 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The purpose of this study is to investigate the optimal framerate (FR) and the use of different counting chambers for improving CASA-Mot technology use in Andrology. Images were captured at 500 fps, then segmented and analyzed in several ranges of FRs (from 25 to 250) to define the asymptotic point that as an optimal FR. This work was replicated using counting chambers based in capillarity (disposable) or drop displacement (reusable) to study their effects on the motility results and kinematic values of the samples under the different experimental conditions. The α value (asymptote corresponding to FRo) of the exponential curve was 150.23 fps, corresponding to a VCL of 130.58 mm/s, far from the value of 98.89 mm/s corresponding to 50 fps (the highest FR used by most current CASA-Mot systems). Our results have shown that, when using reusable counting chambers, type and depth have influence. In addition, different results were obtained depending on the area of image captured inside the different counting chamber types. To have reliable results in human sperm kinematic studies, almost 150 fps should be used for capturing and analyzing and differences between chambers should be considered by sampling from different areas, to obtain a representative value of the whole sample.
Introduction
Infertility is defined like as the inability of a sexually active, non-contracepting couple to achieve pregnancy in one year. The male partner can be evaluated for infertility or subfertility using a variety of clinical interventions, and also from a laboratory evaluation of semen (WHO Semen manual, 5th Edition3). It is estimated that about 25% of couples show infertility in developed countries, where more information is available (Mascarenhas et al. Citation2012). Although male reproductive function is impaired in about half of these infertile couples (Kumar and Singh Citation2015), it depends on the geographical area (Agarwal et al. Citation2015). Semen analysis is the best way to evaluate male fertility ability being centered on sperm concentration, motility, morphology and viability (Tomlinson et al. Citation1999; WHO Citation2010).
Two approaches to perform the seminal analysis ways are coexisting around the world, the subjective and the computer assisted semen analysis (CASA). The subjective method is being used from the middle of past century (MacLeod and Gold Citation1951), and was systematized in the successive WHO (WHO Citation2021), and other manuals (Björndahl et al. Citation2010). The main issue with this approach is the need for high training and the fact that subjectivity introduces a bias in the final results with a high CV amongst the repetitions in the same sample analysis (Cooper et al. Citation2002). Nevertheless, the number of nonconformities to international guidelines is close to 90%, indicating that nonstandard criteria are used when semen analyses are performed (Bailey et al. Citation2007; Palacios et al. Citation2012). This fact has claimed for the necessity to introduce accurate quality control programs to be sure that almost the technician is, at least, working inside some reasonable margins (Cooper et al. Citation2002; Mallidis et al. Citation2012; Filimberti et al. Citation2013; Sánchez-Pozo et al. Citation2013).
The vagaries of the eye-brain system in assessing semen quality can be eliminated by objective assessment where sperm characteristics are defined by a battery of quantitative parameters (Soler et al. Citation2016). This principle was the basis for the development in the 1980’s of Computer Assisted Semen analysis (CASA) technology (Bompart et al. Citation2018). These computer-aided sperm analysis (CASA-Mot) systems were developed to provide more reliable, repeatable, and objective measurements of sperm movement (Rurangwa et al. Citation2004), and to reduce the intertechnical variation in the estimation of sperm concentration, regardless of the type and depth of the counting chamber used. (Lu et al. Citation2007). In addition, CASA-Mot systems are used for obtaining high quantities of spermatozoon data, such as kinematic parameters. The sperm quality of each male could be assessed and bundles of spermatozoa with similar motility features could be defined, by applying subpopulation analysis to the mentioned data (Soler et al. Citation2014). Nevertheless, like any other technology CASA depends on the capabilities of both the hardware and the software used (Talarczyk-Desole et al. Citation2017; Yeste et al. Citation2018). Likewise, it is important to mention other limitations of the CASA systems, such as the absence of defined standard values for variables in the different species and lack of reliable standardization of settings from different equipment (Gill et al. Citation1988; Vantman et al. Citation1988; Hoogewijs et al. Citation2012). Despite these limitations, throughout the last two decades CASA systems have been implemented in a lot of human clinical laboratories showing a good predictive value of the fertility of the samples even considering all of their technological limitations (Holt et al. Citation2018; Tomlinson and Naeem Citation2018).
