Abstract
The first part of the review paper dealt with general information and covered the specific sectors of paper/cardboard and aluminium cans. The present Part 2 should be read in conjunction with Part 1 and assesses the recovery and recycling of additional post-consumer waste sectors, i.e. glass beverage bottles, plastics, scrap metal and steel cans, end-of-life tyres, batteries and household hazardous waste. For each of the sub-sectors, the specific products are described, the recycling processes briefly evaluated and both environmental and research aspects summarised. The waste availability, the existing and further developing recovery and recycling technologies, and the economic rewards stress the importance of this waste management sector.
1. Introduction
The present Part 2 of the review paper on recovery and recycling of post-consumer waste continues with an assessment of the potential in additional product sectors. General background information, as well as the sectors of paper/cardboard and aluminium cans, has been dealt with in Part 1. Both parts should hence be read in conjunction.
2. Target post-consumer waste recovery and recycling sectors
2.1 Glass beverage bottles
2.1.1 General overview
Glass primarily consists of a large fraction of SiO2 (silica), the state of which is characterised by the absence of a crystalline structure and of a distinct melting point. Pure sand is the main feedstock for producing glass. Depending on the application and the production process, various auxiliary compounds are added, i.e. flux salts (Na2CO3, K2CO3, feldspar, fluorspar, etc.), components to improve hardness and chemical resistance (CaCO3, MgCO3, Al2O3, etc.), inorganic colourants, components that increase the refractive index (PbO) and others.
The core of the production process is formed by a melting process, followed by the design of the product (when the final glassy shape is obtained). The melting process is completely reversible, thus allowing the replacement of primary feedstock by waste glass (European Commission Citation2001).
Packaging is the most important use of glass, followed by flat glass. Glass containers represent approximately 2% of the solid waste volume and 7–8% on a weight basis. Removal of glass from the waste decreases the total amount of waste to be land filled and increases the overall efficiency of waste incineration.
Only container glass (common name for glass packaging products, like bottles and jars) is recycled in considerable amounts. Other types of glass (including window panes, light bulbs, mirrors, glassware and ovenware) are considered to be contaminants in the process. The use of post-consumer glass waste is widespread in the production of container glass, and also the production of insulating glass wool incorporates a major fraction of scrap glass. In the European Union, post-consumer glass represents on average over 50% (on a weight basis) of the feedstock for producing container glass. In some companies and for specific applications, this fraction can be as high as 90%. Recycling of glass is specifically interesting because it has the unique property of being 100% recyclable without any loss in quality. Moreover, virtually no waste or by-products are generated during the recycling process. An overview of container glass recycling in European countries is given in European Commission (Citation2001), Waste Online (Citation2009) and Jacobs et al. (Citation2003).
Glass production is very energy intensive, because it involves the melting and phase transition of the inorganic feedstock. The melting process consumes typically over 75% of the total energy consumed in the overall production process. The energy used for re-melting waste glass is lower than that for primary feedstock because the waste glass is already in its non-crystalline phase and, therefore, melts at lower temperatures (hence also increasing the furnace life). Moreover, the required quantity of waste glass is about 20% lower than the equivalent primary feedstock because part of the latter is released during the process, e.g. as CO2.
The production of glass includes the following unit operations (Jacobs et al. Citation2003):
-
storage and transport of the feedstock,
-
mixing and transport,
-
melting and purification,
-
welding,
-
conditioning,
-
coating or surface treatment and
-
handling and packaging.
For the melting process, various types of furnaces are used, depending on the application and the capacity. They are fired by natural gas, fuel oil or electricity.
2.1.2 Recycling process
Prior to melting, the waste glass is broken into small pieces (5–60 mm) and carefully washed. The next step consists of removing the impurities such as porcelain, earthenware, metal caps and cork. The presence of impurities in the melting furnace gives rise to production errors in the new bottles and damages the melting furnace. For the production of clear and white glass, it is of the utmost importance to remove all coloured flakes. The permanent dyes used for producing different coloured glass containers cannot be removed from the molten glass. The coloured glass flakes are detected visually at an early stage of the process (and are even manually removed). Only clean glass flakes are introduced in the melting furnace (Jacobs et al. Citation2003).
2.1.3 The melting furnaces
Regenerative furnaces use regenerative heat recovery systems. Burners are usually positioned in or below combustion air/waste gas ports. The heat in the waste gases is used to preheat the air prior to combustion, by passing the waste gases through a chamber containing refractory material, which absorbs the heat. The furnace only fires on one side at a time. After about 20 min, the firing is reversed and the combustion air is passed through the chamber previously heated by the waste gases. Preheat temperatures up to 1400°C may be attained leading to very high thermal efficiencies. In the cross-fired regenerative furnace, combustion ports and burners are positioned along the sides of the furnace, and the regenerator chambers are located on either side of the furnace. In the end-fired regenerative furnace, the principles of operation are the same; however, the two regenerative chambers are situated at one end of the furnace (European Commission Citation2001, Waste Online Citation2009).
Recuperative furnaces use heat exchangers (termed recuperators) for heat recovery, with continuous preheat of combustion air by the waste gases. Air preheat temperatures are limited to around 800°C even when using refractory steel in fabricating the exchangers. The specific melting capacity (per unit of smelter area) of recuperative furnaces is around 30% lower than that for a regenerative furnace. The burners are located along each side of the furnace, transverse to the flow of glass, and fire continuously from both sides. This type of furnace is primarily used where high flexibility of operation is required with minimum initial capital outlay, particularly where the scale of operation is too small to make the use of regenerators economically viable. It is more appropriate for small capacity installations although higher capacity furnaces (up to 400 tonnes per day) are not uncommon (European Commission Citation2001, Jacobs et al. Citation2003).
Oxy-fuel firing involves the replacement of the combustion air with oxygen (>90% purity). The elimination of the majority of the nitrogen from the combustion atmosphere reduces the volume of the waste gases by about two-thirds, resulting in furnace energy savings because it is not necessary to heat the atmospheric nitrogen to the temperature of the flames. The formation of thermal NOx is also greatly reduced. In general, oxy-fuel furnaces have the same basic design as unit smelters, with multiple lateral burners and a single waste gas exhaust port. However, furnaces designed for oxygen combustion do not utilise heat recovery systems to preheat the oxygen supply to the burners (European Commission Citation2001, Jacobs et al. Citation2003).
Electric furnaces consist of a refractory lined box supported by a steel frame, with electrodes inserted either from the side, the top or more usually from the bottom of the furnace. Energy for melting is provided by resistive heating as the current passes through the molten glass. The technique is commonly applied in small furnaces particularly for special glass. There is an upper size limit to the economic viability of electric furnaces, which depends on the cost of electricity compared with that of fossil fuels. The replacement of fossil fuels in the furnace eliminates the formation of combustion gases (European Commission Citation2001, Jacobs et al. Citation2003).
Combined fossil fuel and electric melting can take two forms: predominantly fossil fuel firing with electric boost, or predominantly electrical heating with a fossil fuel support. Electric boosting is a method of adding extra heat to a glass furnace by passing an electric current through electrodes in the bottom of the furnace.
Discontinuous batch smelters are used where smaller amounts of glass are required, particularly if the glass formulation changes regularly. In these instances, pot furnaces or day tanks are used to melt specific batches of raw material. Basically, a pot furnace consists of a lower section to preheat the combustion air and an upper section which holds the pots and serves as the melting chamber.
The post-melting steps are obviously strongly dependent on the final application of the product.
