Abstract
Biodiesel from waste cooking oil (WCO) requires antioxidants to meet oxidation stability specifications set forth in ASTM D6751 or EN 14214. In contrast, unrefined cottonseed oil (CSO), containing tocopherols and gossypol, produces biodiesel of higher oxidation stability. However, only a portion of these CSO endogenous antioxidants are suspected to be retained in biodiesel. Because the economics of biodiesel manufacturing rely upon inexpensive sources of triglycerides, emphasis was placed on developing improved alternative processing methods where WCO was the main source of methyl esters (WCOME) and CSO was used as a supplemental source of triglycerides and antioxidants in a 4:1 ratio. This study compared four processing methods for their ability to produce biodiesel of increased oxidative stability prepared from a 4:1 ratio of WCO:CSO. Two novel processing methods developed for this study utilise solvent properties of fatty acid methyl esters and glycerol to avoid additional chemical inventory for biodiesel processors. This study concludes that the two new processing methods resulted in biodiesel that had statistically significant improved oxidation stability when compared to two common industrial processing methods. Another significant finding is that high-shear homogenisation during transesterification reduced reaction time from the published one hour to 16 minutes.
Introduction
Transportation of goods is necessary for improved standard of living in modern times. One of the most efficient pairs of chemical storage and energy-converting devices is diesel fuel and the compression-ignition engine. This system converts approximately 40% of the chemical energy into useful mechanical energy (Haywood Citation1988, 887). The compression-ignition engine dominates the world for large, high-use vehicle power plants.
Currently, in many parts of the world, petroleum reserves are the least expensive source of diesel fuel. However, with increased world standard of living, the demand for petroleum has put pressure on supply and increased the side effects of increased petroleum refining such as air and water pollution. A sustainable alternative is to increase the production of biologically-derived oils that can replace a portion of the diesel fuel demand. Triglycerides found in plant oils and animal fats may be converted into alkyl esters (Keera, El Sabagh, and Taman Citation2011, 42–47). These esters behave much like petroleum diesel fuel in terms of viscosity, cetane number, injector dispersion, ignition and heating value (No Citation2011, 131–149). In addition to being biologically derived, these domestic sources are renewable, support agricultural economic development, and reduce dependency upon petroleum.
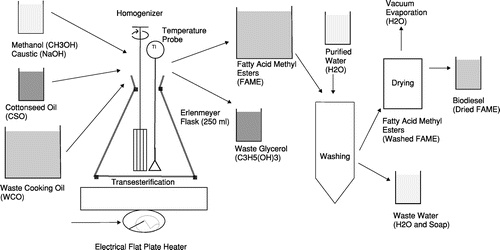
Biodiesel manufacturing is simply understood as a three-step process. First, triglycerides from sustainable plant or animal sources are typically reacted with methanol in the presence of a homogenous base catalyst such as potassium hydroxide to form fatty acid methyl esters (FAMEs) and glycerol. The first waste stream generated is a glycerol phase which contains some methanol, catalyst and water. The second step in manufacturing is purification by washing the FAMEs with water to remove soaps, fatty acids, residual catalyst and methanol. The third and final step is the removal of water and methanol by vacuum heating of the FAMEs.
FAMEs made from plant- and animal-derived triglycerides are termed biodiesel because, as mentioned, their combustion properties resemble petroleum diesel and the main ingredient in their manufacture is biologically-derived renewable triglycerides. In the United States and Europe biodiesel is acquiring market share as a blending ingredient (Ramalho et al. Citation2011, 601–605) in commercially available diesel fuel (Mushrush, Hughes, and Willauer Citation2013, 1764–1768). The acceptability of biodiesel has been in large part accomplished by the establishment of biodiesel fuel standards such as ASTM D6751 in the United States and EN 14214 in the European Union. These standards call for 100% biodiesel to meet physical chemistry properties of kinematic viscosity, density, flash point and cetane number, among numerous others. One such measurable property is oxidation stability, which is the tendency of the biodiesel to oxidise over a period of time when exposed to sunlight, oxygen, water, heat and metals. The result of oxidation is the formation of acids and gums which affect diesel engine injector and fuel filter performance. The approved method for measuring biodiesel oxidation stability is the Rancimat test (EN 14112 or 15751). This method is approved by ASTM D6751 and EN14214 whereby the oxidation of biodiesel is accelerated in a controlled manner (Pullen and Saeed Citation2012, 5924–5950) biodiesel giving an Induction Period (IP) of 6 h by the Rancimat test meets the specification (EN 15751) prescribed in EN 14214 (Sarin et al. Citation2010, 4645–4648).
Biodiesel oxidation would not be a concern if the biodiesel was composed entirely of saturated FAMEs, but the double bonds contained in unsaturated FAMEs are susceptible to oxidation (Knothe Citation2007, 669–677). While the saturated FAMEs are stable against oxidation, they produce biodiesel with poor cold flow properties. Cold flow properties cannot as easily be modified for saturated biodiesel and therefore the industry has utilised unsaturated FAMEs to achieve cold flow properties while adding antioxidants to increase the oxidative stability of biodiesel. It is known that one antioxidant chemical moiety is the aromatic group. The aromatic ring is capable of absorbing a free radical which is released during the autoxidation chain reaction. In addition, many antioxidants have a phenolic moiety from which a liable hydrogen can be abstracted by the peroxyl radical thus terminating the auto oxidative chain reaction (Moser Citation2012, 65–70).
There are two categories of antioxidants that have been shown to increase the oxidation stability of biodiesel: synthetic and natural antioxidants. Synthetic antioxidants such as tert-butyl hydroquinone, 1,2,3-trihydroxybenzene (pyrogallol), 3,4,5-trihydroxybenzoic acid (propyl gallate), 2-tert-butyl-4-methoxyphenol (butylated hydroxyanisole) and 2,6-di-tert-butyl-4-methylphenol (butylated hydroxy toluene) have been shown to increase the oxidation stability of biodiesel to meet ASTM D6751 or EN 14214 standards (Dunn Citation2005, 1071–1085; Tang et al. Citation2008, 373–382). Natural antioxidants such as tocopherols and gossypol have also been shown to increase the oxidation stability of biodiesel to meet these standards (Moser Citation2012, 65–70). Recent industrial correspondence indicates that biodiesel producers incur a cost of $0.01–0.02/gallon biodiesel for the addition of commercial antioxidants to their finished product (DBAEUI Citation2011).
