?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Thermal behavior of honge oil methyl ester (HOME) and its B-20 blend (20% HOME and 80% diesel) is examined by performing calorimetric experiments at 10°C/min heating rate in atmospheric air and oxygen medium. Thermogravitometry (TG) curves indicate two phases of decomposition for diesel and three phases for biofuel. Combustion reaction favors in oxidative atmosphere causing reduction in fuel preparation stage and increase in premixed burning phase reducing peak temperature of combustion and increasing enthalpy with high heat release rate. B-20 blend performance is similar to diesel with combustion index and intensity of combustion and is thermally stable with high offset temperature confirming more combustion duration. Blend of diesel lowers activation energy in initial stage of combustion process, whereas reverse trend is observed in final stage. Ignition index (Di) in air for diesel, HOME, and its B-20 blend is reduced by 70.11%, 34.92% and 42.80% respectively. Burnout index (Db) in air for diesel and B-20 blend reduced by 72% and 61% respectively whereas it increased by 28.5% for HOME. Combustion index (S) is more in air for HOME and its blend. Improved intensity of combustion is observed for diesel and B-20 blend in oxygen whereas reverse trend is observed for HOME.
1. Introduction
High cost petroleum-based fuels and associated problems with global warming lead to the development of alternate energy sources (Manaf et al. Citation2019). Significant performance of biofuel from organic wastes indicates the possibility of utilising in automotives directly or blended with diesel (Ramkumar and Kirubakaran Citation2016; Rajasekar and Selvi Citation2014). 20% blending with petrol saves 13% of petrol. Mixing of 20% biofuel with diesel is efficient and economical (Banapurmath, Tewari, and Hosmath Citation2008; Ramalingam et al. Citation2019; Channappagoudra, Ramesh, and Manavendra Citation2019; Srikanth et al. Citation2020; Atgur et al. Citation2022a). Highly susceptible to auto-oxidation is one of the major concerns for biofuel producers. Oils from plants have different degrees of unsaturation and chemical composition (Vossoughi and El-Shoubary Citation1990). Oxidation initiates with loss of hydrogen atoms from structure leading to alkyl-radicals formation. Alkyl-radicals react with oxygen leading to peroxides formation (Vossoughi and El-Shoubary Citation1990). The reactions intern led to the formation of more alkyl-radicals and hydro peroxides consuming esters. The process ends with formation of aldehydes, esters, alkanes and ketones (Lai, Lin, and Violi Citation2011). Jatropha, pongamia, castor, mahua and neem are promising sources of alternative to diesel. The pongamia (known as karanja and honge) tree grows to 30 m height over all soil types (from stony to clayey except dry sands) in Western India. presents properties of diesel, Honge oil methyl ester (HOME) and its B-20 blend. Composition of fatty acids of HOME is in . Details on oxidation stability, thermal stability and storage conditions are highlighted in Jose and Anand (Citation2016); Christensen, Mccormick, and Con (Citation2014).
Table 1. Properties of biodiesel and diesel (Banapurmath, Tewari, and Hosmath Citation2008).
Table 2. Structure and composition of fatty acids of HOME (Banapurmath, Tewari, and Hosmath Citation2008; Ramalingam et al. Citation2019).
Dwivedi and Sharma (Dwivedi and Sharma Citation2016) examined the oxidation and thermal stability of pongamia biodiesel. Thermal degradation followed the first order reaction. Addition of Pyrogallol antioxidant increased the induction period and activation energy. The trend reversed in case of iron metal contaminants. Shancita et al. (Shancita et al. Citation2016) studied the effect of non-oxidative pongamia and moringa biodiesels. Iron bar dipped in the biodiesel during transesterification process and absorbed oxygen. Non-oxidative process improved the calorific value, oxidation stability, Cetane number of pongamia biodiesel. TG-DSC studies indicated improvement in storage stability, thermal and oxidation process of the non-oxidative biodiesel. Conceio et al. (Conceição et al. Citation2007) studied thermal degradation of castor oil biodiesel in synthetic air atmosphere. Percentage of mass loss, peak temperature and enthalpy recorded with temperature at 10°C/min heating rate. Stability of castor biodiesel observed up to 150°C and degraded at high temperature forming intermediate compounds. Bica et al. (Bică, Cernăianu, and Tutunea Citation2009) studied thermal degradation of the rapeseed biodiesel interpreting TG-DTG and DTA thermograms for B-10 to B-50 blends. Distillation interval reported to be high for the biodiesel and its blends. Samaraae et al. (Al-Samaraae et al. Citation2017) considered the safflower biodiesel and its blends and characterised the fuels with the addition of butanol using FTIR, TGA and DSC techniques. The biodiesel blends with diesel and butanol as ternary blend recommended up to 20%. Abiola et al. (Abiola et al. Citation2013) examined the influence of injection pressure, nozzle hole-diameter and fuel properties (such as boiling point, Cetane number and oxygen content on the spray), ignition and combustion on the palm oil biodiesel and diesel. High boiling point of palm biodiesel produced high burning phase length of flame. High Cetane number and oxygen content in the biodiesel led to shorter ignition delay. Santos et al. (Santos et al. Citation2016) examined thermal stability of oil, biodiesel and B-10 blend of different oilseeds. Andrade et al. (Andrade et al. Citation2012) considered the buriti oil biodiesel and its blends with diesel. Test media in the above investigations are air and nitrogen.
