell concentration greater than 1 × 106/ml was also excluded, as these cells are likely to generate high levels of ROS that have been associated with decreased sperm motility (De Lamirande et al. Citation1997; Athayde et al. Citation2007). Samples were divided into two subcohorts (cohort 1; n = 28 and cohort 2; n = 108). Cohort 1 was used to evaluate the optimal incubation time while cohort 2 was used to assess the different media tested in the course of the study (see below).
Culture medium and supplements
The four different culture media selected represent the most commonly used culture media in Latin American micromanipulation labs and IVF clinics: a continuous single culture medium (CSCM®) containing 10% human serum albumin (HSA®) = CSCMs® group; a SpermRinse medium (SR®) supplemented with fructose and carnitine added immediately after rinsing the sample = SRs® group; an in vitro fertilization medium (G-IVF®) containing fructose and 15% serum substitute supplement (SSS®) = G-IVFs® group. These three media were compared with the standard Human Tubal Fluid (HTF®) medium supplemented with 15% SSS® = HTFs® group. The culture media selected for this study were designed and recommended for use at 37°C in 5% CO2. HTF® and CSCM® were obtained from Irvine Scientific (Santa Ana, CA, USA) while SR® and G-IVF® were obtained from Vitrolife (San Diego, CA, USA). The supplements used were obtained from Irvine Scientific (Santa Anna, CA, USA) for SSS® and HSA®, while fructose and L-carnitine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Media composition is listed in Supplementary Table 2.
Specimen collection and semen analysis
Ejaculates were collected into sterile disposable polypropylene bottles following masturbation after 48 to 72 h of abstinence. The samples were liquefied at 37°C for 30 min. Semen analysis was carried out by two trained biomedical laboratory technicians at the Andrology Laboratory of Androscience, with strict internal and external quality control standards. Semen analysis was performed using a Makler counting chamber (Medical Instruments, Israel). This analysis included evaluation of the following parameters: volume (ml), pH, concentration (million/ml), progressive motility (%) and total motility (%), total sperm count (million), total motile sperm count (million), total progressive sperm count (million) as recommended ((WHO) World Health Organization Citation2010).
Structural sperm parameters: sperm morphology was assessed using two methods: Kruger’s strict criteria and the WHO guidelines. According to Kruger’s strict criteria classification, a spermatozoon is considered normal when the head has a perfectly oval configuration with a well-defined acrosome, covering about 40–70% of the sperm head. Heads with a borderline shape are considered abnormal. The length of normal sperm heads is 5 µm to 6 µm, and the width is 2.5 µm to 3.5 µm. There should be no abnormalities in the midpiece or flagellum. The intermediate piece should be thin, attached to the central part of the body, and less than 1 µm wide, while the length should be about one and a half times that of the sperm head. The cytoplasmic droplet should be no more than half the size of the sperm head, while the flagellum should be uniform, slightly thinner than the intermediate piece, about 45 µm long, and should not be spiral (Kruger et al. Citation1986). According to the WHO criteria for morphological analysis of semen samples ((WHO) World Health Organization Citation2010), the sperm head should be between 4.0 and 5.5 µm in length and 2.5 and 3.5 µm in width, without gross defects in the flagellum and intermediate piece. The midpiece is considered normal with a value of less than 1 µm, while the flagellum should be approximately 45 µm in length. The assessment of sperm DNA integrity was performed using the Sperm Chromatin Structure Assay (SCSA®) as described originally in (Evenson et al. Citation1985; Evenson Citation2016).
