Abstract
It is well known that various human papillomavirus (HPV) genotypes are present in semen specimens. Also, it has been demonstrated that sperm parameters are negatively affected when HPV infection is present in the sperm sample. Besides all these, the effect of cryopreservation on HPV sensitivity and resistance is not known. The aim of the present study is to evaluate first the prevalence of HPV and secondly to elucidate whether cryopreservation of sperm HPV-positive samples has any effect on the viability of HPV. For this purpose, a cohort of 78 sperm specimens was used from a respective number of patients. After giving informed consent, semen analysis was performed. Each sperm sample was divided into four equal aliquots. The first one (fresh) was evaluated for the prevalence of HPV, while the other three aliquots were cryopreserved by adding an equal quantity of cryoprotectant and plunged into the LN. Each of the three aliquots was thawed 3, 6, and 12 months later, respectively, so as to evaluate whether there is a time-resistance period of HPV prevalence. HPV infection was found to be in eleven sperm samples, demonstrating a 14.1% (11/78) HPV prevalence. Among the HPV-positive samples, six of them were high-risk and the remaining were low-risk genotypes. Moreover, the high-risk fresh samples demonstrated higher motility values than the low-risk samples (60% ± 2.7 vs 45.6% ± 3.7, p < .05), while semen volume in the high-risk samples was significantly lower than the respective volume in the low-risk samples (2.26 ± 0.2ml vs 3.5 ± 0.6ml, p < .05). Interestingly, cryopreservation of the HPV-positive samples resulted in the sustainability and time-resistance of HPV in all high-risk HPV-positive samples, something that was not the case with the low-risk HPV-positive samples. Conclusively, sperm samples infected with high-risk HPV, demonstrate lower sperm parameters and time-resistance activity during cryopreservation.
Introduction
Human papillomavirus (HPV) nowadays appears to be one of the most common and known sexually transmitted viruses in both males and females (Faridi et al. Citation2011). HPV is primarily transmitted through direct epithelial contact. The HPV genotypes are classified according to their oncogenic ability and divided into low-risk (LR-HPV) and high-risk (HR-HPV) subtypes (Kaspersen et al. Citation2011; Yang et al. Citation2013). Both HPV 16 and 18 have been found to associate with the development of cervical cancer (Chrysostomou et al. Citation2018; de Lima Bossi et al. Citation2019). Similarly in men, HR-HPV subtypes are highly related to cancer (Tsai et al. Citation2019). Interestingly, HPV may also be detected in semen specimens (Garolla et al. Citation2013; Schillaci et al. Citation2013; Foresta et al. Citation2015). The impact of HPV on sperm parameters, including sperm DNA fragmentation, has become a matter of concern in the last decade. An investigation conducted by Lyu et al. (Citation2017) found a prevalence of HPV infection in 11% of men from a general population and 20% in semen samples coming from fertility clinics. It appears that HPV prevalence in semen samples results in abnormal semen parameters (Connelly et al. Citation2001; Bezold et al. Citation2007; Foresta et al. Citation2010; Garolla et al. Citation2013; Moghimi et al. Citation2019), posing HPV as a possible risk factor for male fertility (Lyu et al. Citation2017; Xiong et al. Citation2018). It seems that HPV is mostly located on the sperm surface by binding to two sites along the equatorial region of the sperm head, involving mainly the capsid protein L1 and the syndecan-1 receptor (Foresta, Patassini, et al. Citation2011; Schillaci et al. Citation2013). Although the exact HPV localization in sperm is still under investigation, a question that directly rises is whether infected sperm cells can act as transmission vectors for the virus and secondly whether HPV DNA can enter the sperm cell and intercalate with the DNA of the sperm cell. In line with the effect of HPV on sperm parameters, the possible effect of HPV on fertility treatments and medical assisted reproduction (MAR) cannot be excluded (Zacharis et al. Citation2018). Recent studies have demonstrated the association of HPV prevalence in sperm specimens with embryos of lower quality and increased miscarriage rates (Tangal et al. Citation2019).