Particularly, the effect of frame rate and counting chamber use has been studied in a variety of species, showing that the sperm behavior is species-specific and the need for accurate evaluation in each species and condition (Bompart et al. Citation2019; Valverde Citation2019; Caldeira et al. Citation2019).
In addition, the effect of the counting chamber used and how it is manipulated has an important effect on what was observed a long time ago (Coetzee and Menkveld Citation2001) and recently analyzed in detail (Bompart et al. Citation2018).
The aim of the present work is to use the most advanced CASA technology to define the optimized protocol conditions for human semen analysis.
Results and discussion
Definition of optimal frame rate for kinematic analysis
As explained in more detail in the materials and methods section, the optimal frame rate was determined from the VCL of the sperm using point-to-point reconstructions of their trajectories at each tested FR. VCL was used to define the FRo (α value of the exponential curve indication asymptotic value) because it is the most sensitive parameter to FR changes. When using disposables chamber, FRo was quite similar despite its depth (132.2 fps for 10 µm depth and 133 fps for 20 µm depth). Nevertheless, when reusable chambers were used, they did show differences (126.9 and 150.2 fps for 10 and 20 µm depth, respectively) (, ).
Figure 1. Sperm curvilinear velocity (VCL. µm/s) obtained at different frame rates in the different counting chambers. Optimum frame rate (FRo) was calculated for the different types of chamber: disposable chambers 10 and 20 µm depth (ISAS®D4C10, ISAS®D4C20) and reusable chambers 10 and 20 µm depth (Spermtrack® 10, Spermtrack® 20). VCL: curvilinear velocity (μm/s).
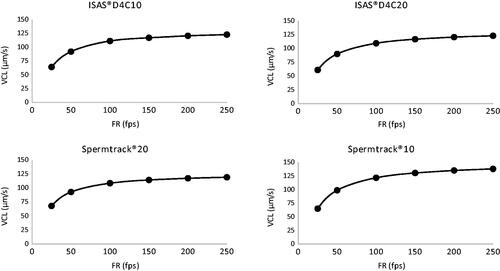
Table 1. Optimum frame rate calculated to have the threshold level for different chambers types and depths.
These data indicate results are influenced by the counting chamber type and depth, the latter only in the case where a reusable chamber is used.
Effect of counting chamber type and area of analysis
Besides the differences in the FRo observed in different counting chamber types, the highest sperm kinematic values were observed in the reusable Spermtack® chamber and even higher with the 10 µm than with 20 µm depth. In the case of the disposable chambers, the depth influence observed was not significant. The movement in reusable chambers was more linear and faster, thus showing higher head lateral displacement (ALH) ().
Table 2. Motility parameters (mean ± SEM) of human spermatozoa determined by CASA system using different counting chambers, and using a frame rate capture of 250 fps.
Reusable chambers tended to decrease sperm kinematic values as we shift from the center to the periphery of the counting area but was only significant for some of the parameters in the most exterior region. This phenomenon was even more sensitive for 20 µm than for 10 µm depth ().
Table 3. CASA motility parameters (mean ± SEM) of human spermatozoa in different areas of the reusable counting chambers Spermtrack®10 and 20 µm depth.
When using 20 µm depth disposable chambers, LIN and BCF were reduced in areas far from the charging point, while VCL and VAP remained invariable along the counting points ().
Table 4. CASA motility parameters (mean ± SEM) of human spermatozoa in different areas of disposable counting chambers ISAS®D4C 10 and 20 µm depth.
Arguing the use of CASA systems based on our results
CASA technology is commercially available from since the 1980’s. However, its presence in human clinical practice is much lower than expected (Tomlinson et al. Citation2010), despite the fact that the relevance of analytical data obtained in different research fields involving spermatozoa has been demonstrated (Yániz et al. Citation2015, Citation2018).