2.1.4 Environmental benefits, sustainability and R&D potential
It has been previously indicated that glass recycling has major energy and emission reduction benefits. The successful recycling of recovered container glass depends, however, on marketing a colour-sorted and contamination-free secondary feedstock, and this in a consistent way both towards quality and in time. Other markets for glass, therefore, need further investigation.
Typical examples are glassphalt using a percentage of crushed glass for road applications, foam glass for construction board and insulation, glass wool insulation, mixtures of glass and plastic polymers, agricultural soil conditioners to improve drainage, artificial sand, fibreglass, abrasives and other applications. Most of these applications have been proven, but further research is needed to enhance the more frequent application.
2.2 Plastics
2.2.1 Mechanical recycling
The environmentally and economically most favourable recycling technique is mechanical recycling. This method contributes for approximately 50% the overall recycling and is the second largest recovery technique for plastic waste after energy recovery. This technique directly recovers mainly clean plastics (production scraps) for reuse in the manufacturing of new plastic products thus being mostly suitable for pre-consumer plastic waste, and mostly applied directly in the companies that manufacture the polymers or in companies that use or transform them into the final product. The pre-consumer products generally consist of a unique feedstock, and are well identified, clean and homogeneous (Plastics Europe Citation2004, Al-Salem et al. Citation2009a).
In contrast, the post-consumer residues are a mixture of different plastics generally contaminated with dirt or other residues thus making recycling much more difficult, although feasible.
2.2.2 Feedstock recycling
Feedstock recycling comprises various advanced recycling technologies to turn solid polymeric wastes into high-value feedstock that can be used as raw materials in the production of new petrochemicals and plastics, without any deterioration in their quality and without any restriction regarding their application. Feedstock recycling has in theory a great potential to boost plastic waste recovery levels (Plastics Europe Citation2004). These processes involve the use of moderate to high temperatures to break the structural bonds of the polymer. They can be carried out in the absence of air (pyrolysis), in the presence of a high partial pressure of hydrogen (hydrocracking) or with a controlled amount of oxygen (gasification) (Al-Salem et al. Citation2010).
2.2.2.1 Pyrolysis
Pyrolysis is a decomposition process carried out in the absence of air. It is a flexible process and thus especially useful when dealing with heterogeneous wastes. Using pyrolysis, the plastic waste is converted into gases, a mixed liquid hydrocarbon fraction and a solid residue (char). Since hydrogen and oxygen are absent during the process, usually high molecular weight and hence high boiling fractions are obtained. These are further processed and refined, resulting in petrochemical feedstock such as naphtha.
When dealing with condensation polymers such as polyesters, polyamides, polyethylene terephthalate (PET) and polymethylmethacrylate, it is possible to use pyrolysis to transfer the plastic into their original synthesis monomers. This process is termed chemical recycling or depolymerisation. Depolymerisation of addition polymers is more difficult, although some studies have shown its feasibility (Scheirs Citation1998, Arena and Mastellone Citation2005, Al-Salem et al. Citation2010).
2.2.2.2 Hydrocracking
A second feedstock recycling process for plastic waste is known as hydrocracking. The plastic waste is exposed to a hydrogen atmosphere at pressures largely in excess of 100 bar. In this process, plastic waste is converted into fragments of hydrocarbons, in appearance and composition similar to crude oil (Scheirs Citation1998, Al-Salem et al. Citation2010).
In hydrocracking, heat fractures molecules into highly reactive free radicals (cracking) that are saturated with molecular hydrogen (hydrogenation) as they form. Cracking and hydrogenation are energetically complementary processes because the cracking reaction is endothermic while hydrogenation is exothermic. Thus, the surplus of heat that is produced can be handled by employing cold hydrogen as a quench for this reaction. The partial pressure of hydrogen must be high enough (about 200 bar) to suppress undesirable coking or re-polymerisation. A catalyst can possibly be used for enhancing the hydrocracking process.
The advantages of hydrogenation include: (i) high-value products are obtained, (ii) the synthetic crude oil can be used without any difficulty in refineries (better feedstock than produced by pyrolysis and gasification), (iii) troublesome hetero-atoms (i.e. Cl, N, O and S) are handled excellently and (iv) no toxic products such as dioxins are produced in or survive the process (Scheirs Citation1998, Arena and Mastellone Citation2005).
2.2.2.3 Gasification
Gasification or partial oxidation of plastic waste is performed with the controlled addition of oxygen. The process essentially oxidises the hydrocarbon feedstock in a controlled way. The primary product is a gaseous mixture of carbon monoxide and hydrogen, with minor percentages of gaseous hydrocarbons also formed. This gas mixture is known as syngas and can be used as a substitute for natural gas or in the chemical industry as feedstock for the production of numerous chemicals. The inorganic ash residue becomes bound in a glassy matrix and can be used as a component in concrete and mortar due to its high acid resistance (Scheirs Citation1998, Al-Salem et al. Citation2009a, Al-Salem et al. Citation2010).
2.2.2.4 Incineration with energy recovery
Energy recovery remains the most common recovery route for post-consumer plastics waste in western Europe with 22.5% of total collectable plastic waste dealt with in this way. Strict legislation has ensured that energy recovery is nowadays endorsed as an environmentally sound option (EU Directive 2000/76/EC). The heat content of plastics compares favourably with that of traditional fuels such as heating oil and natural gas, and plastics can thus be conveniently used in waste-to-energy processes, particularly if they cannot be mechanically recycled because of excessive contamination, separation difficulties or polymer property deterioration (Scheirs Citation1998).
The plastic waste can be burned as such or can be co-fired as a mixed combustible fraction for use in solid fuel fired boilers and power plants. The use of plastic waste as a fuel in cement and lime kilns is widely applied (utilised as a partial substitute for coal or coke).
The energy option is interesting when mechanical recycling is both economically and environmentally costly (Al-Salem et al. Citation2010).
Plastic waste incineration with energy recovery offers major advantages: (i) a reduction in the mass of waste by 90%, (ii) potentially harmful substances in the waste stream are destroyed, (iii) the inorganic fraction of the waste is essentially mineralised by incineration to an inert slag, which can be used as a raw material in the construction of roads, (iv) it is an ideal route for recycling mixed or heavily polluted polymeric substances and (v) it is the best and safest method for handling hazardous plastic waste such as medical plastic waste or hazardous-goods packaging.
Co-incineration of energy-rich plastic and low-calorific municipal solid waste has a positive effect on the incineration process where plastics are a benefit as a fuel that is low in ash and moisture, and an energy source for efficient destruction of pollutants.
Mono-combustion of plastic waste is often used to produce steam for heat and power generation. Modern combustion technologies provide a high degree of combustion control and automation, and appropriate sensors optimise air/fuel demands (Scheirs Citation1998, Al-Salem et al. Citation2010).
Whereas mono-combustion requires specially designed boilers, co-combustion can be performed in existing (multi-fuel) boilers. Burning 100% waste plastics in a grate-fired boiler can be hampered by an uneven distribution of air in the bed and the occurrence of hot-spots, both of which are the result of the inhomogeneous nature of the plastic feedstock. As a consequence, such waste incinerators require extensive flue gas cleaning systems to comply with the strict emission regulations. On the other hand, if the plastic waste is sorted and shredded and then evenly mixed with a primary fuel (e.g. coal or peat), the combustion is more efficient and no extensive flue gas cleaning is required. This is also true when mixing plastic waste with other municipal solid waste before incineration (Scheirs Citation1998).
