Abstract
The ruthenium sawhorse has proven effective in the conversion of trans-cinnamic acid, and substituted trans-cinnamic acids, giving an effective source of biobased styrene and styrene analogues. The reaction is especially versatile, as it achieves product without utilising co-reagents. However, the optimum conditions and substrate scope of the reaction remain unexplored. This report covers the decarboxylation of a series of cinnamates with different structures. Apparent activation energies ranging from 66 to 142 kJ mol−1 were uncovered.
The development of industrially useful polymers from non-petroleum sources is a topic of seemingly everlasting interest (Biermann et al. Citation2000, Citation2011; Bozell et al. Citation2000). The conversion of bio-based components into materials that are identical to those already used in industry, a direct drop-in replacement strategy, may someday lead to a viable biorefinery (Yao and Tang Citation2013). Removing oxygen from biological materials is the key to this goal, especially for industrial use of natural oils. Currently, many different technologies are competing in this transformation (Dawes et al. Citation2015; Foglia and Barr Citation1976; Gooßen and Rodríguez Citation2004; Gooßen et al. Citation2009; Kraus Citation2014; Kraus and Riley Citation2012; Liu et al. Citation2014; Maki-Arvela et al. Citation2006; Miller, Nelson, and Byrne Citation1991, Citation1993; Miranda et al. Citation2012; Murzin et al. Citation2009; Popov and Kumar Citation2015; Snåre et al. Citation2006, Citation2008; Stern and Hillion Citation1985; Tanaka, Shimizu, and Yamamoto Citation1997; van der Klis et al. Citation2011, Citation2012). One of the most promising utilises only heat and a small amount of ruthenium catalyst, and does not require a co-reagent, such as an anhydride, peroxydisulphate (Murray, Doll, et al. Citation2014; Murray, Walter, et al. Citation2014) or the environmentally dreaded lead (Kochi, Sheldon, and Lande Citation1969). The initial report (Murray, Walter, et al. Citation2014) described the process on several different natural oil-based substrates including 10-undecenoic acid, 9-cis-octadecenoic acid (oleic acid) and cinnamic acid (Scheme ); yields ranged from 20 to74%, with the best conversion being from cinnamic acid to styrene.
This high-yield reaction is of interest in the quest for a biobased production route to polystyrene or acrylonitrile butadiene styrene thermoplastics. Natural trans-cinnamic acid is commonly obtained from oil of cinnamon (Klejdus and Kováčik Citation2016), but also in other plants of commercial interest such as shea butter (Maranz, Wiesman, and Garti Citation2003), basil, storax and cocoa (Hoskins Citation1984). It has been widely studied as an anti-bacterial, nutraceutical and cosmetic material (Edris Citation2007). However, there are no significant reports of the industrial uses of this versatile precursor. This report focuses on the catalytic decarboxylation of cinnamic acid, using a systematic approach to elucidate parameters, such as the apparent activation energy of decarboxylation. Additionally, the rate of decarboxylation is observed in substituted cinnamic acid analogs.
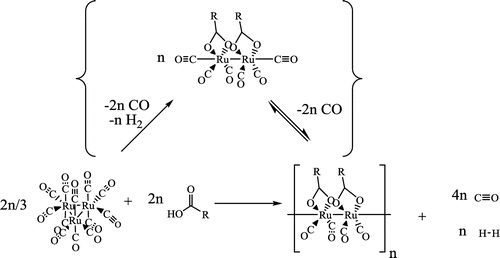
The synthesis of polymeric ruthenium compounds of ruthenium is dated back to the 1960s (Crooks et al. Citation1969). Starting from triruthenium dodecacarbonyl, the orange/yellow [Ru(CO)2(CH3COO)]n and analogous compounds were produced by the addition of carboxylic acid. The addition of ligands, such as pyridines, has allowed the isolation and production of high-quality crystals of the dinuclear compounds which have been termed, ‘sawhorses’ (Kepert et al. Citation2000). As well as in the work described earlier in this paper (Murray, Doll, et al. Citation2014; Murray, Walter, et al. Citation2014), triruthenium dodecacarbonyl and the sawhorse compounds, sometimes with other added ligands, have all been effective precatalysts in isomerisation (Castiglioni et al. Citation1976; Caulton et al. Citation1976; Salvini, Frediani, and Piacenti Citation2000; Sherlock et al. Citation1988; Sivaramakrishna et al. Citation2008; Sleeter Citation1998; Yang et al. Citation2016) and transvinylation (Ziriakus et al. Citation2013) reactions. The synthesis of the precatalyst used in this work, 2-ethylhexyl ruthenium sawhorse, [Ru(CO)2 (CH3(CH2)3C(CH2CH3)HCOO)]n, was performed analogously to the literature methods (Scheme ) where sawhorses containing propanoic and acetic acid groups were prepared (Crooks et al. Citation1969) from triruthenium dodecacarbonyl (Ru3(CO)12 (99%, Acros Organics, Pittsburgh, PA) or (99%, Strem Chemical, Newburyport, MA). Catalysts of this type have been previously synthesised in the literature by this method. (Doll et al. Citation2017; Kepert et al. Citation2000; Murray Citation1991; Yang et al. Citation2016; Ziriakus et al. Citation2013).