One reason for this paradox could be the following: CASA systems have just been used to replace human observations, but follow the same subjective criteria (Yeung et al. Citation1997). This premise can be validated by looking at the different WHO manuals; in the fourth edition of the WHO manual (1999), class “a” of sperm motility, is defined as fast and progressive motility (i.e., ≥25µm/s at 37 °C and ≥20µm/s at 20 °C). But quantifying what "fast" means in these magnitudes is not possible for the human eye and, as a consequence, to be fast just means not to be slow, which is something completely subjective, even if a kinematic limit is proposed; for example, why 25 and not 26? This problem of having four motility classes was skipped in the fifth edition of the WHO manual (2010) by reducing those classes to three. Currently, we have the latest edition of the WHO manual (2021), where the four-speed classes classification have returned.
Another reason is related with the technological limitations of the systems (Bompart et al. Citation2018). It was shown long ago that the FR interferes with the quality of the sperm kinematic results (Mortimer et al. Citation1988; Castellini et al. Citation2011). The most commonly used video cameras could operate from 25 to 60 fps (Robinson et al. Citation2018); nowadays, some commercial systems are able to capture at 100 fps (García-Molina et al. Citation2021). But working with these FRs entails problems because the identification of spermatozoa and their trajectories is not as satisfactory as is required and therefore a manual correction of the results must be made, which then interferes with the final results (Robinson et al. Citation2018).
In the present study, we attempted to standardize the technique used for the motility assessment of human sperm using high-resolution cameras and specific chambers. The first step was to define the FRo for sperm kinematic analysis. In this study, we found a direct positive relationship between capture frequency and VCL. The tracking of the sperm from one dot to the following is allowed by a proper capture frequency, which permit a correct reconstruction of sperm trajectory; thereafter, VCL is calculated from its trajectory (speed = trajectory/time). The maximum frame required is obtained by non-linear regressions, defined as FRo, which represents the threshold (α) from which more captures will not imply a better definition of sperm path.
In recent years, similar studies have been published in different species to find the FRo and to achieve a satisfactory sperm kinematic analysis. This FRo was 300 fps in stallions (Gacem, Bompart, et al. Citation2020), 250 fps in bull (Bompart et al. Citation2019), donkies (Gacem, Catalán, et al. Citation2020) and salmon (Caldeira et al. Citation2019), 225 fps in boar (Valverde et al. Citation2019) and sturgeon (Caldeira et al. Citation2019) and 200 fps in eels (Caldeira et al. Citation2019). In our study, the value obtained for human semen is 150fps, quite lower than in other species, which indicates how slowly and regularly human spermatozoa moves, compared with other species.
The obtained value for the FRo of 150 fps indicates that FRs below this value are offering suboptimal kinematic results. Obviously, the lower the FR, the less clinical and scientific value can be sustained from CASA-Mot analysis, and this is one of the reasons limiting its use and significance (Knuth et al. Citation1987; Knuth and Nieschlag Citation1988; Bompart et al. Citation2018). But it does not mean that all the former work devoted to human sperm kinematics has no value; throughout the history of Science, the improvements on measurement capabilities has overcome meaning of the old results but never meant to invalidate them.
The second step was to analyze the sperm behavior in different chamber types. Everyone knows that use of CASA systems is influenced by a variety of technical factors, such as the use of different counting chambers (Bailey et al. Citation2007; Soler et al. Citation2012; Bompart et al. Citation2018; Valverde et al. Citation2019). One traditional (but inconvenient) alternative is to use just a simple microscope slide with a coverslip but, in this case, it is not possible to standardize the depth of the preparation. Moreover, as the coverslip floats on the sample, it induces additional high drifting sperm movement (Del Gallego et al. Citation2017; Robinson et al. Citation2018). Two alternative counting chamber types have been developed by different manufacturers: drop displacement reusable (Makler®, Spermtrack® …) and capillary loaded disposable (Leja®, ISAS®D …). These chambers are based on different physics diffusion principles, so it is not surprising that the obtained results are also different (Del Gallego et al. Citation2017; Bompart et al. Citation2018; Valverde et al. Citation2019). In the present work, the highest velocities and the most linear motility were observed when Spermtrack® (drop displacement type) of 10 µm depth was used. Therefore, the results of our study demonstrate that the depth of the counting chamber influence the kinematic values. In fact, when comparing between reusable chambers but at different depths, we can observe that the sperm presents higher values for all the kinematic parameters (VCL, VSL, VAP, LIN, STR, WOB, ALH and BCF) in the narrower 10 µm chamber than in the 20 µm chamber.