Although HPV may act as a transmission vector to other cells through the sperm cells, a more intrinsic question that should be answered is whether HPV is resistant during sperm cryopreservation. Answering this question is critical since many fertility clinics use donor sperm samples or even cryopreserve sperm cells for several reasons. The behavior of many viruses during cryopreservation has been elucidated. Viruses may retain their infectivity during cryopreservation and in storage at ultra-cool temperatures (De Paoli Citation2005). Therefore, sperm samples contaminated with viruses could potentially cross-contaminate other specimens during long-term storage, making sperm cryopreservation a potentially risky ART procedure. Although proposed methods for the elimination of the most known viruses during cryopreservation have been demonstrated, including dilution of semen, removal of seminal fluid (Loskutoff et al. Citation2005), or even separate cryopreservation tanks, the impact of cryopreservation on HPV-infected sperm samples as well as the resistance of the virus during cryopreservation is not known. Although there is no clinical evidence that viruses can cross-contaminate when appropriate and standard practice is followed during MAR, the presence of HPV in sperm samples may pose a theoretical or a real risk of cross-contamination (Anifandis et al. Citation2021) during cryopreservation and MAR procedures.
The aim of the present study is to elucidate the impact of cryopreservation on HPV sperm samples and to shed more light on the resistance of the virus during cryopreservation.
Results
Among the 78 patients studied 11 semen specimens were found to be positive for HPV, which accounts for a 14.1% prevalence of HPV. Interestingly, five of them were exclusively LR HPV (5/11, 45.5%), five of them were exclusively HR HPV (single or multiple infections), and the remaining one was co-infected by LR and HR HPV and was counted as HR HPV (overall HR HPV: 6/11, 54.5%). In three cases (3/11, 27.3%), a multiple HPV infection was detected. Furthermore, among the 11 HPV-positive samples detected in semen either with single or multiple infections, it was found that 3 samples were positive for HPV 16 (27.3%), 2 for HPV 33 (18.2%), 2 for HPV 31 (18.2%), 2 for HPV 26 (18.2%) and 6 for HPV 6 (54.5%). There were no significant differences in the demographic data and sperm parameters between the fresh HPV-negative and HPV-positive sperm samples (). Apart from the expected significant associations between concentration and PRM and IM, no other correlations were found between the studied variables. presents the sperm motility values in HPV-negative samples as well as in LR- and HR-HPV-positive samples before freezing and 3, 6, and 12 months post-freezing. Significant differences were observed between the HR-HPV and LR-HPV sperm motility values in the fresh samples, while there were comparable values in all the subsequent time intervals. More interestingly, the decrease in sperm motility in the HPV-negative samples was similarly observed (in an analogous manner) in the HR- and LR-HPV samples, pointing out that cryopreservation impacts sperm motility regardless of the presence of HPV. Moreover, in both HPV-negative and HPV-positive samples, a light reduction (non-significant) in sperm motility was detected, something that can be characterized as normal due to the cryopreservation process ().
Table 1. Demographic characteristics and semen parameters (mean + SEM) between fresh HPV-positive and HPV − negative groups.
Table 2. Sperm motility values (mean ± SEM) between HPV−, LR-HPV, and HR-HPV in fresh and 3, 6, and 12 months post-freezing.
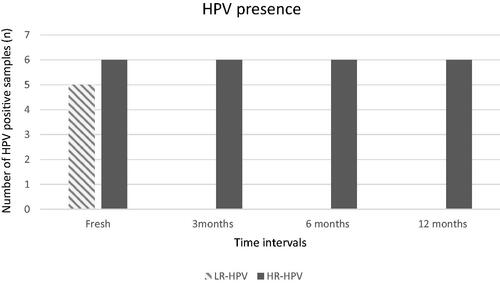
In an attempt to evaluate the resistance of the virus during cryopreservation, all HPV-positive fresh samples were evaluated for the presence of HPV (11 samples) 3, 6, and 12 months post-freezing. Worth mentioning, only the HR- HPV virions remained intact through all time intervals, while the LR- HPV virions presented in the sperm samples were undetectably post-thawed in all time intervals, as depicted in . Noteworthy the co-infected (with LR and HR HPV) sample abolished the LR- HPV virions, while the HR- HPV virions were retained in all time intervals during cryopreservation.
Figure 1. HPV presence in fresh and 3, 6, and 12 months post-freezing samples. It is shown the prevalence of HPV (both LR and HR) in fresh and 3, 6, and 12 months post-freezing samples. LR-HPV was undetectable during cryopreservation in all time intervals of all LR-HPV samples, while HR-HPV infectivity remained during cryopreservation in all time intervals of all HR-HPV samples.