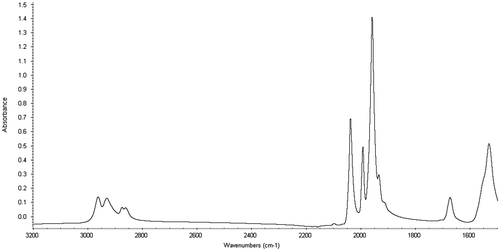
Although many different saturated carboxylic acids could have been chosen, 2-ethylhexanoic was used as the ligand for several reasons. First, 2-ethylhexanoic acid is widely available, being derived from a hydroformylation of propene followed by condensation and oxidation. Next, it is soluble in organic solvents which allowed homogeneous catalysis to take place. Finally, its branched structure has proven useful in the use of agriculturally based industrial products (Cermak, Brandon, and Isbell Citation2006; Doll, Sharma, and Erhan Citation2007). Because 2-ethylhexanoic acid (99%, Sigma-Aldrich, St. Louis, MO) is more difficult to remove than the smaller acids used in the literature, only a single equivalent compared to ruthenium was used, toluene solvent (99.8%, Fisher Scientific, Fairlawn, NJ) was utilised and a nitrogen atmosphere was maintained during the reaction using Schlenk techniques. The sawhorse was characterised by IR spectroscopy (Thermonicolet, Madison, WI,) Nexus 470 spectrophotometer equipped with a Smart Orbit accessory and diamond plate, 32 scans. Peaks in the spectrum are in the expected 2870–2965 cm−1 region, and definitively show the signs of successful sawhorse preparation from the carbonyl signals, 2064, 1994 1959 cm−1 (Figure ).
The protocol selected for this study involved using glass pressure vessels which was done for several reasons. First, the clear glass allowed direct observation of the solution during reaction. Next, the pressure vessels allow sampling during the reaction. Finally, pressure build-up could be safely monitored in this experimental set-up. In this study, reactions typically used ~30 mg of the sawhorse precatalyst which was weighed into a 3 oz glass pressure vessel, (Lab Crest-Andrews Glass, Vineland, NJ). Trans-cinnamic acid (99%, Sigma-Aldrich, St. Louis, MO), ~1 g, was dissolved in ~9 g of 2-ethylhexanoic acid (99%, Sigma-Aldrich, St. Louis, MO), and heated to 65 °C to ensure dissolution. The mixture was transferred to the pressure vessel containing the precatalyst and the vessel was purged with argon. The reactor was then pressurised to 25 lbs in−2 with argon, magnetic stirring was set to 200 RPM and heat was introduced using a silicone oil bath. Aliquots were taken at 1 h intervals using Vici (Baton Rouge, LA) precision sampling pressure lock syringes and analysed by gas chromatography (two different Agilent 7890 (Santa Clara, CA) gas chromatographs were employed. For product identification, a 5975 mass spectrometry detector was used, whereas flame ionisation detection was used for quantitation. Identical columns, Agilent/J&W (Santa Clara, CA) DB35-MS, 30 m x 320 um, 0.25 um film thickness; sample preparation, injections of 1 uL of samples made by diluting ~10 uL of reaction solution in 1 mL of acetone were done by autosampler using a 50:1 split ratio; and temperature/injection programmes, starting at 40 °C for 3 min, then was raised 10 °C min−1 to 190 °C and held for 5 min, then raised 25 °C min−1 to 340 °C; were used in each instrument. A pressure build-up of 35–55 lbs in−2 was observed for reactions with significant conversion.