Sustainable and renewable natural antioxidants found in cold-pressed plant oils are capable of increasing the oxidation stability of biodiesel to meet the 6 h specification (EN 15751) prescribed in EN 14214 (Sarin et al. Citation2010, 4645–4648). One such raw plant oil worth greater consideration for industrial biodiesel application is cottonseed oil (CSO), because of its high concentration of endogenous antioxidants, tocopherols and gossypol. Gossypol is a toxic antioxidant and must be removed from CSO if the oil is to be refined for human consumption. Gossypol is an unwanted impurity in expressed CSO. On the other hand, the antioxidant properties of gossypol are desired in biodiesel and therefore are of additional benefit to the manufacture of biodiesel from CSO. If new processing methods could be identified where CSO antioxidant properties can be incorporated into finished biodiesel having higher oxidative stability then this benefit could increase the market for CSO.
It has been shown that tocopherols and gossypol have a positive influence on oxidative stability when added directly to finished biodiesel (Moser Citation2012, 65–70; Pullen and Saeed Citation2012, 5924–5950). These antioxidants are relatively abundant in cold-pressed CSO, where gossypol ranges in concentration from 4000 to 17,000 ppm in the whole cottonseed kernel, depending upon the species, season, temperature of extraction and other factors (Wang et al. Citation2009, 215–263). When cold-pressed CSO is extracted from the kernel a considerable portion of the gossypol binds to protein. As an estimate, crude cold-pressed CSO has a gossypol concentration of about 5000 ppm (Mirghani and Che Man Citation2003, 625–628).
It has been reported that the direct addition of 500 ppm of gossypol to waste cooking oil methyl esters (WCOME) has an IP of 5.7 h (Moser Citation2012, 65–70). Crude CSO contains sufficient antioxidants, approximately 5000 ppm, to ensure that the resulting biodiesel yields IPs much greater than 6 h, but much lower IPs have been reported of 4.9 h (Fernandes et al. Citation2012, 658–661) and 1.9 h (Rashid, Anwar, and Knothe Citation2009, 1157–1163). A possible reason for low IP of Cottonseed Oil Methyl Ester (CSOME) is due to antioxidants not being retained in the finished biodiesel, although this has not been explicitly measured by any researchers. This lack of research is most likely due to the complexity of the analytical task of measuring the initial and final concentrations of numerous tocopherols and gossypol in the finished biodiesel. After undergoing strong caustic and high temperature reaction conditions it is likely that gossypol and other natural antioxidants undergo chemical modification. No research has yet been done to study the many possible gossypol derivatives present in biodiesel after undergoing the biodiesel transesterification step. Neither does this study seek to address the fate of many natural antioxidants after being subject to the reaction conditions of biodiesel production. It is not clear why biodiesel made from CSO has an IP less than the CSO oil from which it was derived. The endogenous antioxidants found in CSO differ in their abilities to affect IP at various concentrations (Yang et al. Citation2013, 366–375). Each is found in different concentrations in expressed CSO. Each has a different chemical structure, polarity (Obadiah et al. Citation2012, 56–63), and therefore solubility in methyl esters resulting in different concentrations in the biodiesel and waste streams. One explanation for lower than expected CSOME IP measurements is that the endogenous antioxidants are more soluble in glycerol than FAME, therefore as the transesterification reaction proceeds towards completion there is a greater volume of glycerol produced into which antioxidants can solubilise. Previous studies from this laboratory by other researchers reported a decrease in gossypol concentration as reaction completeness increased (Joshi, Toler, and Walker Citation2008, 357–363) and also a decrease in IPs with increased reaction completeness. Careful consideration of these results implies, but does not prove, that lower completeness has a smaller volume of glycerol waste and therefore less volume into which antioxidants can solubilise and be removed from the biodiesel.
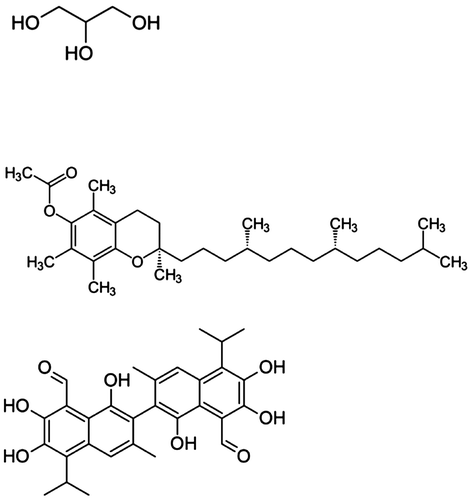
From a chemical solubility standpoint, antioxidants have phenolic moieties which are similar to the glycerol hydroxyl group and not similar to the ester group of the FAMEs. Both antioxidants and glycerol share hydroxyl group functionality which most likely results in a greater solubility of the antioxidants in glycerol. Figure displays the chemical structure of some CSO natural antioxidants and glycerol. On the other hand, it is unlikely that the antioxidants, such as gossypol and tocopherols, are removed from the biodiesel during water washing because these endogenous antioxidants are not water soluble.
Figure 1. Chemical structures of glycerol and two naturally occurring antioxidants found in cottonseed oil: α-tocopherol and gossypol.
This study proposes to compare four biodiesel processing methods using IP, not antioxidant concentration. Antioxidant concentration is a possible explanation of differences between the IP of various processing methods but this is conjecture, not proven. The analysis of all antioxidants in the biodiesel and waste streams is beyond the scope of this study.
