Abstract
The world is facing an energy and climate crisis. Globally, the energy sector emits 26 billion tonnes of CO2 each year, and electricity production alone accounts for 41% of emissions (European Wind Energy Association. 2008. Pure Power: Wind Energy Scenarios up to 2030. European Wind Energy Association. p. 6). Currently, reducing CO2 emissions and curbing climate change have become global priorities, and we only have a narrow window of time left in which to act. The new type of refrigerator can offer an immediate and concrete solution to the many energy and climate challenges that we are facing. This is an account of a new type of refrigerator and its cycle for use in the refrigeration industry. For its cycle, the working substance is air or other gases; its cycle is very similar to that of the reverse Brayton cycle (Hou, Y., Zhao, H. L., Chen, C. Z., and Xiong, L. Y. 2006. “Developments in Reverse Brayton Cycle Cryocooler in China.” Cryogenics 46 (5): 403–407). In the cycle of the new type of refrigerator, the isochoric exothermic process and the isothermal exothermic compression process replace the isobaric exothermic compression process of the reverse Brayton cycle. The new type of refrigerator can produce a net electrical energy, and it is not a perpetual motion machine, because the environmental enthalpy and atmospheric pressure can be utilized as the external energy sources, which cannot be performed by any other conventional refrigerators; therefore, the reliability and significance of this new type of refrigerator is self-evident.
Introduction
| atm | = | standard atmospheric pressure |
| E | = | energy ( |
| = | adiabatic exponent of the air ( | |
| P | = | pressure (atm) |
| = | atmospheric pressure (atm) | |
| = | pressure of the outlet of the turbo expander and of the inlet of air cylinder #1 (atm) | |
| = | pressure of the outlet of air cylinder #1 and of the inlet of the compressor (atm) | |
| = | pressure of the outlet of the compressor (atm) | |
| = | air pressure which is after the isochoric endothermic process (atm) | |
| = | total heat generated during the cycle ( | |
| = | heat of the isobaric endothermic process b → c ( | |
| = | heat of the isochoric exothermic process d → e ( | |
| = | heat of the isothermal exothermic compression process e → a ( | |
| = | heat which extracts from air cylinder #2 or air cylinder #3 ( | |
| = | gas constant of the air ( | |
| = | entropy ( | |
| = | temperature (K) | |
| = | air temperature which is after the adiabatic isentropic expansion process (K) | |
| = | temperature of the environment (K) | |
| = | temperature of the outlet of the turbo expander and of the inlet of air cylinder #1 (K) | |
| = | temperature of the outlet of air cylinder #1 and of the environment (K) | |
| = | temperature of the outlet of the compressor and of the inlets of air cylinder #2 and air cylinder #3 (K) | |
| = | total work of the cycle ( | |
| = | work of the adiabatic isentropic expansion process ( | |
| = | actual work that the new type of refrigerator can produce ( | |
| = | work of the adiabatic isentropic process a → b ( | |
| = | work of the isobaric endothermic process b → c ( | |
| = | work of the conventional heat engine ( | |
| = | work of the adiabatic isentropic process c → d ( | |
| = | work of the isothermal exothermic compression process e → a ( | |
| = | work that the heat of the isochoric exothermic process d → e can transform theoretically | |
| = | work that the atmospheric pressure performs on the piston ( | |
| = | total work that the new type of refrigerator can produce theoretically ( |
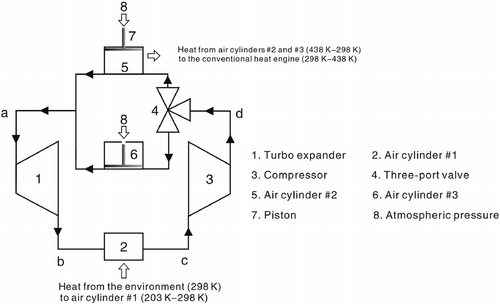
This new type of refrigerator, shown schematically in Figure , has the following main parts: a turbo expander, a generator, three air cylinders (#1, #2 and #3), a three-port valve, a compressor and a conventional heat engine. Air cylinder #1 is similar to the evaporator in a conventional refrigerator (Cengel and Boles Citation2005); air cylinder #2 and air cylinder #3 are similar to the cylinders of the Newcomen atmospheric engine (CitationLira, n.d.).