In an attempt to assess the possible risk of cross-contamination during cryopreservation in all time intervals, the presence of HPV virions was analyzed in several HPV-negative samples. HPV-negative samples, in the vicinity during cryopreservation with HPV-positive samples, even with HR subtypes, remained negative in all time intervals studied.
Discussion
The presence of various HPV subtypes in semen specimens has been established years ago. The prevalence of HPV in sperm samples of infertile patients has been estimated to be approximately 16% (Laprise et al. Citation2014). Moreover, the presence of HPV in sperm samples has been associated with impaired sperm parameters, including mainly reduced progressive motility and increased percentage of sperm DNA fragmentation (Connelly et al. Citation2001; Weinberg et al. Citation2020), indicating indirectly the association of HPV prevalence with male infertility. The possible cryo-resistance of HPV during cryopreservation has been not yet studied. A high percentage of HPV-infected sperm cells was found in sperm cells from cryovials of oncology patients (Foresta, Ferlin, et al. Citation2011). Nevertheless, remains unknown whether HPV retains its infectivity and whether the virus is able to cross-contaminate the cryovials. The present study aims to investigate the prevalence of HPV in 78 sperm samples of individuals examined for fertility to detect any possible effects of HPV in the main sperm parameters and secondly to shed light on the cryo-resistance of the virus during cryopreservation.
The present study showed a prevalence of HPV-positive samples among patients attending a fertility clinic at 14.1%, which is in concordance with previous studies (Laprise et al. Citation2014), indicating that the presence of HPV in semen specimens is associated with fertility problems. Interestingly, HPV-positive sperm specimens demonstrated comparable results (in terms of sperm parameters) with HPV-negative sperm specimens. This observation is in line with several investigations which failed to find any impact of HPV on sperm parameters (Luttmer et al. Citation2016; Cortés-Gutiérrez et al. Citation2017). Most studies, investigating the role of HPV on sperm parameters, found that there is a strong association between HPV prevalence and decreased progressive motility as well as an increased percentage of sperm DNA fragmentation (Connelly et al. Citation2001). A recent meta-analysis also found that the presence of HPV in the sperm had a significant association with decreased sperm concentration, motility, and morphology, but not with semen volume (Weinberg et al. Citation2020). In line with the previous meta-analysis, another meta-analysis found that HPV infection is associated with poor semen parameters and an increased risk for miscarriage (Moreno-Sepulveda and Rajmil Citation2021). The explanation, in terms of mechanism, for the impairment by HPV on sperm parameters had been speculated to be the ASAs and sperm DNA fragmentation, since the presence of HPV in semen was frequently related to IgA and IgG classes (Garolla et al. Citation2013) and an in vitro study showed that sperm cells transfected with exogenous HPV E6/E7, the DNA of the sperm cells had higher percentages of breakages compared to the uninfected controls (Connelly et al. Citation2001). In another meta-analysis, it was found that HPV semen infection is closely related to a reduced percentage of progressive motility (Cao et al. Citation2020), implying that HPV infection is a risk for male infertility and can be a useful tool for male reproductive diagnosis. However, all studies, including the present one, emphasize that the effect of HPV on sperm motility is controversial, while it remains unclear the exact mechanism that HPV impacts sperm motility.
In terms of the LR- and HR-HPV subtypes the results of the present study show that semen samples with HR subtypes demonstrate significantly lower semen volumes, implying that HR subtypes somehow reduce the semen volume. Since a major percentage of semen volume is derived from the prostate, it can be assumed that HR-HPV infection negatively affects the function of the prostate, or it is likely that the secretions from the prostate gland are decreased. On the one hand, this situation has as a result the reduced production of the total sperm count. On the other hand, sperm samples infected with HR subtypes demonstrated higher sperm motility values compared to LR-HPV infected samples (). Although this finding may be random, it cannot be ruled out that HR-HPV and LR-HPV act on spermatozoa through different mechanisms and pathways, exerting different actions on spermatozoa. Literature on the subject is limited and the results are controversial. Specifically, HR HPV was shown to have a more adverse effect on sperm motility compared to LR HPV (Boeri et al. Citation2019). Similarly, HR-HPV infection has been found to be related to poor semen motility values and an increased risk of sperm DNA fragmentation (Boeri et al. Citation2019). LR-HPV infection seems to have no effect on sperm parameters (Cannarella et al. Citation2022). It is likely that either the HR- or LR-HPV subtype contributes differently to the severity of sperm impairment and it is also likely that its subtype has a different implication from another.