2.2.3 Environmental benefits, sustainability and R&D potential
The development of technologies to convert plastic waste into its valuable constituent chemicals is of primary importance, methanolysis of e.g. PET being an example of a chemical route. Another possibility is to thermally ‘crack’ the carbonaceous materials into monomers that can be reused in plastic manufacturing. The latter process moreover enables the recovery of inorganic compounds such as cadmium, lead, glass or other valuable minerals from the pigments or strengtheners added to the plastic. These technologies already exist, but further research is needed to prove their economic viability. Recently, the pyrolysis of waste PET bottles has been investigated with emphasis on the production of a carbonaceous residue with properties of activated carbon (Brems et al. Citation2010). This route needs further investigation both to optimise the process yields and adsorption activity of the char residue, and to assess the applicability to other plastic wastes, with special emphasis on mixed plastics.
2.3 Scrap metal and steel cans
2.3.1 Steel
Steel is one of the most attractive, robust and sustainable materials in the world, that is 100% recyclable. It is, therefore, used in vehicles, buildings, medical applications and household equipment (EUROFER Citation2008).
EUROFER, the European Confederation of Iron and Steel Industries, represents the steel production industry in the European Union. Its objectives are the cooperation of national federations and companies in all matters that contribute to the development of the European steel industry, and the representation of common interests of its members towards third parties (EUROFER Citation2009).
APEAL, the Association of European Producers of Steel for Packaging, is a federation of four multinational producers of steel for packaging in Europe and represents 95% of the total European production of steel for packaging (APEAL Citation2009).
In 2003, approximately 8 million tonnes of crude steel were produced in the EU, which is 18% of the worldwide steel production: 3% of this production is used in the packaging steel consumption. From 1992 to 2001, the generation of steel packaging has increased by 22%, and an increasing part (nearly 33%) of steel packaging is recycled. The disposal rate on the other hand has decreased (Jacobsen et al. Citation2004).
Recycling of metal packages varies between countries. These metal packages include aluminium, but for most countries the use of aluminium in metal packaging is limited. Almost every country has increased the recycling rates over the past decade, with the highest rates found in Belgium and Germany (∼80%), whereas Portugal (∼22%) and Greece (∼10%) score the least. The EU average is, however, close to 50% (Jacobsen et al. Citation2004).
2.3.2 Steel for packaging
There are two types of steel for packaging: tinplate (steel coated on both sides with an ultra-thin layer of tin) and tin-free steel or ECCS (steel coated with chromium and chromium oxide). A new development comprises polymer-coated steels, which are tinplate or ECCS combined with polymer through film lamination or direct extrusion (APEAL Citation2009).
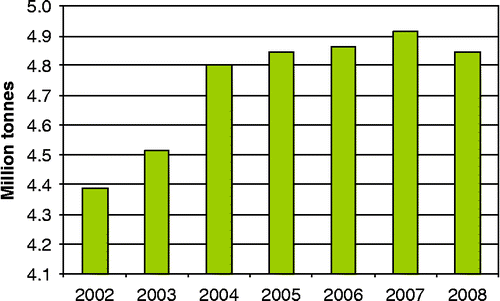
Annually 5 million tonnes of steel for packaging are produced in 12 different European countries as shown in Figure . Applications cover the entire field of industrial and consumer products, though more than half of the production is used in food and drinks industries. Steel for packaging is strong and durable, it preserves its content naturally (by shielding it from water, oxygen and light) and is 100% recyclable. It is a cost-effective way of packaging and enables high-speed operations (BCME Citation2009).
Figure 1 Production of steel for packaging in the European Union (adapted from APEAL Citation2009).
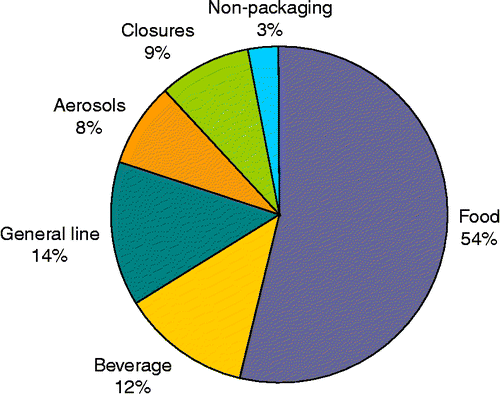
The majority of this annual production is used for packaging human and pet food. These and other applications are shown in Figure .
Figure 2 Steel for packaging applications (adapted from APEAL Citation2009).
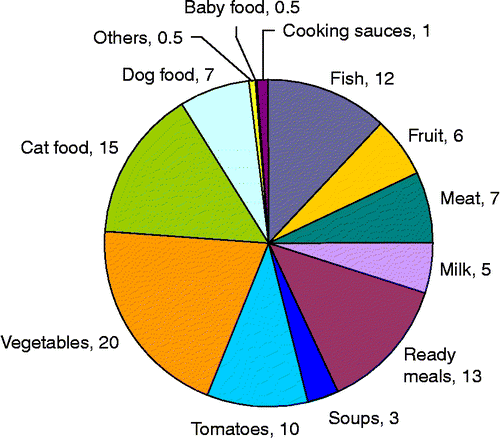
The applications of steel packaging can be divided into five major categories: food cans, beverage cans, aerosols, general line and closures. The nutritional and health value of canned foods has been largely underestimated for many years: essential elements (minerals, vitamins and flavour) in food with high nutritional value remain unaltered. Approximately 51% of steel for packaging was used for food cans in 2003. Also in the pet food segment, cans are the dominant form of packaging, and in 2002 they accounted for 10% of the tinplate consumption. Figure shows the share of steel cans consumption in different market sectors in 2001 (APEAL Citation2009).
Figure 3 Steel cans per market sector in 2001 (adapted from APEAL Citation2009).
2.3.3 Steel beverage cans
About 12% of steel packaging in Europe is used in the manufacture of beverage cans. In 2008, 49 billion units were sold, with the biggest markets in France, the UK and Spain. Sale numbers have almost continuously increased since 2005, when about 38 billion units were produced.
2.3.4 Manufacturing of steel for packaging
Raw steel is produced from raw materials (iron ore and coal) and from used steel scrap. Steel production always requires some scrap, the amount depending on the end-use and the circumstances. By using scrap steel, the industry can save valuable resources. Two steel production processes are well established: integrated and electrical steel making. In the electric arc process, 100% scrap is used. In the integrated process, steel is created from pig iron recovered from ore, coking coal and scrap, by the injection of oxygen. Pig iron contains excess levels of phosphorus and carbon, making it brittle as well as hard and, therefore, unsuitable for shaping processes. The carbon content, however, falls from 3 to 4% to around 0.02%, while phosphorus burns off, when the pig iron is oxidised. During the oxidation, a lot of heat is produced that can be used in the steel manufacturing process. Steel scraps play an important role in lowering the temperature required for steel production to around 1600°C. When production is complete, the steel may be alloyed for further use, although steel for packaging is a non-alloyed product (APEAL Citation2009).
2.3.5 Recycling steel
All steel packaging is totally recyclable, and all new steel packages today are partly made of recycled steel packaging scrap. Recycling rates already far exceed targets set by the European Directive on Packaging and Packaging Waste, and also complies with all essential requirements prescribed. Therefore, steel can be considered a renewable material (APEAL Citation2009).