This paper deals with a comparative study on thermal behaviour of diesel, honge oil methyl ester (HOME) and its B-20 blend (80% diesel plus 20% biodiesel) in air and oxygen. TG-DSC curves are generated at 10°C/min heating rate. Activation energy of fuels is obtained from Arrhenius plots. TG curves indicated the phases of decomposition of fuels.
2. Materials and methods
This section highlights on testing of the produced honge oil methyl ester (HOME) and its B-20 blend, and examining their thermal behaviour from the TG-DSC curves for recommending a suitable alternative to diesel.
2.1 Materials
The University of Agriculture Science (Gandhi Krishi Vignyan Kendra (GKVK) Bangalore, Karnataka, India) supplied the honge oil methyl ester (HOME). Initially, B-20 blend (80% diesel plus 20% biodiesel) of the HOME prepared by magnetic stirrer for 15 min and followed by 20 min in ultrasonicator. Later on, glassworks like pipette, volumetric flask and graduate beaker used.
2.2 Thermogravitometry-Differential scanning calorimetry experiments
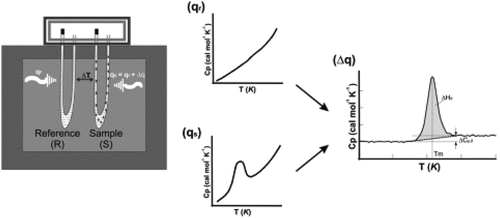
Differential Scanning Calorimetry (DSC) and Thermogravitometry (TG) are popular among thermoanalytical methods. In DSC experiments, physical properties of sample as well as heat flux allied with material transition measured with temperature and time. Heat transfer takes place from thermoelectric disk to pans. Each material exhibits different heat capacity, thereby, different heat absorption. Thermocouple used to measure temperature difference between sample and reference pan. Heat flow measured following the Ohm’s law. Principle of thermogravitometry is as follows. The sample heated at controlled rate in specific environment, viz., air, N2, CO2, He, Ar, and so on. Change in the substance weight recorded with temperature or time. The temperature increased at a constant rate for the known initial weight of the substance. The changes in weights recorded with temperature at different time interval. Weight change with temperature plots referred as the thermogravimetric curves or thermograms. Schematic diagram of TG-DSC system and equipment are in .
TG-DSC experiments performed in air and oxygen using NETZSCH −449F3 simultaneous thermal analyser for 10°C/min heating rate; and the alumina crucible pan with universal cramper for 20 ml/min gas flow rate. Nitrogen is used as protective gas in experiments. Temperature and weight calibrations carried out using sapphire material with different heating rates up to its curie point.
The steps followed during experimentation are as follows.
Selection of pan depending on the sample chemical composition (Aluminium, copper and platinum).
Empty pan weighted initially to get accurate weight of sample.
4 to 8 gms of sample loaded in the pan. The pan sealed hermetically with universal crimper.
Checked sealing to avoid splashing of the sample outside the pans and to safe-guard the sensor.
Temperature range selected with heating rate of 10°C/min.
Purge gas (nitrogen) and balance gas selected.
In pyrolysis experiments, nitrogen selected as balance gas, whereas air selected for combustion with appropriate flow rate.
After experimentation, signals recorded to examine the responses: Time (min), temperature (C), heating flow (W/mg), sample purge gas, and flow rates (ml/min).
The reaction enthalpy obtained by integrating the area of the reaction peak and the interpolated baseline between beginning and end of the reaction.