Worth mentioning, the gradual statistically non-significant decrease in sperm motility during cryopreservation () in both HPV-negative and HPV-positive samples indicates the fact that cryopreservation affects sperm motility, but this issue is independent of the presence of HPV. In other words, the main influence of sperm motility during sperm cryopreservation is coming from the process itself. The explanation for this observation is based on the fact that most protocols of sperm cryopreservation involve the equal dilution of the sample with cryoprotectants, eliminating therefore the presence or the infectivity of the virus. Sperm washing is another method that can be used before any ART procedure for the elimination of several viruses (Mocanu et al. Citation2021), but do not eliminate HPV sperm infection in infertile patients (Foresta, Pizzol, et al. Citation2011). Alternatively, Heparinase-III treatment seems not to affect spermatozoa, and this treatment may be used to prepare sperm from patients who are infected with HPV in order to reduce the risk of HPV infection when using assisted reproduction techniques (Garolla et al. Citation2012). shows the prevalence of both LR and HR HPV in fresh and 3, 6, and 12 months post-thawing HPV-positive samples. LR-HPV was undetectable during cryopreservation in all time intervals of all LR-HPV samples, while HR-HPV detection remained during cryopreservation in all time intervals of all HR-HPV samples. Undetectable levels of LR-HPV in the semen samples after cryopreservation compared to persistent detection of the HR-HPV is a novel finding of crucial significance. Likewise in samples with LR- and HR-HPV coinfections PCR performed 3, 6, and 12 months post-thawing, resulted in the detection only of the HR-HPV types. Studies investigating possible differences in the localization of LR and HR subtypes in spermatozoa do not exist. However, studies with FISH revealed a clear HPV localization at the equatorial region of the sperm head in infected samples (Foresta et al. Citation2010; Schillaci et al. Citation2013), something that explains the possible interference of HPV with the ability of spermatozoon to bind and to penetrate the oocyte (Foresta, Patassini, et al. Citation2011). The location is not likely to be the explanation for the results observed in the present study because otherwise, both subtypes would be undetectable post-thawing. The lack of evidence for the exact mechanism of action of both LR and HR HPV subtypes in the spermatozoa during cryopreservation, allows us to speculate that the different subtypes follow a diverse way of action on spermatozoa. Possibly HR HPV subtypes either penetrate the sperm cytoplasm or integrate into the sperm DNA, providing protection from the cryopreservation procedure. Due to the fact that LR-HPVs are of low oncogenic potential, the investigation into these subtypes has not been prioritized. In general, lesions caused by LR-HPVs are self-restricted and commonly eliminated by the immune system (Egawa and Doorbar Citation2017). The notion that HPV may integrate with the spermatozoa DNA is not to be ruled out, because as it is true in HPV-associated carcinomas HR HPVs are detected integrated into the host cell DNA, episomatic, or both (Kristiansen et al. Citation1994; McBride and Warburton Citation2017). Another explanation for the results of the present study can be the different viral loads. It is possible that LR HPV viral loads in the sperm samples were relatively low and the cryopreservation process, involving abrupt crystallization of the sample, eliminates the detectable viral loads. In contrast, the HR viral load was at first at high levels and the cryopreservation process did not reduce the viral loads to levels that would have been undetectable. Both assumptions can explain the HR-HPV prevalence in post-thaw sperm samples.
Lastly, the presence of the virus poses a ‘theoretical risk’ of cross-contamination during cryopreservation, although it has not been recorded previously with other viruses (Anifandis et al. Citation2021). This possibility needs further evaluation. For this purpose, the possible cross-contamination of HPV-negative samples in a vicinity with HPV-positive samples was investigated. There was no detection of HPV in the HPV-negative samples, implying that the ‘real risk’ of cross-contamination is only theoretical. As it is known LN2 is the only source of contamination or the main substrate and mediator for cross-contamination. However, the hermetically or complete closure of sperm cryovials does not allow the dispersion or the permeation of the virus.
In conclusion, the present study is the first study that deals with HPV subtypes and sperm cryopreservation. The prevalence of HPV-positive samples was 14.1%, in concordance with worldwide epidemiological data. Sperm parameters were comparable between HPV-negative and -positive samples. LR-HPV samples demonstrated higher semen volume compared to HR-HPV samples and interestingly, the detection of HR-HPV DNA remained through the cryopreservation process in all time intervals. HR-HPV screening appears to be a prerequisite for donor sperm. Cross-contamination of neighboring sperm samples was not verified, proposing that the infectivity of the virus remains restricted to the cryovial. Further studies are needed to shed more light on the impact of cryopreservation on HR and LR-HPVs in sperm samples.