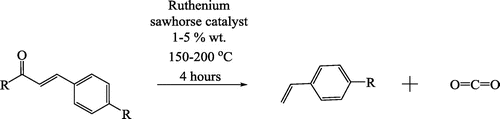
The reaction progress was followed over 4 h at temperatures of 150, 175 and 200 °C. As expected, the observed conversion increased over time, and was more rapid at higher temperatures (Figure ). The conversion was also studied with varied catalyst amounts, where conversion could be increased significantly by increasing catalyst from 1wt.% to 3wt.%, but further increase to 5 wt.% only gave further reaction nearly within experimental deviation of the 3 wt.% results (Figure ). Therefore, the 3 wt.% was used in the substrate studies.
Figure 2. The conversion of trans-cinnamic acid by the 2-ethylhexyl ruthenium sawhorse, 3% wt., at 150, 175 and 200 °C.
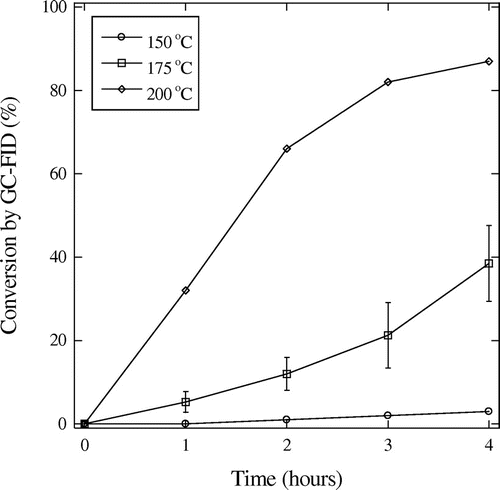
Figure 3. The conversion of trans-cinnamic acid by various concentrations of the 2-ethylhyxyl ruthenium sawhorse, at 175 °C, error bars were calculated as standard deviations with n = 3.
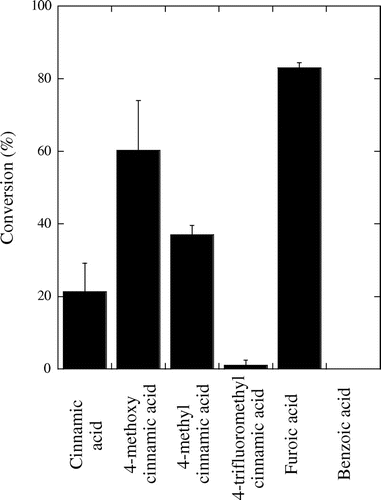
In order to test the substituent effects, cinnamic acid analogues were employed, where methyl, methoxy and trifluoromethyl groups, each on the para position of the cinnamic acid, were called on to give a variety of targets (Figure ). The methyl group, had a limited effect, yielding mildly increased conversion. The trans-4-methoxy cinnamic acid (99%, Sigma-Aldrich, St. Louis, MO) system converted much more rapidly than the other cinnamates. Further, trans-4-trifluoromethyl cinnamic acid (99%, Sigma-Aldrich, St. Louis, MO) system displayed almost no conversion.
Figure 4. The conversion of substrated cinnamic acids and other substrates by 2-ethylhexyl ruthenium sawhorse, 3% wt., at 175 °C over 3 h, error bars were calculated as standard deviations with n = 3.
Scheme 2. The synthesis of ruthenium sawhorse compounds. The carboxylic acids employed here include propanoic acid and 2-ethylhexanoic acid.
A few other interesting but expected facts were observed during this study. First, reactions with the 25 lbs in−2 of added argon gave results within error of non-pressurised reactions ran in the same reaction vessel. Next, there was significant polymerisation of the 4-methoxy styrene, of up to 17% of the GC-FID area at the highest conversions. To further expand the scope of this reaction, 2-furoic acid (99%, Sigma-Aldrich, St. Louis, MO) was also decarboxylated. This substrate, while not containing a linear olefin, does have a double bond in conjugation with the carboxylic acid function. It decarboxylated rapidly (Figure ), but reaction was not observed in benzoic acid (98%, Sigma-Aldrich, St. Louis, MO) which did not decarboxylate at all (Figure ). Levulinic acid (98%, Sigma-Aldrich, St. Louis, MO) also did not decarboxylate, indicating the importance of a double bond in the appropriate proximity to the carboxyl. Attempts to use triosmium dodecacarbonyl (99%, Strem, Newburyport, MA) in an analogous system fashion yielded only 7% conversion of cinnamic acid to styrene.
