Abstract
The Printed Circuit Board (PCB) manufacturing process uses large quantities of water and a wide range of chemicals. If not handled responsibly, many of these materials may ultimately have an impact on the environment or at best generate effluent that needs increasingly costly treatment. There is an opportunity to move towards a more sustainable methodology where waste is minimised, water is recycled and materials are recovered. This paper presents the results from work aimed at enabling PCB manufacturing to become more sustainable. Novel processes evaluated include special electroplating techniques, advanced oxidation methods to remove organic contaminants, and new ion exchange systems. Work has been carried out to develop these processes into viable demonstrators and the results of progress to date are reported. Descriptions of how these individual technologies may be combined to provide an integrated approach to a more sustainable PCB manufacturing methodology are also given.
1. Introduction
The Printed Circuit Board (PCB) manufacturing process uses large quantities of water and a wide range of chemicals in a multistage process. Water consumption represents a significant and increasing cost burden for PCB makers, while many of the materials used may be potentially harmful to the environment if not treated properly (Goosey Citation2000). The chemical processes themselves result in the generation of a significant volume of effluent that needs costly treatment before it can be discharged. The PCB manufacturing process is largely based on the deposition and removal of copper and thus the industry produces large quantities of metal bearing effluent solutions that require expensive treatment before they can be discharged. Similarly, various organic materials such as formaldehyde and the chelating agent ethylene diamine tetra acetic acid (EDTA) are also widely used. These also present a major problem in terms of effluent, since they not only raise the chemical oxygen demand (COD) of the effluent but also make it more difficult and costly to treat. Such materials may also have a negative environmental impact and in some cases represent a waste of valuable resources (Wright et al. Citation2003).
Conventional treatment methodologies for this type of waste often involve multistage processes in which significant volumes of solid waste are generated and sent to landfill. These typically require the use of large quantities of water and treatment chemicals followed by filter pressing to produce a solid waste containing the precipitated metals which can be consigned to landfill. In the early days of the PCB industry there were no trade effluent consent limits and rinse waters were typically consigned directly to sewer while spent chemical solutions went to landfill. As discharge consent levels were introduced and began to increase, compliance was achieved by the separation of high‐copper‐concentration rinses for batch treatment with low‐copper rinses being discharged to sewer in combination with rinses from other processes which effectively diluted the copper content to an acceptable level. Spent chemical solutions were treated by neutralisation and precipitation, with the resultant sludge still being sent to landfill. The subsequent introduction of high efficiency electrolytic recovery cells to remove copper from microetches and neutraliser solutions helped to reduce the volumes of sludge produced and thus the corresponding disposal costs. The filter presses that are used to de‐water sludge can also be used to produce filter cakes with sufficiently high metal contents to make metal recovery a more attractive option than landfilling. However, this later process usually involves shipping large volumes of waste overseas for treatment in countries such as Germany. More details of PCB effluent treatment processes can be found in the Environmental Best Practice Guide produced by the PCIF (Goosey and Kellner Citation1999). While the above approaches offer some significant benefits in terms of a more environmentally friendly process, they still result in the discharge of water and effluent. Consequently, there is an opportunity to enhance further the efficiency of these processes and to move towards a more sustainable methodology where the amount of waste generated is minimised, water is recycled and potentially valuable materials are recovered for use elsewhere. In the ultimate manifestation of this type of approach, the PCB manufacturing process would be truly sustainable, with chemistry and water being reused in a process where only small volumes of new replenishment chemicals would be required.
This paper presents work undertaken on a DTI and industry co‐funded project called ‘Towards Zero Discharge Printed Circuit Board Manufacturing Plant’ (TAZDIS) and also details results from projects undertaken in collaboration with a number of European partners to develop individual elements of the traditional PCB manufacturing process towards a sustainable and zero‐discharge alternative. Details are given of several novel process stages developed and these include the use of special electroplating techniques to capture low levels of metals such as copper and nickel, advanced oxidation methods to remove organic contaminants from rinse waters, and the use of new ion exchange systems as a key enabler for producing viable quantities of treatable elluent. Work has been carried out to develop some of these processes from laboratory scale prototypes to viable demonstrator units that will be used in a manufacturing environment and the results of progress to date are also reported. The paper concludes with descriptions of how these individual technologies have been combined to provide a unique and integrated approach that could enable the PCB industry to adopt more sustainable methodologies in its manufacturing processes. These in turn would help the industry to be more efficient, to reduce the amount of effluent generated and to recover valuable materials for recycling and reuse.