There are different approaches to evaluate the effectiveness of counting chambers such as the use of limits of agreement (Bailey et al. Citation2007), but the purpose of the present work was not to try to define the “real values” for evaluation of kinematics (which is not possible under our point of view) but just to point out how important is to consider the effect of counting chambers type on the final results and how it is not advisable to compare results among different works without considering it this.
Finally, in addition to the type and depth of the chambers, it is necessary to consider the location where sampling is produced inside the counting chamber. Most of the studies done throughout time have never indicated this fact and counts were commonly done in the center of the counting area after putting a volume inside that was not fitted to the actual space (Bompart et al. Citation2018). In a previous study, the same type of reusable counting chambers (Spermtrack®) were tested but at 25 fps (with much lower sensitivity than the one used here, 150fps) and no differences were observed regarding the counting area considered (Soler et al. Citation2012). In this study, the movement was less linear with a significantly lower VSL only in the periphery of the drop displacement chambers, independent of the depth. When capillary loaded disposable chambers were used, the effect was just the opposite, the further away from the sample deposit point, the lower the VSL and the LIN of the movement, being significant from the fifth field. This fact was previously observed in other species such as foxes (Soler et al. Citation2014) or bulls (using Leja and CellVu chambers in these cases, Valverde et al. Citation2019). This may be due to the interaction of the spermatozoa during their passive progressing on the glass surface or due to the surfactants that are added to the glass in these types of chambers. But another not-tested-hypothesis is based on the possibility that a swim-up process could occur inside the tip of the micropipette: the sperm at the bottom of the tip will be pushed to the most advanced areas of the counting chamber, while the cells on the top of the tip (which are the ones with high movement) will remain close to the place of sample deposition.
Materials and methods
Patients
Thirteen semen samples were obtained from adult volunteers (mean age 25.85 ± 8.75, range 19–53). All the volunteers signed informed consent forms to participate in and provide semen samples for the study.
The samples were obtained by masturbation following sexual abstinence for 3-5 days, collected in a clean 60 mL sterile standard container and stored in an incubator at 37 °C for 30 min to allow liquefaction (García-Molina et al. Citation2021).
Frame rate effect
All semen samples were recorded at 500 fps frame rate (FR) for 1 s. This video was segmented into 25, 50, 100, 150, 200 and 250 FR videos. The command used for video segmentation was: [echo off: set fps= 25, 50, 75, 100, 150: for %%i in (.Ä*.avi) do (set fname=%%∼ni) & call: encodeVideo; goto eof: encodeVideo: ffmpeg.exe -i %fname%.avi -r %fps% -clibx264 -preset slow -qp 0 "%fname%_(%fps%fps).avi"; goto eof].
Motility evaluation by CASA-Mot
The assessment of sperm motility and kinematics was carried out with the ISAS®v1 CASA-Mot system (Proiser R + D, Paterna, Spain), connected to an ISAS®-UOP200i negative phase contrast microscope equipped with a heating stage at 37 °C and using a 10× objective (0.25 NA) (Proiser R + D). A high-speed camera (500 fps; M03-CM, Proiser R + D) was used for image capture. The array size of the video frame grabber was 648 × 488 × 8 bits and 256 grey levels. Image resolution was 0.70 µm per pixel in both the horizontal and vertical axes. The tail detection facility of the system was activated to ignore non-sperm particles, with a particle area between 5 and 80 µm2 and a connectivity value of 10 µm. Track recognition mistakes were deleted, when needed, to avoid the introduction of distortions in the final results.
The sperm kinematic parameters analyzed were curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s), average path velocity (VAP, µm/s), linearity (LIN, %), straightness (STR, %), wobble (WOB, %), amplitude of lateral head displacement (ALH, µm) and beat/cross frequency (BCF, Hz).