The collection and recycling of used steel packages was first developed in northern Europe with a strong steel industry presence: France, the UK, Germany, the Netherlands and Belgium. The collection infrastructure at that time consisted mainly of direct magnetic extraction from collected domestic waste, and this is still the most appropriate and cost-efficient way. Today a multi-material kerbside collection scheme operates in most EU countries (APEAL Citation2009, BCME Citation2009).
2.3.6 Environmental benefits, sustainability and R&D potential
Steel is 100% recyclable and is Europe's most recycled packaging material. It can easily and at low cost be separated from other waste streams because of its magnetic properties: the sorting efficiency is close to 100%. Furthermore, steel cans are 35% lighter than those 20 years ago, so they meet the requirement of using less material, which saves resources. The lighter a product's packaging, the less energy it takes to transport, resulting in lower emissions and a significantly reduced carbon footprint. Twenty-four empty beverage cans, for example, equals one empty glass bottle in weight. For every tonne of recycled packaging steel, 2 tonnes of raw materials are saved. Recycling results in energy savings of 70% and water savings of 40% (APEAL Citation2009, BCME Citation2009). Steel packaging reduces resource usage. In the last decade, recycling has increased by 300%, so steel is the most recycled packaging material in Europe. Over the last 30 years, the weight of steel beverage cans has been reduced by 40%, from an average 75 to 45 g for a 33 cl. beverage can. Another way to cut down on raw material and energy consumption per tonne of crude steel produced is to increase recycling. The industry has dramatically improved the environmental profile of its products, by recycling more secondary steel packaging material: recycling in Europe has increased from approximately 700,000 tonnes/year in 1991 to over 2,200,000 tonnes/year in 2004. Steel is the most recycled material in the world (APEAL Citation2009).
Technological changes in the steelmaking process will likely continue to allow more scrap to be used per tonne of steel produced. In addition to energy savings, the Environmental Protection Agency (EPA) has identified other benefits such as a 90% saving on ore, and a reduction by approximately 85% in air pollution.
The industry has been working proactively and constantly to cut down on the impact of its activities on the environment. It has been able to meet the increasing demand from consumers, while reducing the environmental impact by 50%. This is shown by two actual parameters: CO2 emission and energy consumption (Figure ).
Figure 4 Decoupling growth and environmental impact for steel beverage cans (index 100 = year 1990 reference) [adapted from APEAL (Citation2009) and BCME (Citation2009)].
![Figure 4 Decoupling growth and environmental impact for steel beverage cans (index 100 = year 1990 reference) [adapted from APEAL (Citation2009) and BCME (Citation2009)].](/cms/asset/8c1079c1-9375-4016-ae1a-f79d2de4c1e9/tsue_a_507885_o_f0004g.gif)
Between 2001 and 2006, the global consumption of commercially available beverages grew by 3% annually. In 2007, the beverage can market in Europe increased by 10%. That year, over 50 billion cans were filled. The growth in can-filling was experienced in every single country. Western Europe and the USA are the largest markets in the world, although markets in Asia and Central- and South-America are emerging (BCME Citation2009).
2.4 End-of-life tyres
2.4.1 General overview
The annual production of waste tyres (partly worn and end-of-life tyres, ELT) totals 3.4 million tonnes, of which 3 million tonnes are either recycled or recovered. In 2007, the European recovery rate exceeded 91%, making Europe one of the most advanced regions in the recycling of tyres, compared to Japan (89%) or Canada (80%). As a result, a growing variety of new industrial and consumer products have provided the market with innovative and performing articles, responding to an increased demand and thus contributing to create a sustainable market for tyre-derived products (ETRA Citation2000, Al-Salem et al. Citation2009b).
A typical tyre is made up of four parts: (i) the tread, to ‘grip’ the road, (ii) the sidewall, to protect the sides of the tyre, (iii) the liner to prevent air loss and (iv) the carcass, which holds the layers together. The tyre is about 50% rubber by weight. Carbon black (as reinforcing agent), extender oil and the tyre cord in the carcass make up the rest. Cord components are mostly nylon, polyester and steel, which is most popular in radial tyres (Basel Convention Series Citation1999, ETRA Citation2000, Vredestein Citation2009).
The tread ‘grip’ is inversely related to elasticity. Since natural rubber has good elasticity but poor grip, no natural rubber is used in automobile tyre treads. Treads are blended styrene–butadiene rubber (SBR) and poly-butadiene (approx. ratio 1:3). Truck tyres do have natural rubber, between 60 and 100%, to avoid heat build-up and because grip is not so essential in heavy trucks. Aircraft tyres are 100% natural rubber. The carcass requires better flexibility and is a blend of SBR and at least 60% of natural rubber. Sidewalls contain 0–50% of natural rubber. The liner is made of butyl rubber because of its extreme impermeability to air. The carcass contains plies of rubberised fabric (Basel Convention Series Citation1999).
The composition of a typical steel-braced radial car tyre and radial truck tyre is shown in Table . The composition of tyre rubber compound is shown in Table .
Table 1 Approximate proportions of the components in tyres (Basel Convention Series Citation1999, ETRA Citation2000).
Table 2 Typical composition of tyre rubber compound (ETRA Citation2000).
Despite the well-known molecular chemical composition of natural and/or synthetic rubber, the production itself introduces various ‘contaminants’, which are important to be considered in the added-value tyre reclamation actions.
For natural rubber, we cite S-vulcanisation; vulcanisation accelerators, such as derived from benzothiazole and sulphenamides, ZnO and certain fatty acids, reinforcing agents such as carbon black and antidegradants (amines, phenols or phosphates). Synthetic rubber manufacturing applies either Ziegler–Natta catalysis (TiCl3 or TiCl4 in combination with an organometallic compound, Et3Al), peroxide initiators for radical polymerisation or acids for cationic polymerisation. Other metals can also be present, such as sodium (BuNa-S), copper, manganese and nickel (Basel Convention Series Citation1999, Al-Salem et al. Citation2009b, Vredestein Citation2009).
The most common method of waste tyre disposal (over 65%) is dumping them in open storage or in landfill sites, but this is a very unsustainable way (no recovery at all of valuable resources) and causes environmental problems (e.g. through leaching of spores). Therefore, in recent years, more focus is given to reuse or recycle scrap tyres (including its energetic valorisation) (Wu et al. Citation1997, Basel Convention Series Citation1999, Advanced Recycling Sciences Citation2009, Al-Salem et al. Citation2009b).
Scrap tyres are a potentially valuable source of secondary raw materials and energy. The overall calorific value of tyres is about 31–32 MJ/kg, which is about 20% more than that of a typical coal. The polymers and other hydrocarbons in tyres can have calorific values up to 42 MJ/kg.
A variety of treatment and disposal methods for scrap tyres exist and can be categorised into final disposal routes (including landfill and incineration without energy recovery) and methods which involve recycling. The latter can be subdivided into methods for material recovery and energy recovery. Material recovery includes retreading, granulation, rubber reclaim, whole-tyre uses and feedstock recovery. Energy recovery methods include incineration, gasification and pyrolysis (which can also be regarded as a process for feedstock recovery). An overview of the ELT recycling in Europe is presented in Table (ETRA Citation2000).
Table 3 ELT management in Europe (2007), for a total annual ELT of ∼3.4 M tonnes.
2.4.2 Processing of waste tyres
2.4.2.1 Retreading
Some scrap tyres can be reused for their original purpose after retreading. About 35% of scrap tyres are suitable for retreading. The retreading process involves removing the surface from the thread section or the whole outer surface of the casing, and applying a new tread. Although retreaded tyres have a poor reputation, there should not be any safety implications if the process is carried out correctly and they have to comply with strict standards.