2. Capture and recovery technologies
The TAZDIS project was undertaken to develop and implement novel features in plant and equipment for PCB manufacturing processes that would minimise pollution with the aim of achieving near zero environmental pollutant discharge. The objective of the project was to control and minimise pollution at the source via the integration of process control, waste minimisation and recovery techniques. This was to be achieved by minimising the drag over of chemical pollutants from process chemistries into rinse water and also by deploying novel capture and recovery techniques to remove low level metals, anions and organic pollutants from the rinse waters, thus enabling water recycling and a reduction in water consumption.
Ion exchange has been investigated to capture and concentrate metals from these rinse waters. Once saturated, the resins were then regenerated for further use, with the regenerant liquor containing the concentrated metal ions being transferred to an electrochemical cell for recovery and recycling of the metals. The metal‐free regenerant could then be safely discharged to outfall.
Similarly, ion exchange has been deployed to capture and concentrate organic contaminants in the rinse water with the adsorbent resins being regenerated for continued use. The regenerant liquor was then transferred to an organics destruction unit which used advanced oxidation to completely destroy the organics. The organic‐free regenerant liquor could then be safely discharged to outfall.
The above mentioned individual technologies were then combined to provide a novel integrated and sustainable approach to tackling the potential environmental pollution impact of PCB manufacturing processes. This combined approach offers a closed loop system that enables almost total water recycling and re‐use.
2.1 Ion exchange
Increasingly stringent environmental regulations, as well as economic and technical constraints have influenced the decision to use ion exchange technology in this project. These regulations require manufacturers to meet stricter discharge limits for metals, anions and organics and an economic analysis of the treatment costs for the spent process chemistries and rinse waters has highlighted the potential for techniques such as ion exchange. Ion exchange resins have been used within the metal plating and surface finishing industries for many years to rejuvenate plating and pickling baths, to enable recycling of rinse waters and for the treatment of waste effluents (Williams Citation1993). They thus present an established and well‐tested technology that could provide a low risk component of the integrated process being developed in this work. In some cases it is possible to recover and recycle chemicals for their intrinsic value.
In the ion exchange process, ions of a given charge, i.e. either cations or anions, in a solution are adsorbed on a solid material (the ion exchanger) and are replaced by equivalent quantities of other ions of the same charge (Ion Exchange Principles and Applications Citation2001). The ion exchanger may be a salt, acid or base in solid form that is insoluble in water but hydrated. Exchange reactions take place in the aqueous medium. Ion exchange forms the basis of a large number of chemical processes which can be divided into three main categories: substitution, separation and removal.
-
Substitution: a valuable ion (e.g. copper) can be recovered from a solution and replaced by an equivalent charged ion.
-
Separation: a solution containing a number of different ions passes through a column containing an ion exchange resin. The ions are separated and emerge in an order determined by their increasing affinity for the resin.
-
Removal: by using a cation or anion resin, or both, cation and anion ions are removed and replaced by equivalent ions.
Organic scavenger resins are predominantly adsorbent resins and tend to be either strongly basic, weakly basic or inert. They are not ion exchangers but resemble them closely and can be used for the adsorption of non‐ionic or weakly ionised organic species. It is envisaged that the use of novel ion exchange technology will offer an element of a truly sustainable approach to metal recovery and regeneration from copper, nickel, gold and tin processes chemistries within the PCB manufacturing process. Ion exchange will also enable the capture and subsequent destruction of organic contaminants from rinse waters, thus enabling water recycling and minimisation of water consumption and discharge.