This study utilises materials most prevalent in the biodiesel industry, waste cooking oil (WCO), caustic and methanol, and compares the IP of the various methods. WCO was selected because it is a common industrial source of triglycerides due to its low cost. Because WCO and CSO were used in a constant ratio of 4:1, no interference of oxidation stability was expected due to FAME double bond unsaturation differences. The creativity in this study is the development of two new processing methods which are significantly different than the two standard processing methods to which they are compared. This study focuses upon the improved IP resulting from these novel processing methods. The value of this research is to demonstrate the use of CSO as a source of both triglycerides and antioxidants when making biodiesel from the more economical WCO.
When comparing the four different processing methods it is primarily important that quality biodiesel is made of high completeness. New biodiesel processing methods need to meet the biodiesel standards. The inclusion of additional tests on multiple batches verifies the new processing methods are reproducible and producing quality biodiesel. Because biodiesel standards reference IP as a quality measurement for oxidative stability, it stands to reason that IP is the best comparison between processes when considering oxidative stability. Biodiesel standards do not have requirements for antioxidant concentration. The measurement of IP is a measurement which takes into account all types of antioxidants, their concentrations and FAME degree of saturation. The IP measurement gives the true valve as to the oxidation stability of biodiesel.
The experimental plan compared four processes by producing and testing three batches of each of the four processing methods for a total of 12 batches. After all the batches were made and analysed for IP, out of curiosity, gossypol concentration was measured in the starting CSO and in the finished biodiesel. These measurements were not part of the experimental design but were reported in order to suggest future inquiry. Waste glycerol stream samples were not available for analytical measurement at the end of the experimental design.
Experimental
Biodiesel processing methods
Four commercially-viable processes were proposed for comparison. The first two processes are common industrial methods; the third and fourth processes are novel and seek to optimise the oxidative stability of biodiesel made from WCO and CSO in 4:1 ratio. The 4:1 ratio of oils was selected in order to keep the biodiesel producer’s cost of raw materials low, where CSO is more costly than WCO.
Mixed oil process
CSO and WCO were combined and then converted into methyl esters (MEs). The MEs were washed and dried to simulate commercial industrial operations. This process mimicked a biodiesel production facility with two storage tanks of raw oils that can be blended in a 4:1 ratio of WCO to CSO, followed by production of biodiesel from the blended oil.
Separate oil process
CSO and WCO were transesterified separately and washed separately (steps 1 and 2), afterward the biodiesels were combined to form the final biodiesel blend (step 3). This process mimicked an industrial facility where four batches of WCO biodiesel are produced and then one batch of CSO biodiesel is added to the previous 4 batches in a final blend tank.
Reduced WCO glycerol process
WCO was converted to WCOME and separated from the WCO glycerol (step 1), then the WCOME was mixed with CSO and the CSO was converted into CSOME in the presence of the WCOME (step 2). Then the combined CSOME and WCOME mixture was washed to yield the finished biodiesel. This two-step process mimicked a production facility which produces WCOME and separates the WCO glycerol, then adds CSO to the separated WCOME and converts the CSO into CSOME.
WCOME extraction process
WCO was converted into WCOME and separated from the WCO glycerol (step 1). The WCOME was divided into two equal portions for step wise extraction. Next, CSO was converted into CSOME along with a CSO glycerol phase (step 2). Then, the CSO glycerol phase was contacted with WCOMEs in two steps (step 3). Finally, WCOME and CSOME were combined and washed and dried to yield the finished biodiesel (step 4). This process simulated a commercial extraction operation, which uses WCOMEs as the solvent of extraction where antioxidants can be extracted from the CSO glycerol phase.
Materials
All materials were selected to represent industrial grade reagents to mimic commercial operations. Mechanically expelled crude pima CSO was obtained from USDA-ARS Southwestern Cotton Ginning Research Laboratory (SWCGRL, Las Cruces, NM), in cooperation with Eco-Sol, LLC (Castaic, CA). WCO was obtained from Clemson University dining halls as part of the Clemson University Sustainable Biofuels Initiative (Clemson, SC). Industrial grade methanol (99.9%) was obtained from Brenntag Southeast (Durham, NC) in commercial quantity of 55 gallon drums. Deionised water was obtained from Osmotics reverse osmosis technology treatment of potable water. Technical grade potassium hydroxide was obtained from Fisher Scientific (Waltham, MA) having a 90% purity, due to moisture content.
Material preparation
Crude CSO was centrifuged to remove solid particulates and stored in sealed containers until use. Three acid value (AV) titrations were performed following a modified American Oil Chemists’ Society (AOCS) method Cd 3a-63 where 100 mL of isopropyl alcohol replaced 125 mL of isopropyl alcohol-toluene (1:1 v/v) solvent. The average AV of CSO was 3.32 mg KOH/g CSO. The CSO had been stored for two years at room temperature prior to use which accounted for the high AV. WCO was titrated in a similar manner to provide an AV of 3.37 mg KOH/g WCO. When required for batch processing, CSO and WCO were heated to 90 °C at 50 torr for 30 min for water removal. Initially, water vapour bubbled vigorously from the oil until the oil was dry, at which time the bubble formation ceased.
A base catalyst concentration of 0.9 wt% KOH/wt WCO:CSO was chosen to replicate industrial operations. Excess KOH was added to neutralise free fatty acids (FFAs). For a typical 144 g WCO:CSO (4:1 wt/wt) batch, 1.98 g KOH (90%) was added of which 0.54 g KOH (90%) neutralised FFAs.
Methanol was used in a 5:1 M ratio to triglyceride. From the AV determination, FFAs were estimated to be 1.68 wt%. This FFA weight was subtracted from the oil weight to determine the available triglyceride (MW = 810) available for transesterification. A typical 144 g WCO:CSO batch required 28.0 g methanol (35.3 mL). The catalyst was dissolved in methanol before addition to the reaction flask to form potassium methoxide.