2.4.2.2 Granulation
The production of rubber granules, or crumb, is well established, especially in the USA and Canada. The granules of waste tyres are mixed with other rubber wastes and are used for several purposes including brake linings, landscaping mulch, absorbent for oils, hazardous wastes and chemical wastes, carpet backing and sports surfaces. Another use for rubber crumb is as an additive to asphalt in road surfacing and is currently the most important disposal route for rubber crumb. Road surfaces incorporating rubber crumb were found to last about twice as long as conventional roads. For most applications, the rubber crumb needs to be free of metal and textile remains. The separation can be carried out in two ways: (i) the rubber layer is peeled off using a knife and (ii) the tyre is completely shredded and grinded, where the metal and textile fractions, as well as other impurities, are removed. The metal fraction still containing about 2–3% rubber is resent to the metal industry for re-melting and the textile fraction is combusted with energy recuperation (Advanced Recycling Series Citation2009, Al-Salem et al. Citation2009b).
Methods for treating the surface of rubber granules have been proposed, which make the rubber more compatible with virgin rubber. One example is the Air Products' process, which treats the surface of rubber crumb with an oxidising gas containing fluorine, which improves the adhesion and compatibility of the crumb with a liquid polymer which promotes cross-linking with other rubber.
The energy consumption of this treatment method is mainly situated in shredding and granulation. The required power is in the order of magnitude of 250–625 kW for a 5-tonne/h installation.
2.4.2.3 Reclaim/de-vulcanisation
The elastic properties of rubbers are a consequence of the (limited) cross-linking between various polymer chains by sulphur bonds. De-vulcanisation includes the delinking of the sulphur molecules from the rubber molecules, thereby facilitating the formation of new cross-linkages. The resulting material can be used to replace a certain amount of virgin rubber in new rubber products.
Various de-vulcanisation technologies have been developed. Thermal de-vulcanisation is most frequently used. In this process, the rubber is subjected to high temperature and pressure for 6–12 h. In mechanical de-vulcanisation, the required energy is provided by mechanical deformation of the rubber granules. A process using 2-butanol was patented by Goodyear in 1999 and is some form of solvent-based de-vulcanisation. The use of ultrasound and bacteria for de-vulcanisation is being developed.
The product is a powder which is further plasticised by the addition of fillers such as carbon black or clay. However, reclaimed rubber has difficulty in achieving a consistent quality meaning that the product can only be used in down-market applications such as doormats and garden furniture (ETRA Citation2000).
2.4.2.4 Incineration
The thermal recovery as a substitute fuel in cement kilns or in the blast furnaces is responsible for the fraction of energy recovery. With more stringent emission standards applied to this form of combustion of waste, and the recognised emissions of SO2, tars, polycyclic aromatic compounds and other hazardous compounds, these straight combustion processes are now severely assessed, and a need for novel and cleaner thermochemical treatment processes has emerged. The recovery of steel and zinc oxide is feasible during incineration. However, combustion is difficult to control because of the high rate of release of volatile materials from tyres. Combustion must, therefore, necessarily be a two-stage process (ETRA Citation2000).
2.4.2.5 Pyrolysis/gasification
These processes were previously explained under Section 2.2.
Pyrolysis technologies have been developed using a number of different types of furnaces, from rotary kiln and microwave to a thermolysis process using an oil bath. The solid, liquid and gaseous products of pyrolysis all have potential uses. The oily liquid product can be used as a fuel oil or as a chemical feedstock, while the gas can be used for process heating or to produce methanol. The solid products are carbon black, which can be used in new tyres or as a filter medium, and steel which can be used in the scrap metal industry (Wu et al. Citation1997).
By altering the reaction conditions, the products obtained can be controlled, and there are no large volumes of waste gases requiring expensive clean-up. Tyres lend themselves to pyrolysis because they are predominantly hydrocarbon, and so produce relatively consistent products. Problems have been encountered in the scale-up to commercial operation, but pyrolysis continues to attract attention.
The financial benefits stem from (i) the production of a high calorific fuel, which can be used in co-generation/combined Heat and Power to produce not only electricity but also useful heat, (ii) the possible production of hydrogen, which many see as an increasingly valuable resource and (iii) the qualification for Renewable Obligation Certificates (in the UK only).
The operational benefits result from (i) a significantly smaller plant size than the post-combustion flue gas treatment, (ii) less imposing needs for planning permission approval, (iii) all products (liquids, gas and char) can be used as energy source or feedstock for other petrochemical processes and (iv) the fact that no flue gas clean-up is needed prior to utilisation (Al-Salem et al. Citation2009b).
The environmental benefits evident are (i) displacing the use of fossil fuels, (ii) reducing the Green House Gas and CO2 emissions, (iii) enabling the production of a ‘green’ energy and (iv) contributing towards government waste targets and achieving Kyoto protocol commitments.
2.4.3 Environmental benefits, sustainability and R&D potential
A large amount of energy is stored in ELT and hence novel technologies focus on thermolysis and energy recovery. The (co-) combustion of ELTs as a substitute fuel in cement kilns or in the steelmaking industries is traditionally used for energy recovery, but is nowadays largely criticised because of the recognised emissions of polycyclic aromatic compounds, SO2, tars, etc. Therefore, the need for novel, more environmental friendly treatment processes is apparent.
Pyrolysis and gasification, to a lesser extent, are currently under scrutiny because of some beneficial properties. Both produce a high calorific fuel (liquid or gas) that can be used in combined heat and power applications for the generation of electricity and heat. Moreover, the process conditions can be altered to produce hydrogen gas. Operational benefits include a significant reduction in plant size and flue gas treatment (compared to combustion), less imposing needs for planning permission approval and all products (char, liquids and gas) can be used as energy source or feedstock in chemical industry (Al-Salem et al. Citation2009b). Environmental benefits include the displacement of fossil fuels, the reduction of greenhouse gas emissions and the production of energy.
2.5 Batteries
2.5.1 General overview
The disposal of batteries has become an ever-increasing topic of discussion over the past years, mostly due to their use of heavy metals such as mercury, lead and cadmium. In response to these concerns, both battery collection programmes and legislation controlling the production and disposal of batteries have become more stringent. Key questions are what a battery is and why has its disposal become such an issue? To answer these questions, it is best to distinguish between lead-acid automotive-type batteries and typical household-type batteries that are used in consumer items such as flashlights, radios and watches: the means of handling and disposal of these two types of batteries are quite different.
2.5.2 Household-type batteries
2.5.2.1 Types of batteries
Batteries are available in various types, and a distinction should be made between non-rechargeable (primary) and rechargeable (secondary) batteries. Non-rechargeables contribute up to 90% of the total battery consumption in Europe. Large variations in composition are possible among the different producers.
The most representative rechargeable battery is the Ni/Cd cell. In recent years, lithium batteries have generally been used in portable electronic devices such as cell phones, digital cameras and laptops.
2.5.2.2 Separation
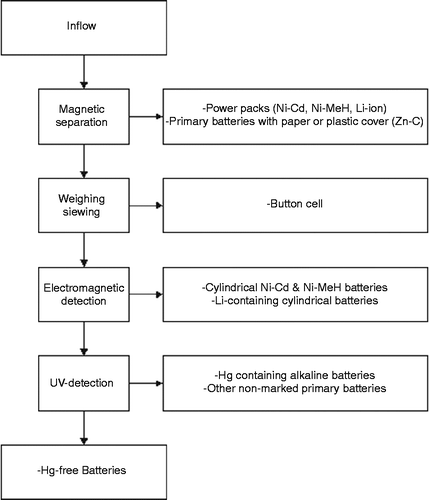
Since every type of battery has its own composition, several techniques need to be used for treating this waste fraction. When more types are collected simultaneously, a separation method is usually necessary. A typical process scheme including the adapted separation methods is presented in Figure .