Functional sperm parameters: sperm viability was assessed by the hypoosmotic swelling test ((WHO) World Health Organization Citation2010) that monitors cell membrane integrity. In samples with low motility (< 40%), the vitality test is helpful to discriminate between immobile dead sperm and immobile live sperm. Ejaculates with a total progressive sperm count greater than 20 million were treated using the discontinuous density gradient technique (Isolate®, Irvine Scientific, Santa Ana/CA, USA) and resuspended with the same medium used for incubation. On the other hand, samples with a total progressive sperm count of < 20 million were treated by a simple washing technique (the seminal sample was centrifuged and resuspended with the same medium used for incubation). In each case, two aliquots were generated to monitor pre- and post-incubation parameters. All samples were maintained under mineral oil and incubated at 37°C in a 5% CO2 atmosphere (SANYO MCO – 5AC, Osaka, Japan). Spermatozoa kinetic parameters, including curvilinear velocity (VCL; μm/s), linear velocity (VSL; μm/s), mean velocity (VAP; μm/s), linearity (LIN; %), straightness (STR; %), oscillation (WOB; %) and hyperactivity (HYP; %) were measured using a semi-automated SCA® platform (MICROPTIC, Barcelona, Spain; Burkman Citation1991; Davis and Katz Citation1992) following the manufacturer’s standard procedures. The analysis was performed at 100 × magnification.
Reactive oxygen species levels were measured by light emission after stimulation with a luminol chemiluminescent probe as originally described by (Aitken et al. Citation1992; Athayde et al. Citation2007).
Mitochondrial activity was assessed by DAB staining according to the method described by Hrudka (Citation1987).
Statistical analysis
Statistical analysis was performed using IBM SPSS 19.0 for Windows (IBM SPSS, Inc., Chicago, IL) to test variance homogeneity and normality of results. For all analyses, P < 0.05 was adopted as a statistically significant difference. To evaluate the differences recorded in the samples when considering each medium and the pre-and post-incubation times, two-way ANOVA analyses were performed. As the variables could eventually be considered dependent or independent, we considered both situations (see Supplementary ). When a parameter was found significantly different between the compared groups, a post-hoc Tuckey test was conducted. Pearson correlation was used to evaluate the parameters of progressive and non-progressive motility with regard to incubation time.
Ethics approval and consent to participate
The Research Ethics Committee approved this study, The University of Sao Paulo Medical School (CAAE No. 42,790,915.9.0000.0065), and all subjects signed the informed consent form.
Authors’ contributions
Participated in the design of the study, performed the experimental procedures in the laboratory, participated in the data analysis and wrote the first draft of the manuscript: CR; participation in the study design, supervision of the clinical investigation, data analysis and writing of the first draft of the manuscript: JRP; data analysis, critical reading and editing of the final manuscript: JRD, JH. Corresponding author (JH) assumes final responsibility for the decision to submit the manuscript for publication.
Consent for publication
All authors contributed to the study. They have all seen the final version and consent to its submission.
Acknowledgments
The authors would like to acknowledge Androscience, Science and Innovation Center in Andrology and High-Complex Clinical and Research Andrology Laboratory, for supporting this study. Authors would like to express gratitude to SCSA®, Diagnostics, Brookings, SD, USA, for providing the SCSA® analysis. We thank Dr. Tiago Antonio de Souza for the biostatistical analysis and thoughtful insights. The authors would like to thank Dr. Muriel Kelly (Scientific Editing Services, Madbury, NH, USA) for her professional proofreading and editing of the manuscript with respect to English syntax and grammar.
Disclosure statement
Collectively, the authors do not declare any competing interests. For the sake of transparency, JRD and JH are scientific advisors to a US-based biotechnology company (Celloxess, Ewing, NJ, USA) that contributes to preventive medicine by focusing on the production of antioxidant dietary supplements. Celloxess did not contribute in any way to this study.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Ahmed I, Abdelateef S, Laqqan M, Amor H, Abdel-Lah MA, Hammadeh ME. 2018. Influence of extended incubation time on human sperm chromatin condensation, sperm DNA strand breaks and their effect on fertilisation rate. Andrologia. 50(4):e12960. doi:https://doi.org/10.1111/and.12960.
- Ahmed TA. 2019. Role of oxidative stress in male infertility: an updated review. J Hum Reprod Sci. 12(1):4–18. doi:https://doi.org/10.4103/jhrs.JHRS_150_18.
- Aitken RJ, Buckingham DW, West KM. 1992. Reactive oxygen species and human spermatozoa: analysis of the cellular mechanisms involved in luminol- and lucigenin-dependent chemiluminescence. J Cell Physiol. 151(3):466–477. doi:https://doi.org/10.1002/jcp.1041510305.