Among kinematic parameters, the VCL is the most sensible to FR changes (Bompart et al. Citation2018; Valverde et al. Citation2019) and, therefore, this was the parameter used for the calculation of the optimum FR.
Counting chambers
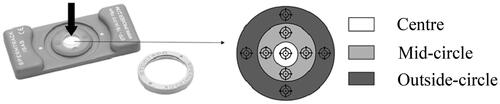
In general, the design of this study was based on the recommendations made by Björndahl et al. (Citation2016). The effects of capillary-loaded, disposable, 10 and 20 µm depth chambers (ISAS® D4C10 and D4C20, respectively, Proiser R + D), and drop-displacement, reusable, 10 and 20 µm depth (Spermtrack®, Proiser R + D) counting chambers were tested. All the chambers were prewarmed to 37 °C in a heating plate before use. Disposable chambers were loaded by capillarity with a volume of 2 and 3 µL (for 10 and 20 µm depth, respectively) maintaining the tip in contact with the coverslip until the fluid reached the end of the counting track. In these chambers, seven microscopic fields (one in each chamber square) were analyzed (). The reusable chamber was loaded placing a 2 and 4 µl drop (depending on the depth) in the center of the base and immediately placing the cover, with no further movement of the cover. In this chamber, nine fields were captured, one in the center (white area), 4 in the mid-circle (light-grey area), and 4 in the outside-circle (dark grey area) (). An average of 500 sperm was captured per field. To avoid possible errors related to biases in examining samples in the different chambers, the counting chambers were assessed randomly. In both cases ( and ), they were the same independent of the depth of the used chamber. Comparison amongst the counting chamber types and counting areas inside each chamber were done at 150 fps.
Figure 2. Disposable counting chambers (ISAS D4C®) and forms used and the fields analyzed. ISAS D4C® is a commercial disposable counting chamber, available in two different depths: 10 µm (D4C10) and 20 µm depth (D4C20). These are counting chambers based in capillarity. The arrow demonstrates the place of drop deposition. Numbers (1–7) inside the chambers demonstrate different capture fields.
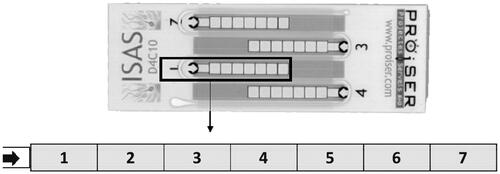
Figure 3. Reusable counting chambers (Spermtrack®) and forms used and the fields analyzed. Spermtrack® is a commercial reusable counting chamber, available in two different depths: 10 µm (SpK 10) and 20 µm depth (SpK 20). These are counting chambers based in drop displacement. The arrow demonstrates the place of drop deposition. Drop deposition is done in the center of the circle (white area); the covers were rapidly but gently put in place to achieve a homogenous distribution of the sample. There are 3 different colors (grayscale) that mark the different capture fields and the symbol indicates the specific point of capture.
Statistical analysis
The data obtained from the evaluations of all ejaculates were analyzed by descriptive statistics. Distribution properties for all variables were also explored using histograms and probability plots to check for a normal distribution. The analysis of variance was further applied to evaluate statistical differences between counting chambers on seminal parameters. Furthermore, the effect of the field within the counting chambers was analyzed, also by analysis of the variance, for all kinematic variables.
The statistical model used was:
Where Xijk = Measured sperm kinematic variable; µ = Overall mean of variable x; Ai = Effect of counting chamber Bj = Effect of field; AB (ij) = Effect of interaction between counting chamber *field; εijk = Residual.
Calculating the optimum frame rate
For regression analyses, the effects of FR were tested in an exponential model, in the form:
Where y is VCL and x is FR, α is the asymptotic level, β is the rate of increase to the asymptote, and exp is the base of natural logarithms. The biological significance of the equation is that the asymptotic values α represent the maximum achievable when the FR is above the threshold level. The threshold level was calculated as the FR needed to obtain 95% of the maximum value. The rate of the approach to the asymptote represents the dependence of the curve on the FR; that is, a high value of β indicates high growth of VCL as FR increases and vice versa.