Its refrigerant is air. The other common refrigerants used in this new type of refrigerator are ammonia, sulphur dioxide and non-halogenated hydrocarbons such as propane.
The relevant cycle includes the following processes: the adiabatic isentropic expansion process, the isobaric endothermic expansion process, the adiabatic isentropic compression process, the isochoric exothermic process and the isothermal exothermic compression process. The new type of refrigerator can extract heat from the environment during the isobaric endothermic expansion process and release heat to the conventional heat engine during the isochoric exothermic process and the isothermal exothermic compression process. Atmospheric pressure can be utilized as the external energy source during the isothermal exothermic compression process. This means that the new type of refrigerator can produce a net electrical energy, whereas other conventional refrigerators must consume electrical energy.
Structure and cycle of the new type of refrigerator
The working cycle of the air comprises the following stages: the turbo expander → the air cylinder #1 → the compressor → the three-port valve → air cylinder #2 and air cylinder #3 → the turbo expander.
A part of air cylinder #1 is the ice chest of the new type of refrigerator; air cylinder #2 and air cylinder #3 are the hot reservoirs of the conventional heat engine.
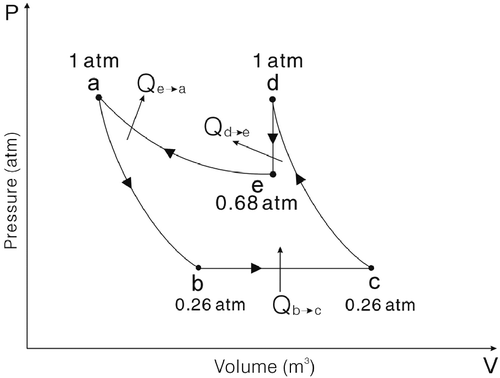
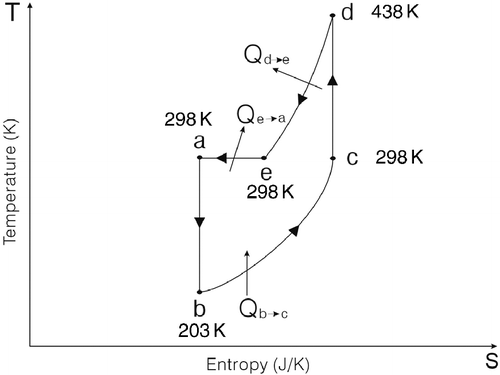
In Figures , ‘a’ depicts the inlet of the turbo expander and the outlets of air cylinder #2 and air cylinder #3; ‘b’ depicts the outlet of the turbo expander and the inlet of air cylinder #1; ‘c’ depicts the outlet of air cylinder #1 and the inlet of the compressor; ‘d’ depicts the outlet of the compressor and the inlets of air cylinder #2 and air cylinder #3; ‘e’ depicts the thermodynamic state after the isochoric exothermic process.
The cycle of the new type of refrigerator is very similar to that of the reverse Brayton cycle.
In the cycle of the new type of refrigerator, the isochoric exothermic process d → e and the isothermal exothermic compression process e → a replace the isobaric exothermic compression process of the reverse Brayton cycle.
a → b depicts that the air flows through the turbo expander, which is an adiabatic isentropic expansion process. The air temperature drops and the air starts to expand, so the kinetic energy of the air is transformed into the kinetic energy of the turbine.
b → c depicts that the air flows through the air cylinder #1, which is an isobaric endothermic expansion process, , where
is the pressure of the outlet of the turbo expander and of the inlet of air cylinder #1, and
is the inside pressure of the outlet of air cylinder #1 and of the inlet of the compressor. Because the air temperature of air cylinder #1 is lower than that of the environment,
, where
is the temperature of the outlet of the turbo expander and of the inlet of air cylinder #1. Air cylinder #1 extracts heat from the environment until its temperature is equal to that of the environment,
, where
is the temperature of the environment and
is the temperature of the outlet of air cylinder #1.