In order to check for potential side reactions such as decarbonylation, the aldehyde, octenal (99%, Lancaster, Ward Hill, MA), was subjected to the reaction conditions, and the only reaction observed was condensation. Aromatic aldehydes such as trans-cinnamaldehyde (99%, Sigma-Aldrich, St. Louis, MO) and benzaldehyde (99%, Sigma-Aldrich, St. Louis, MO) also did not react significantly. In other words, the sawhorse is not an effective decarbonylation catalyst under these conditions.
The scope of this reaction system has been further explored. Nitrogen containing substrates were utilised, including trans-4-dimethylamino cinnamic acid (99%, Sigma-Aldrich, St. Louis, MO) and nicotinic acid (98%, Sigma-Aldrich, St. Louis, MO), the latter being decarboxylated in a stainless steel reactor. In the dimethylamino case, insolubility prevented the reaction from occurring, again demonstrating that this is a truly homogeneous reaction. Nicotinic acid was decarboxylated in 29% yield. As a final comparison, cis-9-octadecenoic acid (oleic acid, (Nu-Check Prep, Elsyian, MN > 99%), trans-9-octadecenoic acid (elaidic acid > 99%, Nu-Check Prep, Elsyian, MN) and cis-6-octadecenoic acid (petroselinic acid (>99%, Nu-Check Prep, Elsyian, MN) were found to have identical reactivity using this catalyst in a stainless steel reactor.
Changing the reaction solvent from 2-ethylhexanoic acid to tetraglyme (99%, Sigma-Aldrich, St. Louis, MO) diminished the reaction conversion by a factor of 12. Mixtures of the two solvents gave results intermediate between the two solvent systems. In other words, ruthenium is the best choice for performing this conversion and it is best used in 2-ethylhexanoic acid.
In order to quantify these results, the initial rate of the first hour of each substrate was used. This method has been shown to give the most accurate value for pseudo-first order rate constants (Woodward and Mesrobian Citation1953), since at the low conversions, the substrate concentration can be considered a constant. The maximum value observed in this work was 0.000447 s−1, for the 4-methoxy substrate at 200 °C. For the three cinnamic substrates where the applicable data were available at a variety of temperatures, apparent activation energies can be calculated using an Arrhenius type calculation (Table ). The values, consistent with the limited available literature (Ortuño, Dereli, and Cramer Citation2016; Popov and Kumar Citation2015), show that ordinary cinnamic acid is more difficult to decarboxylate than the 4-methoxy substituted substrate could be decarboxylated with less than half of the energy.
Table 1. A calculation of apparent activation energies using the Arrhenius equation of rates obtained from the initial rate method, of the decarboxylation of trans-cinnamic acid and 4-substituted trans-cinnamic acids with the addition of 3% wt., 2-ethylhexyl ruthenium sawhorse.
Conclusion
Utilisation of biobased oils to produce high-quality polymers is important. However, even some of the most interesting reports in this area remain mysterious. This report clears some of the obscurity in the sawhorse-based decarboxylation of natural aromatic oils, and also highlights the need for an understanding of the interesting chemistry behind these bio-refinery relevant conversions.
Funding
This work was supported by the Agricultural Research Service [grant number 41000-170-00D].
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Kenneth M. Doll, PhD, is a lead scientist in the Bio-Oils research unit at the National Center for Agricultural Utilization Research. His research focuses on the use of biologically derived oils in industrial applications, specifically focusing on chemical modification of oils through catalytic reactions. He has published 70 articles in journals such as Green Chemistry, Industrial and Engineering Chemistry Research, and the International Journal of Sustainable Engineering.
Erin L. Walter, BS, is a physical science technician in the Bio-oils Research Unit at the National Center for Agricultural Utilization Research in Peoria, IL. The research project she is currently involved in identifies and develops new uses for plant based oils, most commonly soybean oil. Previous publications have appeared in Journal of the American Oil Chemists’ Society, ACS Catalysis, Chemical Engineering Communications, Industrial Crops and Products, and others.
Rex E. Murray, PhD, is a research leader of the Bio-Oils research unit at the National Center for Agricultural Utilization Research. He leads a team of researchers on several different projects including: Industrial Monomers and Polymers from Plant Oils, and Value added Bio Oil Products and Processes. He has published articles in Industrial and Engineering Chemistry Research, ACS Sustainable Chemistry and Engineering, and the Journal of the American Oil Chemists Society.