Transesterification reaction
The conversion of triglycerides into FAMEs was carried out in a 250 mL Erlenmeyer flask equipped with a temperature probe and Polytron PT 1200 CL homogeniser of diameter 10 mm, rotating at a speed of approximately 10,000 RPM. WCO and CSO were added to the flask at approximately 60 °C. Temperature was maintained by use of a hot plate. Room temperature potassium methoxide was added to the flask along with a homogeniser and a temperature probe. The reaction was carried out between 60 and 65 °C, Figure . The narrow neck of the Erlenmeyer flask served to reduce methanol vapour loss at this temperature range. Due to high agitation shear rate the reaction was completed in 12 min. Completeness was qualitatively confirmed by the 27/3 glyceride test where 3 mL of FAME reaction mixture was dissolved in 27 mL of methanol. Lack of a separate diglyceride/triglyceride liquid phase qualitatively indicated reaction completeness. To ensure completeness, 16 min of reaction time was allowed.
Purification
After reaction completion, the biodiesel was poured into centrifugation tubes and the liquid glycerol layer was separated from the FAME phase by decantation and pipette extraction. The FAMEs were then washed with deionised water three times (10 , 15 and 15 mL). The total water wash volume was equal to about 28% by volume of the FAME volume. If soaps were visible after the third wash, an additional wash was conducted. Final solvent removal and water removal was done by mechanical vacuum heating at 90 °C for about 1 h, thus resulting in finished biodiesel. A visual indication of dry biodiesel was the cessation of gas bubbles during vacuum heating.
Analytical procedures for finished biodiesel
Properties were measured following AOCS and American Society for Testing and Materials (ASTM) standard test methods using instrumentation described previously (Joshi et al. Citation2009, Citation2010): acid value (AV, mg KOH/g), AOCS Cd 3d-63; moisture (ppm), ASTM D2709; cloud point (CP, °C), ASTM D5773; free and total glycerol (mass %), ASTM D6584; IP (h), EN 14112; kinematic viscosity (KV, mm2/s), ASTM D445; pour point (PP, °C), ASTM D5949. For a greater degree of precision, PP was measured with a resolution of 1 °C instead of the specified 3 °C increment.
Gossypol concentration was determined by RP-HPLC using a Shimadzu HPLC model LC-10ATvp and controller model SCL-10Avp. The detector was a UV polydiode array model SPD-M10Avp optimised at 247 nm for gossypol. Upgraded software was from LC Solutions. The mobile phase was methanol:water 80:20 applied for 0.2 min and then a gradient ramp until minute 3.0 where the solvent concentration was stabilised at 95:5 methanol:water. The flow rate was constant 1.1 mL/min through a Kinetex column 2.6μ C18 of dimensions 100 mm × 4.6 mm diameter in oven at setpoint of 50 °C.
Peroxide values (PVs) were determined using the IDF method of Shantha and Decker (Citation1994, 421–424) as modified for scale by Hu, McClements and Decker (Citation2004, 5272–5276). Briefly, 10 mg of sample was placed into a test tube. Three millilitres of methanol:butanol (2:1 v/v) was then added and contents were vigorously stirred for 2–4 s. Ammonium thiocyanate (15 μL of 3.94 M solution) was added, and tube was stirred again for 2–4 s. Fifteen microlitres ferrous chloride (0.018 M) was added and tube was agitated for an additional 2–4 s. The solution was then allowed to sit at room temperature for 20 min before being transferred into a plastic cuvette. Absorbance at 510 nm was measured on a Perkin-Elmer Lambda 35 spectrophotometer. PVs were calculated from a standard curve of ferric chloride as described by Shantha and Decker (Citation1994, 421–424). PV is expressed as milliequivalents (meq) of peroxide per kg oil.
Statistical analysis
Four processes were studied for their ability to achieve higher IPs of the finished biodiesel. Each process was replicated three times for a total of 12 batches. A random number generator was used to determine the order in which the batches were produced, thus ensuring a completely randomised design. Each batch was checked for completeness. Free and total glycerol was measured once for each batch and reported. To confirm the biodiesel quality, each batch was also measured twice for AV, and three times for moisture, KV, CP and PP. Mean and standard deviation values were reported.
IP was measured twice for each batch and mean values were reported. Process mean IP and standard deviation were also reported for each process. A one-way analysis of variance (ANOVA) was conducted and means for the four processes were compared using Tukey’s honestly significant difference (HSD) test. Results were considered statistically different if p < 0.05. The equal variance assumption was tested and confirmed but not reported.
PV was measured in triplicate for each batch and mean values reported. Process mean PV and standard deviation were also reported for each process. A one-way ANOVA was conducted and process means were considered statistically different if p < 0.05. The equal variance assumption was tested and confirmed but not reported.
Results and discussion
Reaction completeness
The transesterification reaction conditions used in this experiment followed widely accepted values for catalyst concentration (0.90 wt% KOH/wt oil) and temperature (60–65 °C). However this reaction used a lower methanol:oil ratio of 5:1 rather than the traditional 6:1 ratio reported previously (Freedman, Pryde, and Mounts Citation1984, 1638–1643) and a very high shear rate of approximately 10,000 RPM. The results were a 16 min reaction time with high completeness, far less than published one hour reaction times (Freedman, Pryde, and Mounts Citation1984, 1638–1643; Meneghetti et al. Citation2006, 2262–2265). Table shows that all batches had a very high completeness. Free glycerol and total glycerol were below the limits specified in ASTM D6751 and EN 14214, except for one batch (0.275 mass%) which exceeded the total glycerol specifications listed in both standards. One possible explanation for the increased total glycerol produced by the reduced glycerol process is the dilution of the second step transesterification of CSO with WCOME. The greater overall volume diluted the CSO and methanol reactants and therefore reduced the chemical conversion rate. A longer reaction time could be used in commercial operations to reach higher reaction completeness.
Table 1. Free and total glycerol in batch processes compared to limits specified in ASTM D6751 and EN 14214, and residual glycerides.