- Aitken RJ, Gibb Z, Mitchell LA, Lambourne SR, Connaughton HS, De Iuliis GN. 2012. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod. 87(5):110. doi:https://doi.org/10.1095/biolreprod.112.102020.
- Ashwood-Smith MJ, Hollands P, Edwards RG. 1989. The use of albuminar (TM) as a medium supplement in clinical IVF. Hum Reprod. 4(6):702–705. doi:https://doi.org/10.1093/oxfordjournals.humrep.a136970.
- Athayde KS, Cocuzza M, Agarwal A, Krajcir N, Lucon AM, Srougi M, Hallak J. 2007. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl. 28(4):613–620. doi:https://doi.org/10.2164/jandrol.106.001966.
- Ben WX, Fu MT, Mao LK, Ming ZW, Xiong WW. 1997. Effects of various concentrations of native seminal plasma in cryoprotectant on viability of human sperm. Arch Androl. 39(3):211–216. doi:https://doi.org/10.3109/01485019708987918.
- Biggers JD. 1987. Pioneering mammalian embryo culture. The Mammalian Preimplantation Embryo. New York: Plenum Press; p. 1–22.
- Björndahl L. 2010. The usefulness and significance of assessing rapidly progressive spermatozoa. Asian J Androl. 12(1):33–35. doi:https://doi.org/10.1038/aja.2008.50.
- Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. 2007. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 4:CD004507.
- Bungum M, Humaidan P, Bungum L. 2002. Recombinant human albumin as protein source in culture media used for IVF: a prospective randomized study. Reprod Biomed Online. 4(3):233–236. doi:https://doi.org/10.1016/S1472-6483(10)61811-1.
- Burkman LJ. 1991. Discrimination between non hyperactivated and classical hyperactivated motility patterns in human spermatozoa using computerized analysis. Fertil Steril. 55(2):363–371. doi:https://doi.org/10.1016/S0015-0282(16)54131-4.
- Calamera JC, Fernandez PJ, Buffone MG, Acosta AA, Doncel GF. 2001. Effects of long-term in vitro incubation of human spermatozoa: functional parameters and catalase effect. Andrologia. 33(2):79–86. doi:https://doi.org/10.1046/j.1439-0272.2001.00409.x.
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. 1992. Evidence for decreasing quality of semen during past 50 years. BMJ. 305(6854):609–613. doi:https://doi.org/10.1136/bmj.305.6854.609.
- Cicaré J, Caille A, Zumoffen C, Ghersevich S, Bahamondes L, Munuce MJ. 2014. In vitro incubation of human spermatozoa promotes reactive oxygen species generation and DNA fragmentation. Andrologia. 47(8):861–866. doi:https://doi.org/10.1111/and.12337.
- Ciccone IM, Costa EMF, Pariz JR, Teixeira TA, Drevet JR, Gharagozloo P, Aitken RJ, Hallak J. 2020. Serum vitamin D content is associated with semenparameters and serum testosterone levels in men. Asian J Androl. 22(1):1–7. doi:https://doi.org/10.4103/aja.aja_102_19.
- Clark GF, Schust DJ. 2013. Manifestations of immune tolerance in the human female reproductive tract. Front Immunol. 13(4):26.
- Davis RO, Katz DF. 1992. Standardization and comparability of CASA® instruments. J Androl. 13(1):81–86.
- De Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. 1997. Reactive oxygen species and sperm physiology. Rev Reprod. 2(1):48–54. doi:https://doi.org/10.1530/ror.0.0020048.
- Edwards RG, Steptoe PC, Purdy JM. 1980. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 87(9):737–756. doi:https://doi.org/10.1111/j.1471-0528.1980.tb04610.x.
- Evenson DP. 2016. The Sperm Chromatin Structure Assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 169:56–75. doi:https://doi.org/10.1016/j.anireprosci.2016.01.017.
- Evenson DP, Higgins PJ, Grueneberg D, Ballachey BE. 1985. Flow cytometric analysis of mouse spermatogenic function following exposure to ethylnitrosourea. Cytometry. 6(3):238–253. doi:https://doi.org/10.1002/cyto.990060311.