Acknowledgements
This work was a part of the in-house research of the Agricultural Research Service of the United States Department of Agriculture.
References
- Biermann, U., W. Friedt, S. Lang, W. Lühs, G. Machmüller, J. O. Metzger, M. Rüsch gen. Klaas, H. J. Schäfer, and M. P. Schneider. 2000. “New Syntheses with Oils and Fats as Renewable Raw Materials for the Chemical Industry.” Angewandte Chemie International Edition 39 (13): 2206–2224.10.1002/(ISSN)1521-3773
- Biermann, U., U. Bornscheuer, M. A. R. Meier, J. O. Metzger, and H. J. Schäfer. 2011. “Oils and Fats as Renewable Raw Materials in Chemistry.” Angewandte Chemie - International Edition 50 (17): 3854–3871.10.1002/anie.201002767
- Bozell, J. J., L. Moens, D. C. Elliott, Y. Wang, G. G. Neuenscwander, S. W. Fitzpatrick, R. J. Bilski, and J. L. Jarnefeld. 2000. “Production of Levulinic Acid and Use as a Platform Chemical for Derived Products.” Resources, Conservation and Recycling 28 (3–4): 227–239.10.1016/S0921-3449(99)00047-6
- Castiglioni, M., L. Milone, D. Osella, G. A. Vaglio, and M. Valle. 1976. “Reactions of Dodecacarbonyltriruthenium with Pentenes.” Inorganic Chemistry 15 (2): 394–396.10.1021/ic50156a030
- Caulton, K. G., M. G. Thomas, B. A. Sosinsky, and E. L. Muetterties. 1976. “Metal Clusters in Catalysis: Hydrocarbon Reactions.” Proceedings of the National Academy of Sciences 73 (12): 4274–4276.10.1073/pnas.73.12.4274
- Cermak, S. C., K. B. Brandon, and T. A. Isbell. 2006. “Synthesis and Physical Properties of Estolides from Lesquerella and Castor Fatty Acid Esters.” Industrial Crops and Products 23 (1): 54–64.10.1016/j.indcrop.2005.04.001
- Crooks, G. R., B. R. G. Johnson, J. Lewis, I. G. Williams, and G. Gamlen. 1969. “Chemistry of Polynuclear Compounds. Part XVII. Some Carboxylate Complexes of Ruthenium and Osmium Carbonyls.” Journal of the Chemical Society a: Inorganic, Physical, Theoretical 2761–2766.10.1039/j19690002761
- Dawes, G. J. S., E. L. Scott, J. Le Nôtre, J. P. M. Sanders, and J. H. Bitter. 2015. “Deoxygenation of Biobased Molecules by Decarboxylation and Decarbonylation – A Review on the Role of Heterogeneous, Homogeneous and Bio-Catalysis.” Green Chem 17 (6): 3231–3250.10.1039/C5GC00023H
- Doll, K. M., B. K. Sharma, and S. Z. Erhan. 2007. “Synthesis of Branched Methyl Hydroxy Stearates including an Ester from Bio-Based Levulinic Acid.” Industrial & Engineering Chemistry Research 46 (11): 3513–3519.10.1021/ie070127y
- Doll, K. M., G. B. Bantchev, E. L. Walter, R. E. Murray, M. Appell, J. C. Lansing, and B. R. Moser. 2017. “Parameters Governing Ruthenium Sawhorse-Based Decarboxylation of Oleic Acid.” Industrial & Engineering Chemistry Research 56 (4): 864–871.10.1021/acs.iecr.6b04555
- Edris, A. E. 2007. “Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review.” Phytotherapy Research 21 (4): 308–323.10.1002/(ISSN)1099-1573
- Foglia, T. A., and P. A. Barr. 1976. “Decarbonylation Dehydration of Fatty Acids to Alkenes in the Presence of Transition Metal Complexes.” Journal of the American Oil Chemists’ Society 53: 737–741.10.1007/BF02635473
- Gooßen, L. J., and N. Rodríguez. 2004. “A Mild and Efficient Protocol for the Conversion of Carboxylic Acids to Olefins by a Catalytic Decarbonylative Elimination Reaction.” Chemical Communications 724–725.10.1039/B316613A