In air cylinder #1, due to the work of the compressor, the inside pressure of air cylinder #1 is invariable.
c → d depicts that the air flows through the compressor, which is an adiabatic isentropic compression process. The compressor causes the air to be compressed until its pressure is equal to the atmospheric pressure ;
, where
is the pressure of the outlet of the compressor. The air temperature of the outlet of the compressor is greater than that of the environment,
, where
is the temperature of the outlet of the compressor and of the inlets of air cylinder #2 and air cylinder #3.
A three-port valve is installed at the outlet of the compressor, and it is connected to the inlet of air cylinder #2 and also with that of air cylinder #3.
When the airflow leaves the compressor it enters the air cylinder #2 or the air cylinder #3.
The inlet and outlet of air cylinder #2 have valves. A piston moves along the air cylinder #2. The structure of air cylinder #3 is the same as that of air cylinder #2.
At the beginning, the piston is at the bottom of air cylinder #2, and the inlet of air cylinder #2 opens and connects to the outlet of the compressor.
The piston then pushes on towards the top of air cylinder #2. This movement is similar to that of the intake stroke of the Otto cycle; when the piston reaches the top of the air cylinder #2, the air then enters the air cylinder #2. The valves then close. The piston remains in a fixed position at the top of the air cylinder #2, and then the inlet of this cylinder is disconnected from the outlet of the compressor. Simultaneously, the inlet of air cylinder #3 is then connected to the outlet of the compressor; the movement in the air cylinder #3 is the same as that in the air cylinder #2.
d → e depicts the air stay in the air cylinder #2, which is an isochoric exothermic process. The air temperature drops until it is equal to that of the environment, , where
is the air temperature following the isochoric exothermic process; when the air temperature of air cylinder #2 is equal to that of the environment, the piston will not be in a fixed position.
The conventional heat engine extracts heat from this air, and, initially, the inside pressure of air cylinder #2 drops until the air temperature is equal to that of the environment; the inside pressure of air cylinder #2 is lower than the atmospheric pressure.
e → a depicts that the air stays in the air cylinder #2, which is an isothermal exothermic compression process. At the beginning, because the inside pressure of air cylinder #2 is lower than the atmospheric pressure, the atmospheric pressure pushes the piston to move and compresses the air; consequently, the inside pressure of air cylinder #2 rises until it is equal to the atmospheric pressure. Later, the valve opens and the piston pushes on towards the bottom of air cylinder #2, and the air then exits the air cylinder #2, which is an exhaust process. This movement is similar to the exhaust stroke of the Otto cycle. The air cylinder #2 is then reconnected to the compressor.
The air cylinder #3 undergoes the same processes.
Let us suppose that the air is the ideal gas; the adiabatic exponent of the air is ,
.
To depict the cycle clearly and to simplify the calculation, let us suppose that the temperature of the environment is :
The atmospheric pressure is :
Due to the structure of the compressor, the inside pressure of air cylinder #1, denoted as , can be controlled:
The temperature of the outlet of the turbo expander and of the inlet of air cylinder #1, denoted as , is given by:
The temperature of the outlet of the compressor and of the hot reservoir of the conventional heat engine, denoted as , is given by:
The work of the adiabatic isentropic expansion process a → b is , which can be obtained as follows:
The work of the isobaric endothermic expansion process b → c is , which is given by:
The heat of the isobaric endothermic expansion process b → c is , which can be obtained as follows:
The work of the adiabatic isentropic compression process c → d is , which is given by:
The heat of the isochoric exothermic process d → e is , which can be obtained as follows:
The heat of the isothermal exothermic compression process e → a is , which is given by:
The work of the isothermal exothermic compression process e → a is , the atmospheric pressure performs work on both the piston and the air; for the air,
is negative:
The total heat generated during the cycle is , which releases the heat:
The total work of the cycle is , which is given by:
In 1712, Thomas Newcomen used the isochoric exothermic process and the isothermal exothermic compression process to devise a model of an atmospheric engine, which employed both low-pressure steam and atmospheric pressure. It contained a piston that moved by an atmospheric pressure in a cylinder in which a vacuum was created, which is an isothermal exothermic compression process. The vacuum originated from the usage of the cooling water to condense the steam, which is an isochoric exothermic process (CitationButterman, n.d.).