By magnetic separation, batteries having a plastic or paper cover are separated. This fraction consists typically of ‘Power Packs’ and part of the Zn–C batteries. The latter, having plastic or paper covers, are mostly produced in Asia, the Middle-East and eastern Europe and are primarily used for low-profile applications. They potentially contain mercury. Button cells are separated by means of weighing, or alternatively by sieving. Based on electromagnetic detection, the Zn–C and alkaline fraction is separated from the other fractions. The automatic sorting is in most cases followed by a manual post-sorting of some fractions (European Commission Citation2001).
2.5.2.3 Pyro-metallurgical treatment of primary batteries
Primary batteries contain a large fraction of Fe, Mn and Zn and can hence be added as auxiliary feedstock in typical pyro-metallurgical processes in ferro and non-ferro industries.
The introduction of waste batteries in an Electric Arc Furnace is feasible without the necessity of process changes. The batteries are mixed with the iron scrap without any pretreatment. A maximum dosage of 3% batteries on a weight basis is feasible. Iron, nickel and part of the manganese are recovered in the produced steel, while the remaining part of the manganese concentrates in the slag. Low-melt metals such as Zn, Hg and Cd vaporise in the furnace and solidify again in the cooled flue gases where they concentrate in the fly ashes. Hg partially remains in the gas phase. The fly ashes are filtered from the flue gas, while the gas phase Hg is mostly emitted, unless activated carbon adsorption is used for its abatement. Carbon and organic fractions (paper and plastic) are consumed as a reducing agent or are energetically valorised (support fuel). Other non-metallic components show a different behaviour: NH3 is transformed into gaseous NOx and chlorides/alkali concentrate in the fly ashes.
Zn and Pb are typically recovered from the fly ashes of an Electric Arc Furnace in a Waelz furnace. In a first step, the fly ashes are mixed with sand and furnace slag. The Zn and Pb vaporise at a temperature of 1200°C and are oxidised in the presence of air. After cooling, the ZnO/PbO forms a particulate dust, which is subsequently separated from the waste air by an electrostatic precipitator. The remainder forms a relatively inert slag together with the sand, which can be reused in the building industry. The recovered metal oxide dust can be further treated to remove chlorides, fluorides and alkali and is further processed in the non-ferro industry for the production of Zn and Pb (European Commission Citation2001).
The input of batteries in a typical blast furnace is limited to a maximum concentration of 1–2%. The behaviour of the various compounds in a blast furnace is comparable to that in an electric arc furnace.
2.5.2.4 Pyrolytic treatment of single-use batteries
A pyrolytic process for metal recovery from spent batteries has been developed by Citron (France) under the brand name ‘oxyreducer’. In this process, a stack of batteries is introduced in a cylindrical furnace and is subsequently heated in a reducing atmosphere to a temperature of 1200–1400°C. All organic compounds (paper and plastics) are thermally decomposed and the metal oxides are reduced to their metallic form. This leads to a distillation of low-melt metals (Zn, Hg, Cd and Pb) which are exhausted with the gas phase (European Commission Citation2001, BEBAT Citation2009).
The exhaust air contains not only vaporised metals, but also a large amount of CO. Post-combustion is needed and metals are re-oxidised. ZnO, together with traces of PbO and CdO, is removed from the gas stream by gravitational precipitation at a temperature of 1150°C. The Hg only condensates at lower temperatures and is removed by quenching. The solid residue in the furnace contains mostly iron and manganese and is sold to the ferro industry for reuse.
2.5.2.5 Mercury distillation of button cells
Since 2000, the production of batteries containing a mercury concentration in extent of 5 ppm is prohibited by the European legislation (Directive 98/101/EC). This concentration equals the normal presence of Hg in technical zinc. However, an exception is made for button cells, because Hg-free alternatives are not yet available.
The presence of a considerable amount of Hg (about 20% on average) requires a specific pretreatment of the button cells before treatment. In practice, the button cells are introduced as a whole in the distillation unit and just undergo distillation for the removal of Hg. The Hg vapours (at − 6°C) are condensed and the condensate is sold to the Hg industry. The residue is mostly still disposed of by land fill (European Commission Citation2001, BEBAT Citation2009).
2.5.2.6 Treatment of rechargeable batteries: NiCd and NiMeH
Although NiCd batteries are gradually being replaced by NiMeH batteries, they are still frequently used rechargeable batteries because of their low production cost. The presence of Cd requires a specific treatment, where volatile Cd is distilled. The residue contains mostly Ni and Fe and is a feedstock for the production of inox. The metallic Cd is mainly being reused in battery production itself (Dijkmans and Vercaemst Citation2001).
2.5.2.7 Treatment of rechargeable batteries: Li-ion
The treatment of Li-ion batteries requires specific operating conditions because the presence of traces of water leads to an uncontrollable hydrolysis reaction during which H2 is being formed (an unwanted and explosive reaction). The LiCoO2 salt is moreover highly unstable at temperatures above 80°C. Treatment technologies, therefore, mainly focus on dismantling of the battery and the recovery of high-value components, i.e. Co and the spent electrolyte (Dijkmans and Vercaemst Citation2001, BEBAT Citation2009).
The Toxco-process (US) is based on submerging the battery in liquid nitrogen. The batteries are subsequently cryogenically shredded and the various components are separated. Through mixing the electrode material with water, LiOH and H2 gas are produced in a controllable way. The LiOH is further reformed to the insoluble Li-carbonate and recovered through filtration from the aqueous mixture. Also, the Co is recovered from the liquid (European Commission Citation2001).
The AEA-process (UK) shreds the batteries in a dry, inert atmosphere. The electrolyte is extracted from the electrodes and recovered through evaporation of the solvent in vacuum. The electrode material is separated from the support material (Cu, Al, steel and plastics) by dissolution of the polyvinylidene fluoride (PVDF) binder. The support material is recovered and the electrode particles are separated from the solvent through filtration. The electrode material, consisting of LiCoO2 (cathode) and C (anode), is further treated by electrochemical reduction in the three-valent Co (European Commission Citation2001).
The VAL'EAS procedure has been developed by Umicore (Angleur, Belgium). The procedure is recognised to represent the best available technique for recycling Li-ion batteries which received the ‘Gold Award’ of the European Environmental Press Award in 2004. The collected Li-ion batteries are introduced in a melting furnace without pretreatment. The gas purification is equipped with a plasma generator for preventing the emission of dioxins and furans. Through controlled melting, an inert and pure slag is produced which can be reused in building industry or in concrete production. Co and Ni are refined for obtaining pure substances. The Co is subsequently transformed to LiCoO2 for reuse in the production of new Li-ion batteries. The recovered Ni can optionally be transformed in Ni(OH)2 for the production of NiMeH batteries (European Commission Citation2001).
2.5.3 Lead acid battery recycling
The high concentrations of lead and lead sulphate make this type of battery specifically suitable for material recycling through pyro-metallurgic treatment in the non-ferro industry. The sulphuric acid is removed from the batteries prior to the pyro-metallurgical treatment and batteries are shredded using e.g. a hammer mill. The broken battery pieces are then placed into a vat, where the lead and heavy materials sink and the plastic floats are separated by dense media flotation. At this point, the polypropylene pieces are scooped away and the liquids are drawn off, leaving the lead and heavy metals as heavies. In some applications, the batteries are fed as a whole (after the removal of sulphuric acid; European Commission Citation2001).