Other researchers have shown that higher agitation rates produce higher conversion rates (Meher, Dharmagadda, and Naik Citation2006, 1392–1397). Considering that all of the processes in this study were performed with a lower methanol to triglyceride ratio of 5:1 and that the reaction was completed in about a quarter of the time published in other literature (Freedman, Pryde, and Mounts Citation1984, 1638–1643; Joshi et al. Citation2010, 14–20), it is probable that high-shear homogenisation of the reaction was responsible for high completeness after only 16 min.
Acid value
The removal of FFAs from finished biodiesel was measured by AV. Table displays the AVs for each batch. A low AV was accomplished by adequate washing of the FAMEs with water. In all twelve batches the average AV was well below the maximum limit of 0.50 mg KOH/g specified in ASTM D6751 and EN 14214.
Table 2. Acid values of batch processes compared to limits specified in ASTM D6751 and EN 14214.
Moisture
Table displays the moisture concentration for each batch. Only one batch contained moisture content (502 ppm) greater than the maximum limit of 500 ppm specified in ASTM D6751 and EN 14214. All other batches were below 500 ppm.
Table 3. Moisture content of batch processes compared to limits specified in ASTM D6751 and EN 14214.
Kinematic viscosity
The KVs of the four processes are presented in Table . All batches were in the range of 4.41–4.66 mm2/s, which was an indication that the same ratio of WCO:CSO of 4:1 was maintained in all processes. These results were close to the published results of methyl linoleate (3.65 mm2/s), methyl palmitate (4.38 mm2/s) and methyl oleate (4.51 mm2/s), which are the three most abundant FAMEs found in CSOME (Joshi, Toler, and Walker Citation2008, 357–363; Knothe and Steidley Citation2005, 1059–1065; Rashid, Anwar, and Knothe Citation2009, 1157–1163). All biodiesel batches were within the ranges specified in ASTM D6751 and EN 14214 with respect to KV.
Table 4. Kinematic viscosity of batch processes compared to limits specified in ASTM D6751 and EN 14214.
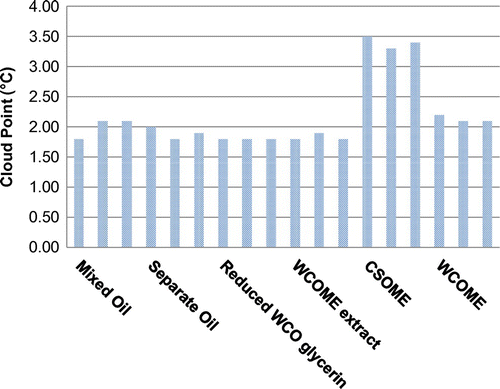
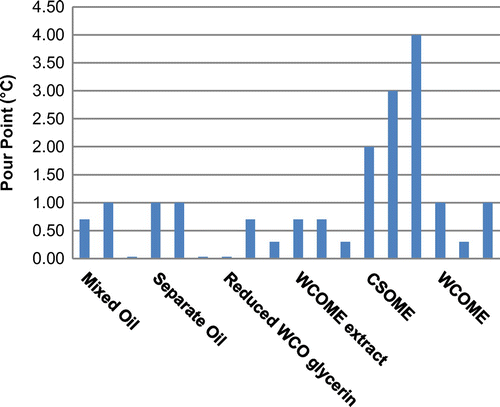
Cloud point and pour point
The CPs and PPs of three batches of each of the four different processes are shown in Figures and , respectively. Also included are the CPs and PPs of biodiesel batches prepared from 100% CSO and 100% WCO. As seen from the figures, all four processes yielded CPs and PPs similar to WCOME. The reason for this is that WCO was used in excess at a ratio of 4:1 (WCO:CSO). Similar CPs and PPs are an indication that the degree of saturation of the methyl esters is similar between the various batches made by the different processing methods. This infers that the difference between the IP of the different processing methods is not related to the degree of saturation of the biodiesel.
Induction period
Considering the oxidation stability of the raw oils before conversion to biodiesel, Table lists the average IP of CSO, WCO and blended 4:1 WCO:CSO. The CSO yielded the highest average IP of 28.52 h whereas WCO had a process average IP of 0.43 h. This large difference could be attributed to either a highly saturated structure of CSO, containing few double bonds, or the presence of endogenous antioxidants present in the CSO. It has been reported that a neat methyl ester blend of 90% methyl stearate and 10% methyl linoleate has an average IP of 3.65 h at 90 °C via the Rancimat method (Pullen and Saeed Citation2012, 5924–5950). If the high CSO average IP was due to chemical structure alone then CSO would be nearly saturated. But CSO is not a solid at room temperature like other saturated fats. Also, if the average IP difference is due to structure alone then the blended 4:1 WCO:CSO oil should have an average IP closer to the average IP of the WCO (0.43 h). But the blended oil had a high average IP (19.93 h) closer to the average IP of CSO (28.52). This data suggested that CSO contained antioxidants that caused the increased oxidation stability of WCO. Also, this data suggested that there was an abundance of endogenous antioxidants in CSO which gave the WCO:CSO blend an average IP much greater than 6 h.
Table 5. Average induction period for raw oils: CSO, WCO, and 4:1 WCO:CSO blend with oil means and standard deviations.
Table lists the IPs obtained from the 12 batches of biodiesel. Each batch was measured twice and the mean values reported. The three averages for each process were then averaged to obtain the ‘Process Mean’. The process mean standard deviation is also reported. For comparison, 100% CSOME had a mean IP of 3.00 h whereas 100% WCOME had a mean IP of 0.94 h. In all WCO batches the resulting biodiesel was below the limit of 3 h prescribed in ASTM D6751.
Table 6. Average induction period for process batches and process means compared to the IP limits specified in ASTM D6751 and EN 14214, with process standard deviations.