Materials and methods
Study group
Seventy-eight healthy men attended the IVF clinic of the University Hospital of Larissa for semen analysis between January 2019 and March 2020. All men took part voluntarily in this study and gave written informed consent. The study was approved both by the Ethics Committee of the Medical School of the University of Thessaly, Greece (protocol number: 28/13.03.2019), and the Scientific Committee of the University Hospital of Larissa, Greece (protocol number: 63969/24.12.2018).
Semen collection and processing
Semen collection was performed within 48 to 72 h of sexual abstinence. For each fresh semen sample, after liquefaction at room temperature, and each aliquot after thawing, semen analysis was performed according to World Health Organization Guidelines (World Health Organization Citation2010). The total amount of semen sample of every patient was treated with the addition of an equal volume of cryoprotectant solution (Sidney IVF Sperm Cryopreservation Buffer, COOK Medical). The mix was divided into three equal aliquots of 0.4 ml and as soon as homogenizing was performed, the samples were immediately plunged into liquid nitrogen (−196 °C). The thawing process was performed at intervals of 3, 6, and 12 months from day 0 of cryopreservation. Each cryovial with the respective sample, after retrieval from the liquid nitrogen, was placed in a 37 °C water bath for 10 min for further processing.
Sperm cells of fresh and their corresponding thawed aliquots were separated from seminal plasma with centrifugation at 2000 rpm for 5 min. Pellet was washed twice with 1× PBS (Phosphate-Buffered Saline, Thermo Fisher Scientific) and subjected to HPV detection and genotyping.
Epidemiological data for all the participants were collected. The smoking status of the men studied was categorized as never smokers (no smokers) or as current smokers (active smokers). Similarly, alcohol consumption was categorized either as abstainers (no alcohol consumption history), or active drinkers (>2 drinks/day) (National Institute of Alcohol Abuse and Alcoholism Citation1995).
HPV detection and genotyping
DNA was extracted from the pellet (containing spermatozoa) derived from both fresh and thawed semen samples by PureLink® Genomic DNA Mini Kit (Invitrogen, Thermo Fisher Scientific, Loughnorough, UK) according to the manufacturer’s instructions. DNA concentration and purity were evaluated by Nanodrop spectrophotometry and agarose gel electrophoresis.
HPV amplification and genotyping were performed using AmoyDx® Human Papillomavirus (HPV) Genotyping Detection Kit (Amoy Diagnostics Co., Ltd, Xiamen, China). PCR reactions were carried out on Rotor-GeneTM 6000 (Corbett Life Science), according to the manufacturer’s protocol. A part of HPV L1 gene is amplified through multiplex PCR. Nineteen high-risk HPV types (HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 68, 70, 73, and 82) and two low-risk HPV types (HPV 6 and 11) were detected through fluorescence signal with a sensitivity cut-off of 100 copies per reaction. A non-rivalry internal control was used to reveal the presence of PCR inhibitors and monitor the accuracy of the experiment.
Statistical analysis
Statistical analysis was performed using SPSS v.22. Data are presented as medians with the standard error. Epidemiological data along with semen parameters were compared between HPV + and HPV − groups using the Mann–Whitney test and the Chi-square test when it was appropriate. A comparison of the HPV subgroups (HR HPV + and LR HPV+) was performed by using the Kruskal–Wallis test. The associations between various clinical variables (e.g., age, BMI, smoking habits, and HPV status) and sperm motility were analyzed. The statistical significance level was determined at less or equal to 0.05 (p ≤ .05).
Ethics approval
The study was approved by the Ethics Committee of the Medical School of the University of Thessaly, Greece (protocol number: 28/13.03.2019), as well as by the Scientific Committee of the University Hospital of Larisa, Greece (protocol number: 63969/24.12.2018). All participants gave written informed consent.