2.5.3.1 Recycling of lead fraction
The lead fraction is re-melted before reuse. The lead is mixed with coke and introduced into a blast furnace. Air is introduced in the bottom part of the furnace, resulting in a partial combustion of the coke and the production of CO. The CO is used as a reducing agent to transform the lead oxides into metallic lead. The metal and slag are continuously or batchwise removed from the furnace.
Other types of furnaces are also being used. Examples are the ISA melting furnace in which the fossil fuels and air are introduced in the melt by a submersed lance, a rotating drum furnace which is in fact a cylindrical furnace placed under a small angle, a rotating furnace which differentiates from the rotating drum furnace because it is used in batch. In an electric furnace, electricity is being used instead of fossil fuels for the heating of the lead mixture. Submersed carbon electrodes are being used as resistances and act as reducing agent for the lead oxides (hence they are consumed during the process). Most electrical furnaces work in a continuous way (Dijkmans and Vercaemst Citation2001, European Commission Citation2001).
2.5.3.2 Recycling of the lead sulphate
Typically, the lead sulphate is being desulphurised through reaction with Na2CO3 or NaOH, after which it is also introduced in the melting furnace. A direct introduction into the furnace is also possible, but since the introduced sulphur will volatilise to the off gases, a thorough flue gas desulphurisation needs to be present. Methods for incorporating the sulphur in the slag have also been developed, but this leads to an increased amount of formed slag.
2.5.3.3 Treatment of the sulphuric acid
The recovered sulphuric acid is reused in new batteries in a mix with new and pure sulphuric acid. It is also being used as a reagent for physic-chemical surface treatment of metals and for the production of metal salts (metal sulphates). Sometimes the acid is processed and converted to sodium sulphate, an odourless white powder used in laundry detergent, glass and textile manufacturing.
2.5.3.4 Treatment of the plastic fraction
The plastic fraction is milled and washed, and can subsequently be used for material recovery. It can also be introduced as a supplementary fuel and as a reducing agent during lead recovery in the blast furnace (Dijkmans and Vercaemst Citation2001).
2.5.4 Environmental benefits, sustainability and R&D potential
In the last couple of years, a lot of research has been carried out to improve the properties of batteries, mainly driven by the increased use of portable electronic devices such as laptops and mobile phones. These devices consume a lot of power and their autonomy should be as high as possible, with rechargeable lithium batteries being very popular because of their high energy density. Research has been focussing on extending the life-time of these batteries (improving the number of loading cycles before battery failure).
In the transportation sector, improved high-power batteries are needed in hybrid electric vehicles and electrically powered vehicles. Most car manufacturers now enter the market using Ni/MH batteries that do not have the optimum characteristics. The development of high-power lithium batteries offers the opportunity to expand the market potential of hybrid electric vehicles because they are lightweight, have a higher efficiency and a longer life.
Many research groups have been pursuing the development of Lithium-oxygen batteries, using lightweight porous carbon electrodes and oxygen drawn from ambient air to replace heavy solid compounds used in Li-ion batteries. Other researches are focused on the development of new electrode materials ranging from alloys of gold and platinum to metallic oxides and less expensive alternatives.
Most importantly, material improvements in batteries are investigated including the substitution of mercury and other hazardous components by non-hazardous substitute materials. A hot topic is the use of carbon nanotubes to store and release electrical energy. Incorporation of these materials will enable the production of batteries that can be recharged quickly and numerous times.
2.6 Household hazardous waste
2.6.1 Definition of household hazardous waste
Household hazardous waste (HHW) arose as an issue in waste management in the 1980s. Various plans, programmes and facilities were developed to keep HHW out of the solid waste stream and to provide options for properly managing these wastes. Without HHW management, most of it would be improperly disposed of in the refuse, down the drain or in the soil.
HHW is defined by EPA as the leftover household products that contain corrosive, toxic, ignitable or reactive ingredients. HHW is a term used to describe hazardous waste of domestic origin, but it can also be applied to municipal hazardous waste. The UK-based National Household Hazardous Waste Forum NHHWF describes HHW as ‘any material discarded by a household which is difficult to dispose of, or which puts human health or the environment at risk because of its chemical or biological nature’. Products such as paints, cleaners, oils, batteries and pesticides. contain potentially hazardous ingredients and, therefore, require special care when disposed of. Improper disposal includes pouring HHW down the drain or on the ground, putting it out with the trash, etc. Such disposal methods can cause environmental pollution and threats for human health (EPA Citation1988, Citation2009; Slack et al. Citation2009).
European legislation currently lacks precise definitions for HHW, however, the term ‘dangerous substance’ has been defined as ‘explosive, oxidising, easily flammable, flammable, toxic, harmful, irritant, dangerous for the environment, mutagenic, toxic for reproduction, dangerous for the environment, corrosive, carcinogenic, etc.’ These dangerous substances can only be placed on the market if the labelling indicates the name and origin of the substance, the appropriate danger symbol and risks arising from such dangers. Examples of hazardous substances are solvents, acids, pesticides, paints, inks, adhesives, resins, detergents, batteries, etc. A possible definition of HHW could be ‘waste that could potentially increase the hazardous properties of municipal solid waste when landfilled, incinerated or composted’ (European Commission Citation2002).
The list of HHW differs from one country to another, which affects the comparability of quantities of HHW collected. Commonly identified categories of HHW in European member states are paints, varnishes, inks and glues; batteries and accumulators; used oils; fluorescent tubes and other equipment containing mercury; pesticides; medicines and aerosols. Annually, approximately 200 million tonnes of municipal solid waste is generated in the EU. Only 1% (by weight) of this amount is represented by HHW. There are, however, great differences between countries, due to different consumption patterns but also due to the different definitions applied as mentioned above.
Reliable detailed records on quantities collected are only available for the Benelux countries. Statistics for Belgium reveal that the average quantity of HHW produced in 2000 amounts to 1.7 kg/person and contains 27% paints (including glue, ink and resin), 34% oils (mineral and vegetable), 4% solvents, 1% cleaning products, 2% fluorescent tubes and other Hg- containing waste, 1% pesticides and fertilisers, 16% batteries, 1% pharmaceuticals, 1% acids, 1% alkalis, 6% packaging of hazardous products, 1% aerosols, 1% X-rays and photo chemicals, 1% flame extinguishers and 3% unspecified. Apart from batteries and waste oil, the largest proportion of HHW collected includes paint residues (21–38%). The total annual rate of collection varies between 1.3 and 3.5 kg HHW per person, which represents a collection rate varying between 56 and 70% of estimated HHW quantities arising (European Commission Citation2002).
A three-tier classification structure is adopted to identify toxicity levels in HHW. Hazardous household products are hereby divided into three categories (European Commission Citation2002):
-
Tier 1 products have a significant hazardous potential for the solid waste streams,
-
Tier 2 products are now banned or strictly controlled within the EU but adequate disposal practices might still not be in place and
-
Tier 3 products are products for which concerns have been expressed about their potential negative impacts on health and environment but for which there is a lack of information to confirm their hazardous potential.