- Gooßen, L. J., C. Linder, N. Rodríguez, P. P. Lange, and A. Fromm. 2009. “Silver-Catalysed Protodecarboxylation of Carboxylic Acids.” Chemical Communications 47: 7173–7175.10.1039/b912509d
- Hoskins, J. A. 1984. “The Occurrence, Metabolism and Toxicity of Cinnamic Acid and Related Compounds.” Journal of Applied Toxicology 4 (6): 283–292.10.1002/(ISSN)1099-1263
- Kepert, C. M., G. B. Deacon, L. Spiccia, G. D. Fallon, B. W. Skelton, and A. H. White. 2000. “A Facile and Benign Synthesis of Binuclear Ruthenium(I) ‘Sawhorse’ Complexes.” Journal of the Chemical Society, Dalton Transactions 16: 2867–2873.10.1039/b002304n
- Klejdus, B., and J. Kováčik. 2016. “Quantification of Phenols in Cinnamon: A Special Focus on ‘Total Phenols’ and Phenolic Acids including DESI-Orbitrap MS Detection.” Industrial Crops and Products 83: 774–780.10.1016/j.indcrop.2015.11.060
- Kochi, J. K., R. A. Sheldon, and S. S. Lande. 1969. “Rates of Photochemical and Thermal Decarboxylation of Acids by Lead (IV) Tetraacetate.” Tetrahedron 25 (6): 1197–1207.10.1016/S0040-4020(01)82693-4
- Kraus G. A. 2014. Method for Producing Olefins. US Patent 8629312, filed April 25, 2013.
- Kraus, G. A., and S. Riley. 2012. “A Large-Scale Synthesis of Α-Olefins and Α,ω-Dienes.” Synthesis 44 (19): 3003–3005.10.1055/s-00000084
- Liu, Y., K. E. Kim, M. B. Herbert, A. Fedorov, R. H. Grubbs, and B. M. Stoltz. 2014. “Palladium-Catalyzed Decarbonylative Dehydration of Fatty Acids for the Production of Linear Alpha Olefins.” Advanced Synthesis & Catalysis 356 (1): 130–136.10.1002/adsc.201301109
- Maki-Arvela, P., I. Kubickova, M. Snare, K. Eranen, and D. Y. Murzin. 2006. “Catalytic Deoxygenation of Fatty Acids and Their Derivatives.” Energy & Fuels 21 (1): 30–41.
- Maranz, S., Z. Wiesman, and N. Garti. 2003. “Phenolic Constituents of Shea (Vitellaria paradoxa) Kernels.” Journal of Agricultural and Food Chemistry 51 (21): 6268–6273.10.1021/jf034687t
- Miller J. A., J. A. Nelson, and M. Byrne 1991. Process of Making Olefins. US Patent 5077447, filed December 31, 1991.
- Miller, J. A., J. A. Nelson, and M. P. Byrne. 1993. “A Highly Catalytic and Selective Conversion of Carboxylic Acids to 1-Alkenes of One Less Carbon Atom.” The Journal of Organic Chemistry 58 (1): 18–20.10.1021/jo00053a008
- Miranda, M. O., A. Pietrangelo, M. A. Hillmyer, and W. B. Tolman. 2012. “Catalytic Decarbonylation of Biomass-Derived Carboxylic Acids as Efficient Route to Commodity Monomers.” Green Chemistry 14 (2): 490–494.10.1039/c2gc16115j
- Murray R. E. 1991. Transvinylation Reaction. US Patent 4981973, filed January 1, 1991.
- Murray, R. E., K. M. Doll, and Z. Liu. 2014. Process for Isomerization and Decarboxylation of Unsaturated Organic Compounds with a Metal Catalyst or Catalyst Precursor. US Patent 2014/0275592, filed September 18, 2014.
- Murray, R. E., E. L. Walter, and K. M. Doll. 2014. “Tandem Isomerization-Decarboxylation for Converting Alkenoic Fatty Acids into Alkenes.” ACS Catalysis 4 (10): 3517–3520.10.1021/cs501019t
- Murzin, D. Y., I. Kubickova, M. Snare, P. Maki-Arvela, and J. Myllyoja. 2009. Method for the Manufacture of Hydrocarbons. US Patent 7491858, filed February 17, 2009.