- Fácio CL, Previato LF, Machado-Paula LA, Matheus PC, Filho EA. 2016. Comparison of two sperm processing techniques for low complexity assisted fertilization: sperm washing followed by swim-up and discontinuous density gradient centrifugation. JBRA Assist Reprod. 20(4):206–211. doi:https://doi.org/10.5935/1518-0557.20160040.
- Fitzpatrick JL, Lüpold S. 2014. Sexual selection and the evolution of sperm quality. Mol Hum Reprod. 20(12):1180–1189. doi:https://doi.org/10.1093/molehr/gau067.
- Franken DR, Van Wyk R, Stoumann C, Avari K. 2011. Temperature controlled centrifugation improves sperm retrieval. Andrologia. 43(3):217–221. doi:https://doi.org/10.1111/j.1439-0272.2010.01136.x.
- Gadea J, Selles E, Marco MA, Coy P, Mata C, Romar R, Ruiz S. 2004. Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 62(3–4):690–701. doi:https://doi.org/10.1016/j.theriogenology.2003.11.013.
- Gallup GG, Finn MM, Sammis B. 2009. On the Origin of Descended Scrotal Testicles: the Activation Hypothesis. Evolutionary Psychol. 7(4):517–526. doi:https://doi.org/10.1177/147470490900700402.
- Gardner DK. 2008. Dissection of culture media for embryos: the most important and less important components and characteristics. Reprod Fertil Dev. 20(1):9–18. doi:https://doi.org/10.1071/RD07160.
- Gardner DK, Lane B. 1997. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 3(4):367–382. doi:https://doi.org/10.1093/humupd/3.4.367.
- Gardner DK, Lane M. 1993. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 48(2):377–385. doi:https://doi.org/10.1095/biolreprod48.2.377.
- Gonzalez-Cuevas J, Navarro-Partida J, Marquez-Aguirre AL, Bueno-Topete MR, Beas-Zarate C, Aemendariz-Borunda J. 2011. Ethylenediaminetetraacetic acid induces antioxidant and anti-inflammatory activities in experimental liver fibrosis. Redox Rep. 16(2):62–70. doi:https://doi.org/10.1179/174329211X13002357050851.
- Hallak J. 2017a. A call for more responsible use of Assisted Reproductive Technologies (ARTs) in male infertility: the hidden consequences of abuse, lack of andrological investigation and inaction. Transl Androl Urol. 6(5):997–1004. doi:https://doi.org/10.21037/tau.2017.08.03.
- Hallak J. 2017b. Utility of sperm DNA fragmentation testing in different clinical scenarios of male reproductive abnormalities and its influence in natural and assisted reproduction. Transl Androl Urol. 6(4):S509–S512. doi:https://doi.org/10.21037/tau.2017.06.29.
- Hallak J, Veras MM, Saldiva PHN. 2018. How environmental and air pollution disrupt spermatogenesis and male reproductive health: a mechanistic approach. In: Bioenvironmental issues affecting men’s reproductive and sexual health. p. 5–32.
- Hinton BT. 1990. The testicular and epididymal luminal amino acid microenvironment in the rat. J Androl. 11(6):498–505.
- Hossain A, Aryal S, Osuampke C, Phelps J. 2010. Human sperm bioassay for reprotoxicity testing in embryo culture media: some practical considerations in reducing the assay time. Adv Urol:136–898.
- Hosseini A, Khalili MA. 2017. Improvement of motility after culture of testicular spermatozoa: the effects of incubation timing and temperature. Transl Androl Urol. 6(2):271–276. doi:https://doi.org/10.21037/tau.2017.03.43.
- Hrudka F. 1987. Cytochemical and ultracytochemical demonstration of cytochrome c oxidaese in spermatozoa and dynamics of its changes accompanying ageing or induced by stress. Int J Androl. 10(6):809–828. doi:https://doi.org/10.1111/j.1365-2605.1987.tb00385.x.
- Kleijkers SH, van Montfoort AP, Bekers O, Coonen E, Derhaag JG, Evers JL, Dumoulin JC. 2016. Ammonium accumulation in commercially available embryo culture media and protein supplements during storage at 2–8°C and during incubation at 37°C. Hum Reprod. 31(6):1192–1199. doi:https://doi.org/10.1093/humrep/dew059.
- Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, Van Zyl JA, Smith K. 1986. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 46(6):1118–1123. doi:https://doi.org/10.1016/S0015-0282(16)49891-2.
- Kuo YL, Tzeng WL, Chiang HK, Ni RF, Lee TC, Young ST. 1998. New system for long-term monitoring of sperm motility: EDTA effect on semen. Arch Androl. 41(2):127–133. .
- Lane M, Gardner DK. 2007. Embryo culture medium: which is the best?. Best Pract Res Clin Obstet Gynaecol. 21(1):83–100. doi:https://doi.org/10.1016/j.bpobgyn.2006.09.009.
- Leese HJ, Donnay I, Thompson JG. 1998. Human assisted conception: a cautionary tale. Lessons from domestic animals. Hum Reprod. 13(4):184–202. doi:https://doi.org/10.1093/humrep/13.suppl_4.184.
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. 2017. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 23(6):646–659. doi:https://doi.org/10.1093/humupd/dmx022.
- Lichtenfels AJ, Gomes JB, Pieri PC, Msg EK, Hallak J, Saldiva PHN. 2007. Increased levels of air pollution and a decrease in the human and mouse male-to-female ratio in São Paulo, Brazil. Fertil Steril. 87(1):230–232. doi:https://doi.org/10.1016/j.fertnstert.2006.06.023.
- Liu DY, Liu ML, Baker HW. 2011. ‘Quinn’s advantage fertilization medium enhances zona pellucida-induced acrosome reaction compared with human tubal fluid medium. Reprod Biomed Online. 23(6):735–739. doi:https://doi.org/10.1016/j.rbmo.2011.07.013.
- Lumley AJ, Michalczyk Ł, Kitson JJ, Spurgin LG, Morrison CA, Godwin JL, Dickinson ME, Martin OY, Emerson BC, Chapman T, et al. 2015. Sexual selection protects against extinction. Nature. 522(7557):470–473. doi:https://doi.org/10.1038/nature14419.
- MacKenna A, Crosby J, Huidobro C, Correa E, Duque G. 2017. Semen quality before cryopreservation and after thawing in 543 patients with testicular cancer. BRA Assist Reprod. 21(1):31–34.
- Mansour RT, Serour MG, Abbas AM, Kamal A, Tawab NA, Aboulghar MA, Serour GI. 2008. The impact of spermatozoa preincubation time and spontaneous acrosome reaction in intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 90(3):584–591. doi:https://doi.org/10.1016/j.fertnstert.2006.11.176.
- Marchesi DE, Biederman H, Ferrara S, Hershlag A, Feng HL. 2010. The effect of semen processing on sperm DNA integrity: comparasion of two techniques using the novel Toluidine Blue Assay. Eur J Obstet Gynecol Reprod Biol. 151(2):176–180. doi:https://doi.org/10.1016/j.ejogrb.2010.05.003.
- Marin-Briggiler CI, Tezon JG, Miranda PV, Vazquez-Levin MH. 2002. Effect of incubating human sperm at room temperature on capacitation-related events. Fertil Steril. 77(2):252–259. doi:https://doi.org/10.1016/S0015-0282(01)02982-X.
- Martínez‐Soto JC, Landeras J, Gadea J. 2012. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology. 1(3):365–375. doi:https://doi.org/10.1111/j.2047-2927.2012.00040.x.
- Matson P, Tardif S. 2012. A preliminary search for alternatives to albumin as a medium supplement for the culture of human sperm. Biol Reprod. 12(3):329–331. doi:https://doi.org/10.1016/j.repbio.2012.09.006.
- Matsuura R, Takeuchi T, Yoshida A. 2010. Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J Androl. 12(5):753–759. doi:https://doi.org/10.1038/aja.2010.46.
- Meintjes M, Chantilis SJ, Ward DC, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, Barnett BD, Madden JD. 2009. A randomized controlled study of human serum albumin and serum substitute supplement as protein supplements for IVF culture and the effect on live birth rates. Hum Reprod. 24(4):782–789. doi:https://doi.org/10.1093/humrep/den396.