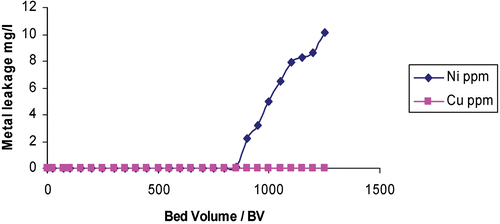
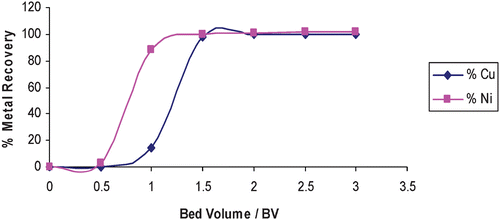
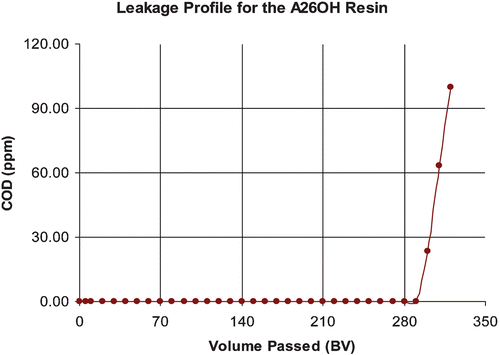
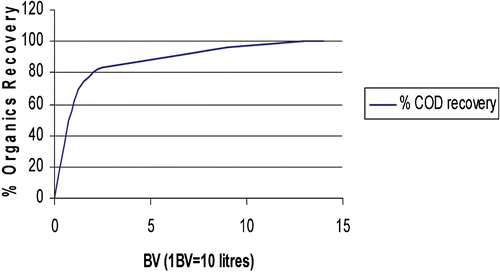
In laboratory‐scale, small pilot plant and large scale field tests of production capable demonstrator systems developed within this project, the recovery of low‐levels of copper and nickel in model rinse streams and real process chemistry from an Electroless Nickel Immersion Gold (ENIG) process has been effectively demonstrated using commercially available resins supplied by Rohm and Haas Company. Copper and nickel ions were captured and concentrated on cationic exchange resins. The resins were then acid regenerated once saturated. Figure shows the leakage of metal ions versus the volume of rinse water treated during a pilot plant test to recover copper and nickel from rinse streams. Leakage is measured in mg L−1, and volume is measured in bed volumes (BV, where 1 BV = 1 m3 solution per m3 of resin). Figure shows the regeneration of copper and nickel using 1M sulphuric acid expressed as per cent metal recovery versus volume of regenerant.
2.2 Electrochemical recovery
A number of established techniques are available for the treatment of process and wastewater streams containing metal ions, including electrodeposition, precipitation, ion exchange, adsorption and reverse osmosis. The preferred method is electrodeposition which can recover elemental metals directly from aqueous solutions over a wide range of metal ion concentrations. However, electrochemical recovery is inefficient at the low concentrations typically found in the types of effluent addressed by this project. Therefore, in order to achieve high plating efficiencies, the relatively low levels of metal ions were effectively concentrated using ion exchange. In many cases, the plated metals can then be directly recycled. In this project an ‘Enhanced Fluidised Bed Electrolytic Recovery System’ (Chemelec cell) was evaluated. This type of cell displays high levels of non‐specificity for a range of metal recovery applications and has a high tolerance towards interfering chemical species such as chelating agents.
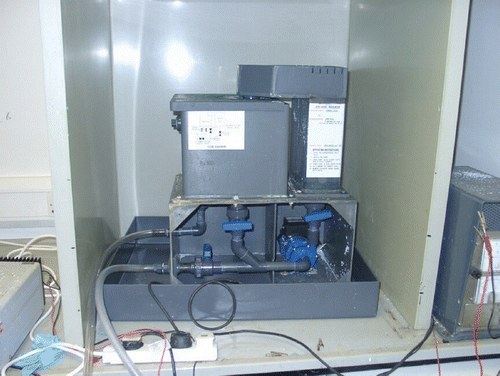
This method has been used to remove copper and nickel in concentrated regenerant liquors from the ion exchange treatment process. Metal levels can be reduced to sufficient levels for the regenerant to be reused or safely discharged down stream. The materials that can be used for column regeneration are typically low concentration acid solutions (e.g. 2 to 4% hydrochloric and sulphuric acid). The possibility of achieving selective metal recovery is also being evaluated by a variety of techniques. Figure shows a laboratory‐scale fluidised bed electrochemical cell that has been used during these evaluations.
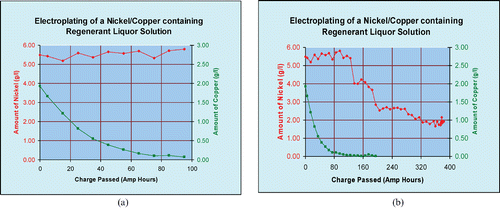
The electro‐winning of copper and nickel has been effectively demonstrated on regenerant liquor during pilot plant scale tests. In one such experiment a sample containing copper (2 g L−1) and nickel (5.5 g L−1) was subjected to treatment in a Chemelec Cell at a current of 10 A and a current density of ∼3.0 A dm−2 (30 A ft−2). Copper levels were reduced effectively to below 20 mg L−1 within 20 hours. Figure shows the recovery of copper from regenerant liquor. Nickel did not plate out at the same time as copper. This was possibly due to a preference for copper deposition under the conditions used. The regenerant was highly acidic and more favourable for copper deposition. It is well known that nickel deposition is more favourable at a pH of 3 to 4. The nickel required a pH adjustment of 3 to 4 before effective recovery could be achieved. Figure shows the recovery of nickel after pH adjustment.