The one-way ANOVA F-test indicated there was a significant difference in the average IP between process treatments (F(3, 8) = 26, p = 0.0002). Tukey’s HSD test indicated that there was a statistically significant difference (p < 0.05) in the average IP between the mixed oil process (0.64 h) and both the reduced WCO glycerol process (1.74 h) and the WCOME extraction process (1.95 h). There was a statistically significant difference (p < 0.05) in the average IP between the separate oil process (1.14 h) and both the reduced WCO glycerol process (1.74 h) and the WCOME extraction process (1.95 h), which did not significantly differ. There was not a statistically significant difference (p < 0.05) between the average IPs of the mixed oil process (0.64 h) and the separate oil process (1.14 h).
Differences in average IP were a result of processing method. A high ratio of WCO to CSO (4:1) was used which diluted the effect of possible antioxidants available to be incorporated into the blended biodiesel.
A possible explanation for the high IP of the WCOME extraction process is that endogenous antioxidants were solubilised in the glycerol phase. In this study, the antioxidant concentration in the glycerol was not measured but after contacting the CSO glycerol with WCOME a high IP was obtained. This would infer that antioxidants present in the glycerol were extracted into the WCOME and carried over into the final biodiesel. This extraction process led to the highest IP values among the processes studied.
Another observation which supports the possibility that antioxidants were solubilised into the glycerol is to consider the lowest average IP was a result of the mixed oil process. Even the IP of 100% WCOME biodiesel (0.94 h), with no CSO, had a higher average IP than the mixed oil process (0.64 h). This can be explained if glycerol is considered to be a better solvent for antioxidants than FAMEs. In the mixed oil process, the WCO and CSO were mixed in a 4:1 ratio, and then biodiesel was made from the combined oil. Intuitively this process seemed like a facile method to add antioxidants from CSO into the biodiesel blends. But when the mixed oil FAMEs were produced there was also an increase in the total volume of glycerol in contact with the FAMEs. It can be inferred that the large volume of glycerol from the WCO had significant capacity to solubilise the antioxidants present in the CSOME. Theoretically, a greater volume of glycerol solvent will hold a greater share of CSO antioxidants, thus leading to the lowest average IP among all processes studied.
The significantly higher average IP of the reduced glycerol process can also be explained by the possibility that glycerol is a good solvent for antioxidants. In this process the WCO was converted to WCOME and the WCO glycerol phase was removed. The WCOME was added to CSO before transesterification of CSO into CSOME was completed. This is similar to a single step extraction where all of the WCOME is contacted with the small volume of CSO glycerol. After discarding the CSO glycerol, the final WCOME:CSOME exhibited a statistically higher average IP (1.74 h) than the mixed oil process (0.64 h) and the separate oil process (1.14 h). The contact of the WCOME with the waste glycerol is therefore a valid method of increasing the IP of the final biodiesel.
It is also reasonable to conclude that the extraction process, which was a two-step extraction, would have a higher average IP than the reduced glycerol process because a two-step extraction should be more efficient than a single-step extraction. This is in fact observed as the extraction process average IP (1.95 h) was higher than the reduced oil process average IP (1.74 h), although these processes were not significantly different (p < 0.05). Because the antioxidants in the glycerol and biodiesel were not measured, the higher average IPs imply, but do not prove, that the CSO glycerol phase was a good solvent for antioxidants and that these antioxidants can be captured into the final biodiesel by extraction using WCOME.
Considering the separate oil process whereby the WCO and CSO were converted separately into WCOME and CSOME, their glycerol layers removed and the finished biodiesels combined and washed. The IP of this process averaged 1.14 h, in comparison to 100% WCOME and 100% CSOME which had average IPs of 0.94 and 3.00 h, respectively. Because CSO is used in a 4:1 ratio, the addition of four parts 100% WCOME and one part 100% CSOME had a weighted IP average of 1.35 h (4 × 0.94 h + 1 × 3.00 h/5). The weighted average (1.35 h) was close to the separate oil process average IP (1.14 h). The average IP of 100% WCOME was increased by the addition of higher average IP 100% CSOME in the separate oil process. This process is considered to be the baseline for IP comparison among all the processes studied and yields a result similar to a numerical weighted average.
In summary, the two new processing methods studied did produce high-quality biodiesel of statistically significant higher IP than biodiesel made by two standard processing methods. These improved processing methods could result in biodiesel which requires less antioxidant additives to meet ASTM and EN standards and lead to cost savings for biodiesel producers. It appears likely that antioxidants were removed from the biodiesel production process in the glycerol phase. Visually this was observed by the dark brown colour of the glycerol layer separated from the FAMEs after transesterification and centrifugation. The dark brown glycerol colour is indicative of aromatic hydrocarbons, such as gossypol, which absorb light in the visible 200–600 nm range.
Peroxide value
The extent of autoxidation was quantified by PV. Higher PVs are indicative of greater oxidative degradation before a maximum PV is ultimately reached. After the maximum, PV decreases as peroxide intermediates decompose further to other more stable oxygenated species (Shantha and Decker Citation1994).
Table lists the results of the average PVs obtained from the 12 batches of biodiesel. Each batch was measured three times and the mean values reported. The averages for each process are listed as well as the process mean standard deviation. The one-way ANOVA F-test indicated that there was not a significant difference between process treatments (F(3, 8) = 0.4690, p = 0.7121). Correspondingly, these results indicated that the various processing methods did not result in variation of initial autoxidation of the finished biodiesel. Lastly, neither ASTM D6751 nor EN 14214 specifies limits for PV.
Table 7. Peroxide values of batch process averages, process means and process standard deviations.
Gossypol
At the end of this study, after the processing methods were compared using IP, gossypol concentrations were tested in the raw material oil, CSO, and in the finished biodiesel FAMEs. It was hoped that some difference in gossypol concentration could be identified between the different processing methods to account for their differences in IP. However, gossypol was not detected in any of the finished biodiesel samples, the limit of detection by our HPLC method being 100 ppm. Possibly, gossypol was converted into other chemical structures during transesterification and avoided detection. Or possibly, gossypol was removed from the process in the glycerol phase due to the fact that its’ chemical moiety is very similar to glycerol. In any case, this study did not intend to discover the fate of gossypol during CSO biodiesel production but to propose and confirm alternative processing strategies which would increase biodiesel IP.