Similarly, during the isothermal exothermic compression process e → a, because the inside pressure of air cylinder #2 is , where
, and the atmospheric pressure is
, where
(
), the atmospheric pressure pushes the piston to move and compresses the low-pressure air, and the inside pressure of air cylinder #2 rises until it is equal to the atmospheric pressure. The new type of refrigerator does not need any additional fuel to compress the air; the atmospheric pressure performs the work on the piston and the displacement of the piston is increased during the isothermal exothermic compression process e → a, as the inside pressure of air cylinder #2 drops below the atmospheric pressure. The work that the atmospheric pressure performs on the piston is
, which is a positive work and is numerically equal to
. This work can be utilized by the new type of refrigerator:
As is a positive work, therefore, the actual work that the new type of refrigerator produces is
:
Because , a conventional heat engine works between the high temperature
and the low temperature
, which extracts heat from the isochoric exothermic process d → e. The heat of the isochoric exothermic process d → e is
:
Let us suppose that the conventional heat engine consists of an isochoric endothermic process and an adiabatic isentropic expansion process. The isochoric endothermic process extracts heat from air cylinder #2 or air cylinder #3, and the adiabatic isentropic expansion process transforms the heat into work.
The heat which the isochoric endothermic process extracts from air cylinder #2 or air cylinder #3 is , which is equal to
:
Before the isochoric endothermic process commences, the air pressure is and the air temperature is
.
After the isochoric endothermic process, the air temperature is and the air pressure is
:
An adiabatic isentropic expansion process commences after the isochoric endothermic process. After the adiabatic isentropic expansion process, the air temperature is and the air pressure is
:
The work of the adiabatic isentropic expansion process is :
The work of the conventional heat engine is :
The new type of refrigerator can produce a net positive work, which is :
The isochoric endothermic process and the adiabatic isentropic expansion process are repeated until the air temperature is equal to the air temperature of the environment after the adiabatic isentropic expansion processes.
By the isochoric endothermic process and adiabatic isentropic expansion process, the heat of the isochoric exothermic process d → e can be transformed into work theoretically:
The summary results are presented in Table .
Table 1 Summary results.
Conclusion
Some readers may think that the new type of refrigerator is a perpetual motion machine and so may ask the question what is the external energy source? The following statements should therefore provide the answer:
First, according to Equations 1718(19), the new type of refrigerator does not violate the first law of thermodynamics; therefore, it is not the first perpetual motion engine. | |||||
Second, it does not violate the second law of thermodynamics as well. | |||||
In fact, the new type of refrigerator has both a hot reservoir and a cold reservoir. | |||||
During the isobaric endothermic expansion process b → c, the environment is a hot reservoir, because the temperature of the environment is higher than that of air cylinder #1. The air temperature of the environment is 298 K and the air temperature of air cylinder #1 ranges from 203 to 298 K; therefore, air cylinder #1 can extract heat from the environment. | |||||
During the isochoric exothermic process d → e and the isothermal exothermic compression process e → a, the conventional heat engine and the environment are cold reservoirs, because the temperature of air cylinder #2 ranges from 438 to 298 K, the temperature of the environment is 298 K and the temperature of the conventional heat engine is between 298 and 438 K. The air can release heat to the conventional heat engine and the environment. | |||||
The environmental enthalpy and atmospheric pressure can be utilized as the external energy sources; therefore, the new type of refrigerator can produce a net electrical energy. | |||||
Future work of an experimental nature is needed to evaluate the practicality of the refrigeration cycle described herein.
Acknowledgements
The author thanks the reviewer for the valuable comments and suggestions. The author also thanks Dr Dorothy Middleton, Durham University, and Dr Joe Ho for their help.
REFERENCES
- Butterman, E.n.d.Thomas Newcomen. ASME. https://www.asme.org/engineering-topics/articles/history-of-mechanical-engineering/thomas-newcomen.
- Cengel, Y. A., and M. A.Boles. 2005. Thermodynamics an Engineering Approach. 5th ed.New York: McGraw Hill Higher Education. p. 609.
- Lira, C. T.n.d.Brief History of the Steam Engine. Michigan State University. http://www.egr.msu.edu/∼lira/supp/steam/index.htm.