Figures illustrate that while electrochemical recovery of copper is relatively straightforward in acid conditions, the recovery of nickel is more sensitive to the process conditions. In any commercial application of this approach where there is likely to be a mixture of copper and nickel, it will be necessary to introduce pH adjustment and control, so that after initial copper recovery has been achieved, the nickel can also be electroplated out. This type of pH adjustment is routinely employed in existing effluent treatment plants and is unlikely to present any major difficulties in terms of incorporation into an industrial manifestation of the process detailed in this paper.
2.3 Advanced oxidation
Many of the chemical processes used in the fabrication of PCBs and metal finishing products include various organic species which, whilst being essential to the process, are undesirable from an effluent and waste treatment perspective. Some of these organics can inhibit the recovery of metal ions, whilst others are potentially harmful to the environment and must be removed before the effluent can meet the demands of increasingly strict legislation and regulation. There are numerous applications where organic materials are used directly and many of these are undesirable from an environmental perspective while some are also considered to be toxic. Chelating agents, in particular, pose downstream incompatibility problems with wastewater treatment, metals reduction and water recycling processes, due to their ability to complex heavy metal ions and their low biodegradabilities (Tucker et al. Citation1999).
Existing methods for the removal of persistent organic compounds, such as those employing activated carbon, air stripping, and reverse osmosis create secondary waste problems and are normally applied as ‘end of pipe’ treatments (i.e. not as an integral part of the process) (Lankford and Eckenfelder Citation1990). The deployment of an effective treatment technology will therefore be required to address these problems. An advanced oxidation technique capable of treating this problem was developed by the authors as part of a previous project (Bains et al. Citation2003).
Advanced oxidation techniques are based on the use of ozone and/or hydrogen peroxide in combination with UV radiation. In the case of UV and ozone, an ozonised gas stream, produced by corona discharge, is bubbled or injected into the aqueous medium to be treated. UV radiation, the most commonly used sources emit radiation at 254 nm, is readily absorbed by ozone, resulting in the formation of highly reactive hydroxyl radicals (.OH). These radicals are more powerful oxidising agents than either ozone or hydrogen peroxide, and are capable of converting virtually all organic compounds to carbon dioxide, water and similar species. In the case of hydrogen peroxide, this also produces hydroxyl radicals when treated with UV radiation. Hydrogen peroxide can be dosed into the aqueous medium alone or in combination with ozone. Hydroxyl radicals are short‐lived species formed when ozone in the presence of UV radiation (254 nm) forms oxygen (O2) and an excited oxygen atom (O*). The excited oxygen atoms combine with water to form both hydrogen peroxide and hydroxyl radicals. Hydrogen peroxide subsequently forms hydroxyl radicals in the presence of UV radiation. The basic equations leading to the generation of hydroxyl radicals by this route are as follows:
There are a number of alternative reactions that can take place and it is known that high concentrations of both ozone and hydrogen peroxide need to be avoided as hydroxyl radicals are not selective in reactivity and can react with excess oxidants. The oxidation rates achieved with hydroxyl radicals are much greater than those attainable from conventional oxidants such as ozone, hydrogen peroxide and hypochlorite. In some cases, reaction rates achieved using hydroxyl radicals are 106 to 109 times larger than the rates achieved using ozone alone (Galbraith Citation1992, Skorska and Davis Citation1992, Yue Citation1992, Schulte and Bayer Citation1995, Kou Citation1999). In this project, a UV‐ozone based advanced oxidation system was utilised to destroy organics in‐situ. The organics were captured and concentrated onto special regenerable adsorbent resins (Amberlyst A26OH supplied by Rohm and Haas Company). Advanced oxidation could then be used to destroy the organics in the regenerant liquors. An additional benefit of this approach is that the treated regenerant solution can be safely discharged to outfall without the need for further waste treatment or dilution. Figure shows the leakage of organic contaminants captured from an electroless nickel rinse. Organic capture and recovery has been demonstrated on a pilot scale. In these examples organic contaminants with an approximately 130 ppm (mg L−1) COD level were captured onto an organic acid scavenging resin.