Conclusions
Two novel methods for producing biodiesel using CSO have been identified. The improved processes are capable of producing high-quality biodiesel and have statistically significant higher IPs when compared to other standard processing methods. The four processing methods compared were: a mixed oil process, separate oil process, reduced WCO glycerol process and a WCOME extraction process. Each process produced high-quality biodiesel which, on average, met ASTM D6751 and EN 14214 specifications for free and total glycerol, AV, moisture, KV, CP and PP, where applicable. When these four processes were examined for average IP, the extraction process and the reduced glycerol process produced biodiesel with higher average IPs. This result can best be explained if the solubility of natural antioxidants is assumed to be higher in the glycerol phase than the biodiesel phase after transesterification. The WCOME extraction process yielded the highest average IP for the resulting biodiesel. It is suspected that this process facilitated the greatest amount of antioxidant transfer from the CSO glycerol phase. Similarly, the reduced WCO glycerol process afforded a high average IP. This process resembled a single stage contact extraction where the WCO glycerol is removed before WCOME is contacted with the CSO and final transesterification.
The result of this study has been the development of two new processing methods for raw material blends of WCO and CSO. Biodiesel producers can achieve statistically significant higher IPs of their finished biodiesel when using the processing methods described herein. Cottonseed oil is known to have a high concentration of natural antioxidants which the authors suspect is responsible for the higher IPs attained by the novel processing methods.
A finding, independent of this study, is the non-detection of gossypol in any of the finished biodiesel samples regardless of the processing method. The fate of gossypol was not the purpose of this experimental plan, but this finding is reported to help guide future research.
Another finding, independent of this research, is the improvement of the transesterification reaction laboratory set-up. This improved set-up obtains high reaction completeness in a short period of time when using a homogeniser in an Erlenmeyer flask in place of magnetic stirrer. Such high-sheer agitation reduced the reaction time for all processes studied from literature values of 1 h to 16 min. This valuable finding can reduce laboratory time required for further experimentation, and, if applied to industry, can increase equipment productivity.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Notes on contributors
Gregory S Lepak, MS, is a professional chemical engineer and works as a biochemical engineer with Fluor Enterprises in Greenville, South Carolina, USA. His main research interests are biotechnology plant capacity optimization and clean-in-place strategies for biomanufacturing. He has designed both chemical and pharmaceutical process facilities and has led manufacturing and cleaning design efforts for Eli Lilly, Merck, Amgen, Eastman, Milliken, Bayer and Genentech facilities.
Bryan R Moser, PhD, works as a research chemist with the United States Department of Agriculture in Peoria, Illinois, USA. His main research interests are fuel and industrial applications of biological materials, with an emphasis on chemical modification of plant oils. Previous publications (> 85) have appeared in Energy & Environmental Science, RSC Advances, ACS Sustainable Chemistry & Engineering, Bioresource Technology, Biomass & Bioenergy, and the Journal of Natural Products, among numerous others.
Erica L Bakota, PhD, is a method development chemist at the Harris County Institute of Forensic Sciences in Houston, Texas, USA. She currently leads method development and validation for analytical GC-MS and LC-MS/MS methods. Prior to that, she was a research chemist at the United States Department of Agriculture, Peoria, Illinois, USA, where she studied oxidative stability of edible oils. Previous publications have appeared in Journal of the American Chemical Society, Biomacromolecules, Journal of Food Science and many others.
Julia Sharp, PhD, is an associate professor of Statistics at Colorado State University, Fort Collins, Colorado, USA. Her main research interests include statistical application and methodology in other fields such as agriculture, public health, engineering and education. Recent publications have appeared in International Journal of Environmental Health Research, Energy Systems, and Journal of Statistical Theory and Practice.
C David Thronton is a microbrewery entrepreneur. Previously he has operated the Clemson University cooking oil waste to biodiesel refinery and been employed by other biodiesel producers in North Carolina. His research interest is discovering wild forms of yeast for beverage production.
Terry Walker, PhD, works as a Professor of Biosystems Engineering in the College of Engineering, Computing and Applied Science at Clemson University. His main research interests are in bioprocess engineering of algae and fungi to biofuels and bioproducts with emphasis on biodiesel production. Previous publications have appeared in Algal Research, Bioresource Technology, Biotechnology Letters and Biomass and Bioenergy and others.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by SC Cotton Board.
Acknowledgements
The authors are very grateful to the SC Cotton Board for their financial support for laboratory materials and supplies. Also gratitude is extended to Julie Anderson and Benetria Banks (USDA ARS NCAUR) for excellent technical assistance.
References
- DBAEUI (Department of Biological and Agricultural Engineering at University of Idaho). 2011. “Comparison of Oxidative Stability Additives for Biodiesel.” Biodiesel Technology Notes, May.
- Dunn, R. O. 2005. “Effect of Antioxidants on the Oxidative Stability of Methyl Soyate (Biodiesel).” Fuel Processing Technology 86: 1071–1085.10.1016/j.fuproc.2004.11.003
- Fernandes, D. M., D. S. Serqueira, F. M. Portela, R. M. N. Assunção, R. A. A. Munoz, and M. G. H. Terrones. 2012. “Preparation and Characterization of Methylic and Ethylic Biodiesel from Cottonseed Oil and Effect of Tert-Butylhydroquinone on its Oxidative Stability.” Fuel 97: 658–661.10.1016/j.fuel.2012.01.067
- Freedman, B., E. H. Pryde, and T. L. Mounts. 1984. “Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils.” Journal of the American Oil Chemists Society 61: 1638–1643.10.1007/BF02541649
- Haywood, J. B. 1988. Internal Combustion Engine Fundamentals. New York: McGraw-Hill.