- Molina M, Segura JA, Aledo JC, Medina MA, Nunez De Castro NI, Marquez J. 1995. Glutamine transport by vesicles isolated from tumour-cell mitochondrial inner membrane. Biochem J. 308(2):629–633. doi:https://doi.org/10.1042/bj3080629.
- Moncla BJ, Chappell CA, Debo BM, Meyn LA. 2016. The effects of hormones and vaginal microflora on the glycome of the female genital tract: cervical-vaginal fluid. PLoS One. 11(7):e0158687. doi:https://doi.org/10.1371/journal.pone.0158687.
- Monteiro RAC, Pariz JR, Pieri PC, Hallak J. 2016. An easy, reproducible and cost-effective method for andrologists to improve the laboratory diagnosis of nonobstrutive azoospermia: a novel microcentrifugation technique. Int Braz J Urol. 42:132–138. doi:https://doi.org/10.1590/S1677-5538.IBJU.2015.0090.
- Morbeck DE, Paczkowski M, Fredrickson JR, Krisher RL, Hoff HS, Baumann NA, Moyer T, Matern D. 2014. Composition of protein supplements used for human embryo culture. J Assist Reprod Genet. 31(12):1703–1711. doi:https://doi.org/10.1007/s10815-014-0349-2.
- Nabi A, Khalili MA, Halvaei I, Roodbari F. 2014. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia. 46(4):374–379. doi:https://doi.org/10.1111/and.12088.
- Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. 1985. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 260(17):9699–9705. doi:https://doi.org/10.1016/S0021-9258(17)39295-5.
- Palasz AT, Rodriguez-Martinez H, Beltran-Brena P, Perez-Garnelo S, Martinez MF, Gutierrez-Adan A, De la Fuente J. 2006. Effects of hyaluronan, BSA, and serum on bovine embryo in vitro development, ultrastructure, and gene expression patterns. Mol Reprod Dev. 73(12):1503–1511. doi:https://doi.org/10.1002/mrd.20516.
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. 1992. Pregnancies after intracitoplasmic injection of single spermatozoa into an oocyte. Lancet. 340(8810):17–18.
- Palini S, Primiterra M, Stefani S, Pedna MF, Sparacino M, Farabegoli P, Benedetti S, Bulletti C, Sambri V. 2016. A new micro swim-up procedure for sperm preparation in ICSI treatments: preliminary microbiological testing. JBRA Assist Reprod. 20(3):93–97. doi:https://doi.org/10.5935/1518-0557.20160022.
- Parinaud J, Milhet P, Vieitez G, Richoilley G. 1998. Human sperm capacitation and in vitro fertilization in a chemically defined and protein-free medium SMART1. Hum Reprod. 13(9):2579. doi:https://doi.org/10.1093/humrep/13.9.2579.
- Pariz JR, Ranéa C, Drevet JR, Hallak J. 2021. Dysplasia of the fibrous sheath with axonemal and centriolar defects combined with lack of mitochondrial activity as associated factors of ICSI failure in primary ciliary dyskinesia. Int Braz J Urol. 47(3):617–626. doi:https://doi.org/10.1590/s1677-5538.ibju.2019.0362.
- Pasha YY, Jorsaraei SG, Zeinalzadeh M, Amiri MG, Ramaji A. 2012. Evaluation of preincubation time interval in testicular biopsy to obtain optimum sperm parameters. Cell J. 14(1):1–6.
- Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. 2006. Cigarette smoking is related to a decrease in semen volume in a population of fertile men. Int Braz J Urol. 97(2):324–326. doi:https://doi.org/10.1111/j.1464-410X.2005.05906.x.
- Petrella C, Hsieh J, Blake E, Thrift K, Zacur H, Zhao Y. 2003. Human sperm can survive at room temperature for weeks: measured by motility and viability of sperm maintained under various conditions. Fertil Steril. 80:210. doi:https://doi.org/10.1016/S0015-0282(03)01468-7.
- Petrella C, Hsieh J, Thrift K, Jarow JP, Zacur H, Zhao Y. 2005. Optimizing incubation conditions for the preservation of sperm motility in processed semen samples. Fertil Steril. 84(2):513–515. doi:https://doi.org/10.1016/j.fertnstert.2005.01.138.