Figure shows data for the recovery of organics and regeneration of the resin for reuse using a sodium hydroxide solution (1M).
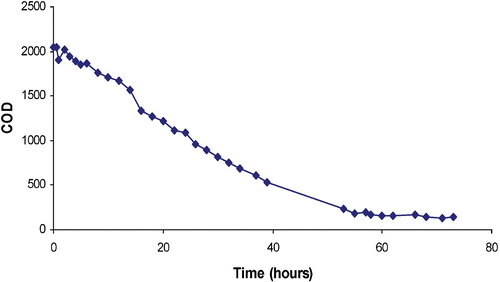
Regenerant liquor obtained from the regeneration of the resin was transferred to an advanced oxidation plant for organics destruction. In one such example a 140 litre sample of liquor containing approximately 2000 ppm of COD was treated using UV and ozone advanced oxidation. The COD level was reduced to less than 150 ppm and well below most consent to discharge limits. Figure shows the depletion of organics content over time when regenerant liquor was subject to advanced oxidation.
The individual process treatment technologies detailed above have been individually assessed as potential elements of a combined effluent treatment process that will enable PCB manufacturing to operate on a more sustainable manner. Work has also been undertaken to integrate and further develop the technology and full size pilot equipment has been built and evaluated in a true production environment provided by two of the project partners.
3. Integrated pollution control
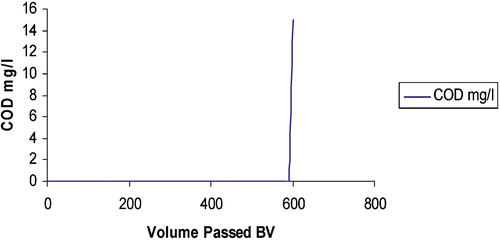
The combined recovery of low level metals such as copper and nickel and organics removal from electroless nickel and immersion gold process waste streams has been demonstrated at laboratory scale and further proven successfully during field trials under true production conditions. Using a combination of organic scavenging, cationic, anionic and chelating resin modules, a closed loop supply of rinse water has been maintained at high purity and ‘deionised water’ quality during production cycles. An electrochemical cell and advanced oxidation plant have been integrated into the complete water treatment and pollution control system. Laboratory scale tests showed that conductivity readings below 10 µS cm−1 could be maintained at least for 600 bed volumes of rinse water treated. Similarly, during production trials, rinse water conductivity was maintained below 50 µS cm−1 for one month with no effect on PCB production quality. Figure shows the combined capture of metals and organics during laboratory scale testing.
An electrochemical cell has been deployed for the recovery and recycling of copper and nickel metals. An advanced oxidation plant has been deployed to treat organics captured during water treatment and recycling.
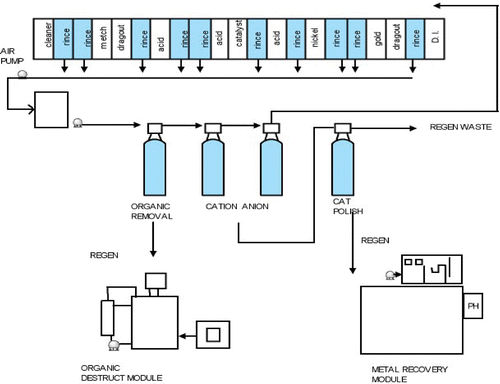
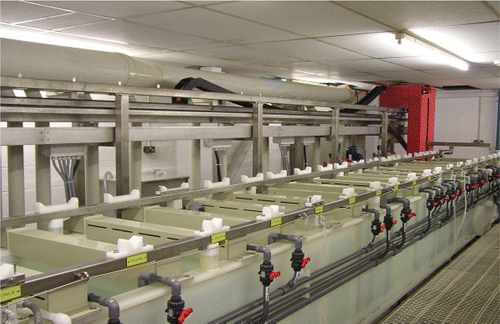
A process plant has been built, installed and commissioned at a project partner's manufacturing facility. The plant incorporates the technologies and methodologies developed as a part of this project. It is equipped with drag‐out minimisation, pollutant capture and treatment and water recycling in a closed loop system. (The design is shown schematically in Figure and with actual images of the equipment in Figures and ).