- Hu, M., D. J. McClements, and E. A. Decker. 2004. “Antioxidant Activity of a Proanthocyanidin-Rich Extract from Grape Seed in Whey Protein Isolate Stabilized Algae Oil-in-Water Emulsions.” Journal of Agricultural and Food Chemistry 52: 5272–5276.10.1021/jf049486j
- Joshi, H., B. R. Moser, J. Toler, and T. Walker. 2010. “Preparation and Fuel Properties of Mixtures of Soybean Oil Methyl and Ethyl Esters.” Biomass and Bioenergy 34: 14–20.10.1016/j.biombioe.2009.09.006
- Joshi, H., J. Toler, B. R. Moser, and T. Walker. 2009. “Biodiesel From Canola Oil Using a 1:1 Molar Mixture of Methanol and Ethanol.” European Journal of Lipid Science and Technology 111: 464–473.10.1002/ejlt.v111:5
- Joshi, H. C., J. Toler, and T. Walker. 2008. “Optimization of Cottonseed Oil Ethanolysis to Produce Biodiesel High in Gossypol Content.” Journal of the American Oil Chemists’ Society 85: 357–363.10.1007/s11746-008-1200-7
- Keera, S. T., S. M. El Sabagh, and A. R. Taman. 2011. “Transesterification of Vegetable Oil to Biodiesel Fuel Using Alkaline Catalyst.” Fuel 90: 42–47.10.1016/j.fuel.2010.07.046
- Knothe, G. 2007. “Some Aspects of Biodiesel Oxidative Stability.” Fuel Processing Technology 88: 669–677.10.1016/j.fuproc.2007.01.005
- Knothe, G., and K. R. Steidley. 2005. “Kinematic Viscosity of Biodiesel Fuel Components and Related Compounds. Influence of Compound Structure and Comparison to Petrodiesel Fuel Components.” Fuel 84: 1059–1065.10.1016/j.fuel.2005.01.016
- Meher, L. C., V. S. S. Dharmagadda, and S. N. Naik. 2006. “Optimization of Alkali-Catalyzed Transesterification of Pongamia Pinnata Oil for Production of Biodiesel.” Bioresource Technology 97: 1392–1397.10.1016/j.biortech.2005.07.003
- Meneghetti, S. M. P., M. R. Meneghetti, C. R. Wolf, E. C. Silva, G. E. S. Lima, L. de Lira Silva, T. M. Serra, F. Cauduro, and L. G. de Oliveira. 2006. “Biodiesel from Castor Oil: A Comparison of Ethanolysis versus Methanolysis.” Energy & Fuels 20: 2262–2265.10.1021/ef060118m
- Mirghani, M. E. S., and Y. B. Che Man. 2003. “A New Method for Determining Gossypol in Cottonseed Oil by FTIR Spectroscopy.” Journal of the American Oil Chemists’ Society 80: 625–628.10.1007/s11746-003-0749-2
- Moser, B. 2012. “Efficacy of Gossypol as an Antioxidant Additive in Biodiesel.” Renewable Energy 40: 65–70.10.1016/j.renene.2011.09.022
- Mushrush, G. W., J. M. Hughes, and H. D. Willauer. 2013. “Blends of Soybean Biodiesel with Petroleum Diesel: Advantages.” Industrial & Engineering Chemistry Research 52: 1764–1768.10.1021/ie302865x
- No, Soo-Young. 2011. “Inedible Vegetable Oils and their Derivatives for Alternative Diesel Fuels in CI Engines: A Review.” Renewable and Sustainable Energy Reviews 15: 131–149.10.1016/j.rser.2010.08.012
- Obadiah, A., R. Kannan, A. Ramasubbu, and S. V. Kumar. 2012. “Studies on the Effect of Antioxidants on the Long-Term Storage and Oxidation Stability of Pongamia Pinnata (L.) Pierre Biodiesel.” Fuel Processing Technology 99: 56–63.10.1016/j.fuproc.2012.01.032
- Pullen, J., and K. Saeed. 2012. “An Overview of Biodiesel Oxidation Stability.” Renewable and Sustainable Energy Reviews 16: 5924–5950.10.1016/j.rser.2012.06.024
- Ramalho, E. F. S. M., J. R. Carvalho Filho, A. R. Albuquerque, S. F. de Oliveira, E. H. S. Cavalcanti, L. Stragevitch, I. M. G. Santos, and A. G. Souza. 2011. “Low Temperature Behavior of Poultry Fat Biodiesel: Diesel Blends.” Fuel 93: 601–605.
- Rashid, U., F. Anwar, and G. Knothe. 2009. “Evaluation of Biodiesel Obtained from Cottonseed Oil.” Fuel Processing Technology 90: 1157–1163.10.1016/j.fuproc.2009.05.016
- Sarin, A., N. P. Singh, R. Sarin, and R. K. Malhotra. 2010. “Natural and Synthetic Antioxidants: Influence on the Oxidative Stability of Biodiesel Synthesized from Non-Edible Oil.” Energy 35: 4645–4648.10.1016/j.energy.2010.09.044
- Shantha, N. C., and E. A. Decker. 1994. “Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Value of Food Lipids.” Journal of AOAC International 77: 421–424.
- Tang, H., A. Wang, S. Salley, and K. Y. Ng. 2008. “The Effect of Natural and Synthetic Antioxidants on the Oxidative Stability of Biodiesel.” Journal of the American Oil Chemists’ Society 85: 373–382.10.1007/s11746-008-1208-z
- Wang, X., C. P. Howell, Feng Chen, J. Yin, and Y. Jiang. 2009. “Gossypol – A Polyphenolic Compound from Cotton Plant.” Advances in Food Research 58: 215–263.10.1016/S1043-4526(09)58006-0
- Yang, Z., B. P. Hollebone, Z. Wang, C. Yang, and M. Landriault. 2013. “Factors Affecting Oxidation Stability of Commercially Available Biodiesel Products.” Fuel Processing Technology 106: 366–375.10.1016/j.fuproc.2012.09.001