- Ortuño, M. A., B. Dereli, and C. J. Cramer. 2016. “Mechanism of Pd-Catalyzed Decarbonylation of Biomass-Derived Hydrocinnamic Acid to Styrene following Activation as an Anhydride.” Inorganic Chemistry 55 (9): 4124–4131.10.1021/acs.inorgchem.5b02664
- Popov, S., and S. Kumar. 2015. “Rapid Hydrothermal Deoxygenation of Oleic Acid over Activated Carbon in a Continuous Flow Process.” Energy and Fuels 29 (5): 3377–3384.10.1021/acs.energyfuels.5b00308
- Salvini, A., P. Frediani, and F. Piacenti. 2000. “Alkene Isomerization by Non-Hydridic Phosphine Substituted Ruthenium Carbonyl Carboxylates.” Journal of Molecular Catalysis a: Chemical 159 (2): 185–195.10.1016/S1381-1169(00)00153-9
- Sherlock, S. J., M. Cowie, E. Singleton, and M. M. D. V. Steyn. 1988. “A Convenient Route to an Uncommon Class of Polymeric and Binuclear Ruthenium(I) Complexes Containing Diphosphine and Dithioether Ligands.” Organometallics 7 (7): 1663–1666.10.1021/om00097a037
- Sivaramakrishna, A., P. Mushonga, I. L. Rogers, F. Zheng, R. J. Haines, E. Nordlander, and J. R. Moss. 2008. “Selective Isomerization of 1-Alkenes by Binary Metal Carbonyl Compounds.” Polyhedron 27 (7): 1911–1916.10.1016/j.poly.2008.02.026
- Sleeter, R. T.. 1998. Method of Conjugating Double Bonds in Drying Oils. US Patent 5719301, filed February 17, 1998.
- Snåre, M., I. Kubičková, P. Mäki-Arvela, K. Eränen, and D. Y. Murzin. 2006. “Heterogeneous Catalytic Deoxygenation of Stearic Acid for Production of Biodiesel.” Industrial & Engineering Chemistry Research 45 (16): 5708–5715.10.1021/ie060334i
- Snåre, M., I. Kubičková, P. Mäki-Arvela, D. Chichova, K. Eränen, and D. Y. Murzin. 2008. “Catalytic Deoxygenation of Unsaturated Renewable Feedstocks for Production of Diesel Fuel Hydrocarbons.” Fuel 87 (6): 933–945.10.1016/j.fuel.2007.06.006
- Stern, R., and G. Hillion. 1985. Process for Manufacturing a Linear Olefin from a Saturated Fatty Acid or Fatty Acid Ester. US Patent 4554397, filed November 19, 1985.
- Tanaka, S., K. Shimizu, and I. Yamamoto. 1997. “Syntheis of 1-Nonene Form Decanoic Acid by Polymer-Bound Palladium Complexes.” Chemistry Letters 26: 1277–1278.10.1246/cl.1997.1277
- van der Klis, F., M. H. van den Hoorn, R. Blaauw, J. van Haveren, and D. S. van Es. 2011. “Oxidative Decarboxylation of Unsaturated Fatty Acids.” European Journal of Lipid Science and Technology 113 (5): 562–571.10.1002/ejlt.201000126
- van der Klis, F., J. Le Nôtre, R. Blaauw, J. van Haveren, and D. S. van Es. 2012. “Renewable Linear Alpha Olefins by Selective Ethenolysis of Decarboxylated Unsaturated Fatty Acids.” European Journal of Lipid Science and Technology 114 (8): 911–918.10.1002/ejlt.201200024
- Woodward, A. E., and R. B. Mesrobian. 1953. “Low Temperature Autoxidation of Hydrocarbons. the Kinetics of Tetralin Oxidation 1,2.” Journal of the American Chemical Society 75: 6189–6195.10.1021/ja01120a024
- Yang, J. D., X. Wang, C. X. Song, W. Q. Zhang, G. F. Zhang, Z. Gao, J. Fan, and H. M. Sun. 2016. “The Thermolysis of Ru3(CO)12 with Carboxylic Acids Revisited: Stepwise Assembly of Ru2 to Ru6 Cluster Frameworks.” Chemistry Select 1 (17): 5397–5403.
- Yao, K., and C. Tang. 2013. “Controlled Polymerization of Next-Generation Renewable Monomers and beyond.” Macromolecules 46 (5): 1689–1712.10.1021/ma3019574
- Ziriakus, J., T. K. Zimmermann, A. Pöthig, M. Drees, S. Haslinger, D. Jantke, and F. E. Kühn. 2013. “Ruthenium-Catalyzed Transvinylation – New Insights.” Advanced Synthesis & Catalysis 355 (14–15): 2845–2859.10.1002/adsc.v355.14/15