Following Mortimer et al. (Citation2015) only one decimal was expressed for most of the parameters. Statistical significance was considered at p < 0.05. Furthermore, pairwise comparison between means effects of counting chambers and fields were performed by the Tukey–Kramer test. The results are presented as the mean ± standard error of the mean (SEM). All data were analyzed with IBM SPSS package, version 23.0 for Windows (SPSS Inc., Chicago, IL, USA).
Ethics approval
All work with semen samples was carried out with patients approved by the Ethic Committee of the IVI- University of València (1705-UV-035-AG). All signed informed consent forms to participate in and provide semen samples for the study. These forms indicate the purpose of the study and the fact that the samples will not be used for any other purpose as they would be destroyed after their use for the study.
Authors’ contributions
Conceptualization: AGM, NG, CS; methodology and investigation: AGM, CC, NN; validation: AGM, SS; formal and statistical analysis: AV; writing: AGM, CS. All authors have read and agreed to the published version of the manuscript.
| Abbreviations | ||
| WHO | = | World Health Organization |
| CASA | = | computer assisted semen analysis |
| CV | = | coefficient of variation |
| VCL | = | curvilinear velocity |
| FR | = | frame rate |
| ALH | = | amplitude of lateral head displacement |
| BCF | = | beat/cross frequency |
| VAP | = | average path velocity |
| CASA-Mot | = | CASA-Motility |
| VSL | = | straight-line velocity |
| NA | = | numerical aperture |
| LIN | = | linearity |
| STR | = | straightness |
| WOB | = | wobble |
| fps | = | frames per second |
| SEM | = | standard error of the mean |
| SpK | = | reusable counting chamber (Spermtrack®) |
| D4C | = | disposable counting chamber |
Acknowledgment
This study did not receive any specific funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Agarwal A, Mulgund A, Hamada A, Hamada A, Chyatte MR. 2015. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 13:37–37.
- Bailey E, Fenning N, Chamberlain S, Devlin L, Hopkisson J, Tomlinson M. 2007. Validation of sperm counting methods using limits of agreement. J Androl. 28(3):364–373.
- Björndahl L, Mortimer D, Barratt CL, Castilla JA, Menkveld R, Kvist U, Haugen TB. 2010. A practical guide to basic laboratory andrology. Cambridge University Press.
- Björndahl L, Barratt CLR, Mortimer D, Jouannet P. 2016. How to count sperm properly: checklist for acceptability of studies based on human semen analysis. Hum Reprod. 31(2):227–232.
- Bompart D, García-Molina A, Valverde A, Caldeira C, Yániz JL, Núñez de Murga M, Soler C. 2018. CASA-Mot technology: how results are affected by the frame rate and counting chamber. Reprod Fert Develop. 30(6):810–819.
- Bompart D, Vázquez RF, Gómez R, Valverde A, Roldán ERS, García-Molina A, Soler C. 2019. Combined effects of type and depth of counting chamber, and the rate of image frame capture, on bull sperm motility and kinematics. Anim Reprod Sci. 209:106–169.
- Caldeira C, Hernández-Ibáñez S, Valverde A, Martin P, Herranz-Jusdado JG, Gallego V, Asturiano JF, Dzyuda B, Pšenicka M, Soler C. 2019. Standardization of sperm motility analysis by using CASA-Mot for Atlantic salmon (Salmo salar), European eel (Anguilla Anguilla) and Siberian sturgeon (Acipenser baerii). Aquaculture. 502:223–231.
- Castellini C, Dal Bosco A, Ruggeri S, Collodel G. 2011. What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil Steril. 96(1):24–27.
- Coetzee K, Menkveld R. 2001. Validation of a new disposable counting chamber. Arch Androl. 47(2):153–156.
- Cooper TG, Björndahl L, Vreeburg J, Nieschlag E. 2002. Semen analysis and external quality control schemes for semen analysis need global standardization. Int J Androl. 25(5):306–311.