Figure 11 Resin modules for pollution capture and water treatment(a) and electrochemical cell for metal recycling (b).

At the time of writing, the equipment detailed above was installed at the premises of PWT Circuits, a small PCB manufacturing company based in Welwyn Garden City. As part of the funding conditions, it was agreed that access to this equipment would be made available to those with an interest.
4. Conclusions
The PCB industry uses a wide range of chemicals in its manufacturing processes and these can lead to the generation of large quantities of effluent. This effluent contains potentially harmful materials that could damage the environment if not properly discharged and which are increasingly expensive to waste treat. The PCB industry in Europe is also under intensive competitive pressure from low cost manufacturing countries such as China and any new technologies that can help reduce costs by enhancing the efficiency of the manufacturing would be welcomed. In recent years, the authors have participated in a number of UK and European funded collaborative projects. These have included the development of novel effluent treatment technologies specifically aimed at helping the PCB industry to reduce costs, minimise its environmental impact and recycle valuable raw materials. They have addressed individual aspects of the PCB manufacturing process and its effluent but the work carried out in this project represents a successful integration of the technology developed during these projects and other best available technologies into an integrated approach that attempts to take the PCB manufacturing process as close as possible to a zero discharge situation. For the future, there are still a number of other key areas that could also be addressed if the PCB industry is to further improve its environmental performance. Examples include the replacement of materials such as formaldehyde and thiourea, which are used in various plating solutions, and a move to additive circuit formation processes that generate less waste. There is also a desire to reduce the energy consumption of the processes used in PCB manufacturing and this could be achieved by developing new low‐temperature chemistry and equipment combinations. This latter topic is currently being investigated and it hoped that it will be possible to detail the results of the work in a future paper. Overall, the PCB industry has made significant progress in developing sustainable processes, but there is still much more that needs to be done.
References
- Bains , N. , Goosey , M. and Hayer , R. 2003 . Evaluation of an enhanced oxidation method for the destruction of ethylene diamine tetra‐acetic acid (EDTA) and related compounds in aqueous solution. . Circuit World , 29 (2) : 15 – 19 .
- Galbraith , M. , Minshu , M. , Davis , S. and Masten , S. 1992 . A second generation enhanced oxidation process for the destruction of waterborne organic contaminants. . Hazardous Industrial Wastes , 24 (34) : 411 – 420 .
- Goosey , M. 2000 . Environmental best practice in the PCB industry. . Circuit World , 26 (3) : 30 – 33 .
- Goosey , M. and Kellner , R. 1999 . The printed circuit board industry – an environmental best practice guide , London : PCIF (now part of Intellect) .
- Ion Exchange Principles and Applications . 2001 . published by Rohm and Haas Company, IER PA 002 GB‐12/2001
- Kou , W.‐S. 1999 . Destruction of toxic organics in water by an injection‐type down flow UV/O3 oxidation reactor. . Ozone: Science & Engineering , 21 (5) : 539 – 550 .
- Lankford , P. W and Eckenfelder Jr , W. W , eds. 1990 . Toxicity reduction in industrial effluents , New York : Van Nostrand Reinhold .
- Schulte , A. and Bayer , F. 1995 . H2O2/O3, H2O2/UV and H2O2/Fe2+ processes for the oxidation of hazardous waste. . Ozone: Science & Engineering , 17 : 119 – 134 .
- Skorska , M. B. and Davis , W. T. A critical evaluation of the ozone/UV technology for treatment of water contaminated with organic pollutants. Proceeding of 47th annual purdue university industrial waste conference , Vol. 47 , pp. 293 – 299 . Boca Raton, FL : CRC Press . May 1992, West Lafayette, IN
- Tucker , M. D. 1999 . Treatment of waste containing EDTA by chemical oxidation – an industrial view. . Waste Management , 19 (7) : 477 – 482 .
- Williams , M. Performance efficiency of a closed‐loop water recycling system. Proceedings of 43rd electronic components and technology conference , pp. 584 – 589 . Piscataway, NJ : IEEE. Issue 1–4 . June 1993, Orlando, FL
- Wright , N. , Potter , M. , Bains , N. and Goosey , M. 2003 . A new method for destroying organic contaminants and recycling waste water from printed circuit board manufacturing process effluent streams. . Circuit World , 29 (4) : 34 – 41 .
- Yue , P. L. 1992 . Degradation of organic pollutants by advanced oxidation. . The UV Trans IChemE , 70, Part B3 : 145 – 148 .