Authors’ contributions
Conceptualization: GA; writing—original draft preparation: MA, MS, ET, CIM, KC, ED, GA; statistical analysis: MA; molecular experiments: MA, MS; writing—review and editing: KD, AD, GK, GA; supervision: GA. All authors have read and agreed to the version of the manuscript.
| Abbreviations | ||
| ASA | = | Anti-sperm antibody |
| ART | = | Assisted reproductive technology |
| BMI | = | Body mass index |
| HPV | = | Human papilloma virus |
| HR | = | High-risk |
| IM | = | Immobility |
| LN | = | Liquid nitrogen |
| LR | = | Low-risk |
| MAR | = | Medical assisting reproduction |
| NPM | = | Non-progressive motility |
| NS | = | No significant |
| PCR | = | Polymerase chain reaction |
| PRM | = | Progressive motility |
| WHO | = | World Health Organization |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anifandis G, Taylor HT, Messini CI, Chatzimeletiou K, Daponte A, Ioannou D, Tempest GH. 2021. The impact of SARS-CoV-2 on sperm cryostorage theoretical or real risk? Medicina. 57(9):946.
- Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. 2007. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 87(5):1087–1097.
- Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, Pozzi E, Clementi M, Viganò P, Montanari E, et al. 2019. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod. 34(2):209–217.
- Cannarella R, Aversa A, Condorelli AR, Cristofaro DS, Greco E, Grillo A, Calogero EA, Vignera LS. 2022. Impact of seminal low-risk human papillomavirus infection on sperm parameters of adult men. Aging Male. 25(1):17–22.
- Cao X, Wei R, Zhang X, Zhou J, Lou J, Cui Y. 2020. Impact of human papillomavirus infection in semen on sperm progressive motility in infertile men: a systematic review and meta-analysis. Reprod Biol Endocrinol. 18(1):38.
- Chrysostomou AC, Stylianou DC, Constantinidou A, Kostrikis LG. 2018. Cervical cancer screening programs in Europe: the transition towards HPV vaccination and population-based HPV testing. Viruses. 10(12):729.
- Connelly DA, Chan PJ, Patton WC, King A. 2001. Human sperm deoxyribonucleic acid fragmentation by specific types of papillomavirus. Am J Obstet Gynecol. 184(6):1068–1070.
- Cortés-Gutiérrez IE, Dávila-Rodríguez IM, Fernández LJ, O-Pérez OL, Garza-Flores EM, Eguren-Garza R, Gosálvez J. 2017. The presence of human papillomavirus in semen does not affect the integrity of sperm DNA. Andrologia. 49(10):e12774.
- de Lima Bossi R, Valadares JBF, Puerto HL, del Rocha MGL, Braga LC, Sampaio MAC, et al. 2019. Prevalence of human papillomavirus (HPV) in the semen patients submitted to assisted reproductive technology treatment in a private clinic in Brazil. JBRA Assist Reprod. 23(3):205–209.
- De Paoli P. 2005. Bio-banking in microbiology: from sample collection to epidemiology, diagnosis and research. FEMS Microbiol Rev. 29(5):897–910.
- Egawa N, Doorbar J. 2017. The low-risk papillomaviruses. Virus Res. 231:119–127.
- Faridi R, Zahra A, Khan K, Idrees M. 2011. Oncogenic potential of human papillomavirus (HPV) and its relation with cervical cancer. Virol J. 8(1):269.
- Foresta C, Ferlin A, Bertoldo A, Patassini C, Zuccarello D, Garolla A. 2011. Human papilloma virus in the sperm cryobank: an emerging problem? Int J Androl. 34(3):242–246.
- Foresta C, Garolla A, Parisi S, Ghezzi M, Bertoldo A, Di Nisio A, De Toni L. 2015. HPV prophylactic vaccination in males improves the clearance of semen infection. EBioMedicine. 2(10):1487–1493.
- Foresta C, Patassini C, Bertoldo A, Menegazzo M, Francavilla F, Barzon L, Ferlin A. 2011. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One. 6(3):e15036.
- Foresta C, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Garolla A. 2011. Semen washing procedures do not eliminate human papilloma virus sperm infection in infertile patients. Fertil Steril. 96(5):1077–1082.
- Foresta C, Pizzol D, Moretti A, Barzon L, Palu G, Garolla A. 2010. Clinical and prognostic significance of human papillomavirus DNA in the sperm or exfoliated cells of infertile patients and subjects with risk factors. Fertil Steril. 94(5):1723–1727.
- Garolla A, Lenzi A, Palù G, Pizzol D, Bertoldo A, De Toni L, Foresta C. 2012. Human papillomavirus sperm infection and assisted reproduction: a dangerous hazard with a possible safe solution. Hum Reprod. 27(4):967–973.