- Del Gallego R, Sadeghi S, Blasco E, Soler C, Yániz JL, Silvestre MA. 2017. Effect of chamber characteristics, loading and analysis time on motility and kinetic variables analysed with the CASA-Mot system in goat semen. Anim Reprod Sci. 177(Suppl. C):97–104.
- Filimberti E, Degl’Innocenti S, Borsotti M, Quercioli M, Piomboni P, Natali I, Fino MG, Caglieresi C, Criscuoli L, Gandini L, et al. 2013. High variability in results of semen analysis in andrology laboratories in Tuscany (Italy): the experience of an external quality control (EQC) programme. Andrology. 1(3):401–407.
- Gacem S, Bompart D, Valverde A, Catalán J, Miró J, Soler C. 2020. Optimal frame rate when there were stallion sperm motility evaluations and determinations for kinematic variables using CASA-Mot analysis in different counting chambers. Anim Reprod Sci. 223:106643.
- Gacem S, Catalán J, Valverde A, Soler C, Miró J. 2020. Optimization of CASA-Mot analysis of donkey sperm: optimum frame rate and values of kinematic variables for different counting chamber and fields. Animals. 10:1993.
- García-Molina A, Valverde A, Bompart D, Caldeira C, Vendrell A, Soler C. 2021. Human kinematic and morphometric sperm subpopulation analysis using CASA technology: a new approach to spermatozoa classification. Rev Int Androl. 20(4):257–265.
- Gill HS, Van Arsdalen K, Hypolite J, Levin RM, Ruzich JV. 1988. Comparative study of two computerized semen motility analyzers. Andrologia. 20(5):433–440.
- Holt WV, Cummins JM, Soler C. 2018. Computer-assisted sperm analysis and reproductive science; a gift for understanding gamete biology from multidisciplinary perspectives. Reprod Fert Dev. 30(6):iii–v.
- Hoogewijs MK, de Vliegher SP, Govaere JL, de Schauwer C, de Kruif A, van Soom A. 2012. Influence of counting chamber type on CASA outcomes of equine semen analysis. Equine Vet J. 44(5):542–549.
- Knuth UA, Nieschlag E. 1988. Comparison of computerized semen analysis with the conventional procedure in 322 patients. Fertil Steril. 49(5):881–885.
- Knuth UA, Yeung CH, Nieschlag E. 1987. Computerized semen analysis: objective measurement of semen characteristics is biased by subjective parameter setting. Fertil Steril. 48(1):118–124.
- Kumar N, Singh AK. 2015. Trends of male factor infertility, an important cause of infertility: a review of the literature. J Hum Reprod Sci. 8(4):191–196.
- Lu JC, Xu HR, Chen F, Huang YF. 2007. Primary investigations on the quality control for semen analysis in Nanjing city. Zhonghua Nan Ke Xue. 13(1):37–41.
- MacLeod J, Gold RZ. 1951. The male factor in fertility and infertility. II spermatozoön counts in 1000 men of known fertility and 1000 cases of infertile marriages. J Urol. 66(3):436–449.
- Mallidis C, Cooper TG, Hellenkemper B, Lablans M, Ückert F, Nieschlag E. 2012. Ten years’ experience with external quality control program for semen analysis. Fertil Steril. 98(3):611–616.e4.
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. 2012. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Med. 9(12):e1001356.
- Mortimer D, Serres C, Mortimer ST, Jouannet P. 1988. Influence of image sampling frequency on the perceived movement characteristics of progressively motile human spermatozoa. Gamete Res. 20(3):313–327.
- Mortimer ST, van der Horst G, Mortimer D. 2015. The future of computer-aided sperm analysis. Asian J Androl. 17(4):545–553.
- Palacios ER, Clavero A, Gonzalvo MC, Rosales A, Mozas J, Martínez L, Ramírez JP, Björndahl L, Morancho-Zaragoza J, Fernández-Pardo E, et al. 2012. Acceptable variability in external quality assessment programmes for basic semen analysis. Hum Reprod. 27(2):314–322.
- Robinson C, Roberts P, Matson P. 2018. Sperm motility assessment using computer assisted semen analysis (CASA): a comparison of standard microscope slides and coverslips and the 20µm MicroCellTM. J Reprod Biotechnol Fertil. 7:1–7.