- Pool TB. 2004. Development of culture media for human assisted reproductive technology. Fertil Steril. 81(2):287–289. doi:https://doi.org/10.1016/j.fertnstert.2003.10.012.
- Quinn P. 2004. The development and impact of culture media for assisted reproductive technologies. Fertil Steril. 81(1):27–29. doi:https://doi.org/10.1016/j.fertnstert.2003.10.003.
- Rehman F, Zhao C, Shah MA, Qureshi MS, Wang X. 2013. Semen extenders and artificial insemination in Ruminants. Vet. 1:1–8.
- Sallam KI. 2007. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate and sodium citrate in refrigerated sliced salmon. Food Control. 18(5):566–575. doi:https://doi.org/10.1016/j.foodcont.2006.02.002.
- Sangeeta S, Arangasamy A, Kulkarni S, Selvaraju S. 2015. Role of amino acids as additives on sperm motility, plasma membrane integrity and lipid peroxidation levels at pre-freeze and post-thawed ram semen. Anim Reprod Sci. 161:82–88. doi:https://doi.org/10.1016/j.anireprosci.2015.08.008.
- Schjenken JE, Robertson SA. 2014. Seminal Fluid and Immune Adaptation for Pregnancy – comparative Biology in Mammalian Species. Reprod Domestic Animals. 49(suppl(3)):27–36. doi:https://doi.org/10.1111/rda.12383.
- Setchell BP, Hinks NT, Voglmayr JK, Scott TW. 1967. Amino acids in ram testicular fluid and semen and their metabolism by spermatozoa. Biochem J. 105(3):1061–1065. doi:https://doi.org/10.1042/bj1051061.
- Shulman A, Hauser R, Lipitz S, Frenkel Y, Dor J, Bider D, Mashiach S, Yo Gev L, Yavetz H. 1998. Sperm motility is a major determinant of pregnancy outcome following intrauterine insemination. J Assist Reprod Genet. 15(6):381–385. doi:https://doi.org/10.1023/A:1022585000740.
- Si Y. 1997. Temperature-dependent hyperactivated movement of hamster spermatozoa. Biol Reprod. 57(6):1407–1412. doi:https://doi.org/10.1095/biolreprod57.6.1407.
- Sposito C, Camargo M, Tibaldi DS, Barradas V, Cedenho AP, Nichi M, Bertolla RP, Spaine DM. 2017. Antioxidant enzyme profile and lipidperoxidation products in semen samples of testicular germ cell tumor patients submitted to orchiectomy. Int Braz J Urol. 43(4):644–651. doi:https://doi.org/10.1590/s1677-5538.ibju.2016.0323.
- Szczykutowicz J, Kałuża A, Kaźmierowska-Niemczuk M, Ferens-Sieczkowska M. 2019. The potential role of seminal plasma in the fertilization outcomes. Biomed Res Int. 2019:1–10. doi:https://doi.org/10.1155/2019/5397804
- Thijssen A, Klerkx E, Huyser C, Bosmans E, Campo R, Ombelet W. 2014. Influence of temperature and sperm preparation on the quality of spermatozoa. Reprod Biomed Online. 28(4):436–442. doi:https://doi.org/10.1016/j.rbmo.2013.12.005.
- Tosic J, Walton A. 1946. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect on respiration. Nature. 158(4014):485. doi:https://doi.org/10.1038/158485a0.
- (WHO) World Health Organization. 2010. WHO laboratory manual for the examination and processing of human semen. 5th. Geneva: World Health Organization.
- Wright C, Milne S, Leeson H. 2014. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 28(6):684–703. doi:https://doi.org/10.1016/j.rbmo.2014.02.004.
- Yavas Y, Selub MR. 2004. Intrauterine insemination (IUI) pregnancy outcome is enhanced by shorter intervals from semen collection to sperm wash, from sperm wash to IUI time, and from semen collection to IUI time. Fertil Steril. 82(6):1638–1647. doi:https://doi.org/10.1016/j.fertnstert.2004.04.061.