- Garolla A, Pizzol D, Bertoldo A, De Toni L, Barzon L, Foresta C. 2013. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil Steril. 99(1):125.e2–131.e2.
- Kaspersen MD, Larsen PB, Ingerslev HJ, Fedder J, Petersen GB, Bonde J, Höllsberg P. 2011. Identification of multiple HPV types on spermatozoa from human sperm donors. PLos One. 6(3):e18095.
- Kristiansen E, Jenkins A, Holm R. 1994. Coexistence of episomal and integrated HPV16 DNA in squamous cell carcinoma of the cervix. J Clin Pathol. 47(3):253–256.
- Laprise C, Trottier H, Monnier P, Coutlee F, Mayrand MH. 2014. Prevalence of human papillomavirus in semen: a systematic review and meta-analysis. Hum Reprod. 29(4):640–651.
- Loskutoff NM, Huyser C, Singh R, Walker DL, Thornhill AR, Morris L, Webber L. 2005. Use of a novel washing method combining multiple density gradients and trypsin for removing human immunodeficiency virus-1 and hepatitis C virus from semen. Fertil Steril. 84(4):1001–1010.
- Luttmer R, Dijsktra MG, Snijders PJF, Hompes PGA, Pronk DTM, Hubeek I. 2016. Presence of human papillomavirus in semen in relation to semen quality. Hum Preprod. 31:280–286.
- Lyu Z, Feng X, Li N, Zhao W, Wei L, Chen Y, Yang W, Ma H, Yao B, Zhang K, et al. 2017. Human papillomavirus in semen and the risk for male infertility: a systematic review and meta-analysis. BMC Infect Dis. 17(1):714.
- McBride AA, Warburton A. 2017. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 13(4):e1006211.
- Mocanu E, Drakeley A, Kupka MS, Lara-Molina EE, Le Clef N, Ombelet W, Patrat C, Pennings G, Semprini AE, Tilleman K, et al. 2021. ESHRE guideline: medically assisted reproduction in patients with a viral infection/disease. Hum Reprod Open. 4(4):hoab037.
- Moghimi M, Zabihi-Mahmoodabadi S, Kheirkhah-Vakilabad A, Kargar Z. 2019. Significant correlation between high-risk HPV DNA in semen and impairment of sperm quality in infertile men. Int J Fertil Steril. 12:306–309.
- Moreno-Sepulveda J, Rajmil O. 2021. Seminal human papillomavirus infection and reproduction: a systematic review and meta-analysis. Andrology. 9(2):478–502.
- National Institute of Alcohol Abuse and Alcoholism. 1995. The physicians’ guide to helping patients with alcohol problems. Washington (DC): U.S. Department of Health and Human Services, National Institutes of Health.
- Schillaci R, Capra G, Bellavia C, Ruvolo G, Scazzone C, Venezia R, Perino A. 2013. Detection of oncogenic human papillomavirus genotypes on spermatozoa from male partners of infertile couples. Fertil Steril. 100(5):1236–1240.
- Tangal S, Taşçı Y, Pabuçcu EG, Çağlar GS, Haliloğlu AH, Yararbaş K. 2019. DNA fragmentation index and human papilloma virus in males with previous assisted reproductive technology failures. Turk J Urol. 45(1):12–16.
- Tsai SCS, Huang JY, Lin C, Liaw YP, Lin FCF. 2019. The association between human papillomavirus infection and head and neck cancer: a population-based cohort study. Medicine. 98(7):e14436.
- Weinberg M, Nahshon SSC, Feferkorn I, Bornstein J. 2020. Evaluation of human papilloma virus in semen as a risk factor for low sperm quality and poor in vitro fertilization outcomes: a systematic review and meta-analysis. Fertil Steril. 113(5):955.e4–969.e4.
- World Health Organization 2010. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. Cambridge, UK: Cambridge University Press.
- Xiong YQ, Chen YX, Cheng MJ, He WQ, Chen Q. 2018. The risk of human papillomavirus infections for male fertility abnormality: a meta-analysis. Asian J Androl. 20(5):493–497.
- Yang Y, Jia CW, Ma YM, Zhou LY, Wang SY. 2013. Correlation between HPV sperm infection and male infertility. Asian J Androl. 15(4):529–532.
- Zacharis K, Messini IC, Anifandis G, Koukoulis G, Satra M, Daponte A. 2018. Human papilloma virus (HPV) and fertilization: a mini review. Medicina. 54(4):50.