- Rurangwa E, Kime DE, Ollevier F, Nash JP. 2004. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 234:1–28.
- Sánchez-Pozo MC, Mendiola J, Serrano M, Mozas J, Björndahl L, Menkveld R, Lewis SEM, Mortimer D, Jørgensen N, Barratt CLR, Special Interest Group in Andrology of the European Society of Human Reproduction and Embriology, et al. 2013. Proposal guidelines for the appraisal of SEMen QUAlity studies (SEMQUA). Hum Reprod. 28(1):10–21.
- Soler C, Cooper TG, Valverde A, Yániz JL. 2016. Afterword to Sperm morphometrics today and tomorrow special issue in Asian Journal of Andrology. Asian J Androl. 18(6):895–897.
- Soler C, Fuentes MC, Sancho M, García A, Núñez de Murga M, Núñez de Murga J. 2012. Effect of counting chamber on seminal parameters, analyzing with the ISASv1®. Rev Int Androl. 10(4):132–138.
- Soler C, García A, Contell J, Segervall J, Sancho M. 2014. Kinematics and subpopulations’ structure definition of blue fox (Alopex lagopus) sperm motility using the ISASv1® CASA system. Reprod dom Anim. 49(4):560–567.
- Talarczyk-Desole J, Berger A, Taszarek-Hauke G, Hauke J, Pawelczyk L. Jedrzejczak 2017. Manual vs computer-assisted sperm analysis: can CASA replace manual assessment of human semen in clinical practice? Ginekol Polska. 88(2):56–60.
- Tomlinson MJ, Kessopoulou E, Barratt C. 1999. The diagnostic and prognostic value of traditional semen parameters. J Androl. 20(5):588–593.
- Tomlinson M, Naeem A. 2018. CASA in the medical laboratory: CASA in diagnostic andrology and assisted conception. Reprod Fert Dev. 30(6):850–859.
- Tomlinson MJ, Pooley K, Simpson T, Newton T, Hopkisson J, Jayaprakasan K, Jayaprakasan R, Naeem A, Pridmore T. 2010. Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms. Fertil Steril. 93(6):1911–1920.
- Valverde A, Areán H, Fernández A, Bompart D, García-Molina A, López-Viana J, Soler C. 2019. Combined effect of type and capture area of counting chamber and diluent on Holstein bull sperm kinematics. Andrologia. 51(4):e13223.
- Valverde A, Madrigal M, Caldeira C, Bompart D, Núñez de Murga J, Arnau S, Soler C. 2019. Effect of frame rate capture frequency on sperm kinematic parameters and subpopulation structure definition in boars, analysed with a CASA-Mot system. Reprod Dom Anim. 54(2):167–175.
- Vantman D, Koukoulis G, Dennison L, Zinaman M, Sherins R. 1988. Computer-assisted semen analysis: evaluation of method and assessment of the influence of sperm concentration on linear velocity determination. Fertil Steril. 49(3):510–515.
- World Health Organization. 2021. WHO laboratory manual for the examination and processing of human semen. 6th ed. Cambridge: World Health Organization.
- World Health Organization. 1999. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: World Health Organization.
- World Health Organization 2010. WHO laboratory manual for the examination and processing of human semen. 5th ed. Cambridge: World Health Organization.
- Yániz JL, Silvestre MA, Santolaria P, Soler C. 2018. CASA-Mot in mammals: an update. Reprod Fert Dev. 30(6):799–809.
- Yániz JL, Soler C, Santolaria P. 2015. Computer assisted sperm morphometry in mammals: a review. Anim Reprod Sci. 156:1–12.
- Yeste M, Bonet S, Rodríguez-Gil JE, Rivera del Álamo MM. 2018. Evaluation of sperm motility with CASA-Mot: which factors may influence our measurements? Reprod Fert Dev. 30(6):789–798.
- Yeung CH, Cooper TG, Nieschlag E. 1997. A technique for standardization and quality control of subjective sperm motility assessments in semen analysis. Fertil Steril. 67(6):1156–1158.