2.6.2 HHW recycling
In the USA, recycling regulations were developed by EPA to promote the reuse and reclamation of useful materials in a manner that is safe and protective of human health and the environment. Reuse, recycling and reclamation can avoid environmental hazards (by reducing air, water and soil pollution), protect scarce natural resources, provide economic benefits and reduce the nation's reliance on raw materials and energy (EPA Citation2009).
The recycling potential is determined by the product concerned. A few important examples include paint, oil, solvents and batteries.
Paint can be divided into two basic categories with different recycling and treatment alternatives: latex (water-based) and oil-based paint. Latex paint recycling is a common practice, usually by low-grade recycling: the paint is collected and reprocessed by a paint manufacturer into a recycled product. This paint is generally used for graffiti control and other outside applications. High-grade recycling is also possible and involves bulking the paint for reprocessing into a saleable product. Some latex paint, however, is not recyclable because of the poor quality or hazardous constituents such as mercury, lead or other heavy metals. Lead was used as a pigment in paint until it was banned in 1973. Recycling oil-based paint is not commonly practiced. It has, however, shown to be technically feasible, and may provide greater alternatives in the future (EPA Citation1988).
Recycling used motor oil is probably the oldest and most common HHW recycling practice. It is collected at automotive service stations, recycling centres, transfer stations and landfills. More complete collection, however, is still a big challenge: the US EPA has estimated that about half of the used oil is put in the garbage, poured on the ground or down sewers. Collected used oil can be readily recycled: it is bulked and can be refined into lubricating oil many times with only minor losses. Re-refining used oil takes only one-third of the energy required for refining crude oil. Used oil can, moreover, also be processed into fuel oil. Used oil will normally contain some contaminants from its use in an engine (water, gasoline, sediments and heavy metals), but it is important to guard against unusual contaminants such as PCBs and chlorinated solvents (EPA Citation1988).
Solvent recycling is a common practice for industrial hazardous wastes and can also be employed for HHW. The solvents are bulked for storage and shipment, with separate bulks for chlorinated and non-chlorinated solvents as well as polar and non-polar solvents. The bulked solvents are processed, and they may also be used as supplementary fuel.
For the recovery and recycling of batteries, the reader is referred to Section 2.5.
2.6.3 Environmental impact of HHW, sustainability and R&D potential
The potential impacts of HHW in landfills can be evaluated by examining the leachate at these sites. The US EPA has measured the concentrations of various hazardous organic constituents in leachate from 53 landfills, and these data are shown in Table .
Table 4 Concentrations of organic constituents in leachate from landfills in the USA (adapted from EPA Citation1988).
Methylene chloride was found at the highest concentrations (220,000 parts per billion) and at the greatest number of sites (60%) (EPA Citation1988).
Research in the field of HHW focuses upon enhancing the recycling of individually collected HHW components, such as waste oil (upgrading), solvents (separation), grease and fat (pyrolysis to bio-diesel additive). These topics require enhanced research to better add value to HHW.
3. Conclusions
Recycling of post-consumer waste materials is gaining increased interest due to public awareness, legislative promotion and imposition, economic benefits and appropriate technologies being available.
The present Part 2 of the review paper dealt with recycling the reuse potential, recycling technologies used, problems and solutions for target post-consumer waste component, i.e. glass beverage bottles, scrap metal and steel cans, scrap tyres, batteries and HHW. The assessment of the waste availability, the existing and currently developed recovery and recycling technologies, and the economically rewarding markets while recycling, stress the technical, economic and environmental importance of this waste management sector.
Together with Part 1 of the paper, a review of the potential and current practice is given for the most important post-consumer waste component.
References
- Advanced Recycling Sciences, 2009. Available from: www.arsciences.com
- Al-Salem , S.M. , Lettieri , P. and Baeyens , J. 2009a . Recycling and recovery routes of plastic solid waste (PSW): a review . Waste Management , 29 ( 10 ) : 2625 – 2643 .
- Al-Salem , S.M. , Lettieri , P. and Baeyens , J. 2009b . Kinetics and product distribution of end of life tyres (ELTs) pyrolysis: a novel approach to poly-isoprene and SBR thermal cracking . Journal of Hazardous Materials , 172 ( 2–3 ) : 1690 – 1694 .
- Al-Salem , S.M. , Lettieri , P. and Baeyens , J. 2010 . The valorization of plastic solid waste (PSW) by primary to quaternary routes: from re-use to energy and chemicals . Progress in Energy and Combustion Science , 36 ( 1 ) : 103 – 129 .
- APEAL, 2009. Association of European Producers of Steel for Packaging. Steel: the sustainable packaging solution
- Arena , U. and Mastellone , M.L. 2005 . “ Fluidized bed pyrolysis of plastic wastes ” . In Feedstock recycling and pyrolysis of plastic wastes , Edited by: Scheirs , J. and Kamimnsky , W. Hoboken, NJ : Wiley .
- Basel Convention Series, 1999. Technical guideline on the identification and management of used tyres, submitted by industry for consideration by the Technical Working Group of the Basel Convention on the transboundary movement of hazardous waste and their disposal, No. 00/03
- BCME, 2009. Beverage Can Makers Europe. Available from: www.bcme.org
- BEBAT, 2009. Available from: www.bebat.be
- Brems , A. 2010 . Polymeric cracking of waste polyethylene-terephthalate to chemicals and energy . Journal of the Air and Waste Management Association , submitted
- Dijkmans , R. and Vercaemst , P. 2001 . VITO, BBT voor de non-ferro nijverheid . EMIS , (in Dutch)
- EPA, 1988. US Environmental Protection Agency. Report to Congress on Waste Disposal in the US, EPA/530-SW-88-011B, Washington DC, October
- EPA, 2009. US Environmental Protection Agency. Available from: www.epa.gov
- ETRA . 2000 . Tyre recycling after 2000: status and options . European Tyre Recycling Association , Available from: www.etra-www.org
- EUROFER, 2008. European Confederation of Iron and Steel Industries. Annual report
- EUROFER, 2009. European Confederation of Iron and Steel Industries. Available from: www.eurofer.org
- European Commission, 2001. Integrated Pollution Prevention and Control (IPPC). Reference document on best available techniques in the glass manufacturing industry, December
- European Commission – Directorate – General Environment, 2002. Study on hazardous household waste HHW with a main emphasis on hazardous household chemicals HHC. Final report
- Jacobs , A. , Wellens , B. and Dijkmans , R. 2003 . Gids afvalverwerkingstechnieken, VITO , 2nd edn (in Dutch)
- Jacobsen , H. 2004 . Inventory of existing information on recycling of selected waste materials , Copenhagen, Denmark : European Environment Agency EEA, European Topic Centre on Waste and Material Flows .
- Plastics Europe . 2004 . An analysis of plastics consumption and recovery in Europe (2002–2003) , Brussels, Belgium : Association of Plastic Manufacturers in Europe .
- Scheirs , J. 1998 . Polymer recycling , 591 p New York : Wiley .
- Slack , R.J. , Gronow , J.R. and Voulvoulis , N. 2009 . The management of household hazardous waste in the United Kingdom . Journal of Environmental Management , 90 : 36 – 42 .
- Vredestein, 2009. Rubber recycling. Available from: www.vredestein-rr.com
- Waste Online, 2009. Glass recycling information sheet. Available from: http://www.wasteonline.org.uk/resources/InformationSheets/Glass.htm
- Wu , S.Y. , Su , M.F. and Baeyens , J. 1997 . The fluidized bed pyrolysis of shredded tyres: the influence of carbon particles, humidity, and temperature on the hydrodynamics . Powder Technology , 93 : 283 – 290 .