2.3 Combustion characteristics of biodiesels
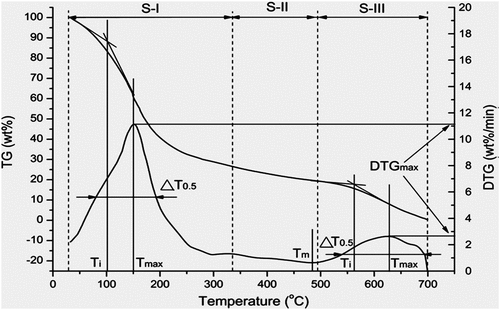
Combustion characteristics of the biodiesels studied in air and oxygen. depicts stages of combustion in a typical TG-DTG curve for estimation of ignition temperature (Ti).
The temperature at DTGmax is denoted by . Tangent lines on TG-curve at the beginning and at
intersected at the ignition temperature (Ti) as in stages S-I and S-III (Ma et al. Citation2006). At burn-out temperature (Tb), 98% of conversion (α) occurs at the single stage of combustion process (Li et al. Citation2006). The conversion
defined from the weights at the beginning and end of combustion (
and
) and the weight (
) at temperature (T) as (Li et al. Citation2006)
Ignition index and burnout index
evaluated from (Atgur et al. Citation2022b)
Here, ΔT0.5 is the half peak width of the DTG curve
Combustion index (S), DTGmean, and intensity of combustion evaluated from
Low value of with high ignition and burnout characteristics indicate better combustion properties.
3. Results and discussion
This section presents TG-DSC analysis results on thermal behaviour of produced HOME and its B-20 blends, their suitability in the category of alternative fuels to diesel.
3.1 Differential scanning calorimetry (DSC) analysis
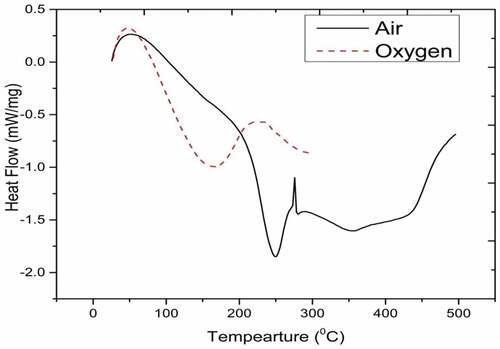
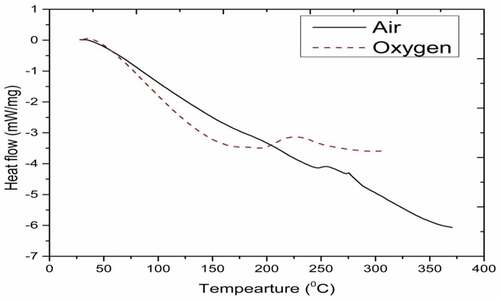
The DSC curves in represent for diesel in air and oxygen. Generally, hydrocarbons combustion takes place in three stages, namely, distillation, low temperature oxidation (i.e. cracking reaction), and combustion process. Initially, diesel exhibits fuel preparation stage (which is endothermic in nature) from 30 to 104°C in air medium while in the oxidative atmosphere, it is from 30 to 82.69°C. Further, it continues heat flow to the environment signifying exothermic reaction (Zhao et al. Citation2012; Dantas et al. Citation2011).
DSC thermogram of diesel (see ) indicates the endothermic reaction in the evaporation zone of 34–100°C. As fuel prepares for combustion, small fraction of diesel volatilise up. Only one step endothermic cracking reaction occurs (which is termed as distillation of the diesel). Combustion curve of hydrocarbons exhibits endothermic reaction initially in evaporation zone. Heat flow continues to environment indicating exothermic reaction. Higher heat release rate with premixed burning phase occurs during diesel combustion resulting in high thermal efficiency.
Peak temperature of combustion and enthalpy in air are 250°C and 130.4 J/g. In oxygen, they are 224.2°C and 140.7 J/g. The carbon number in mineral diesel is in the range of 5–12 with boiling temperature varying from 184 to 370°C (Febrero et al. Citation2014; Vinay Atgur, Desai, and Nageswara Rao Citation2021). Oxidative atmosphere favours the combustion reaction by reducing the duration of fuel preparation and temperature by 22%. Premixed burning phase in oxidative atmosphere is more than that of air. There by, reducing peak temperature of combustion and increasing enthalpy with high heat release rate and better thermal efficiency.
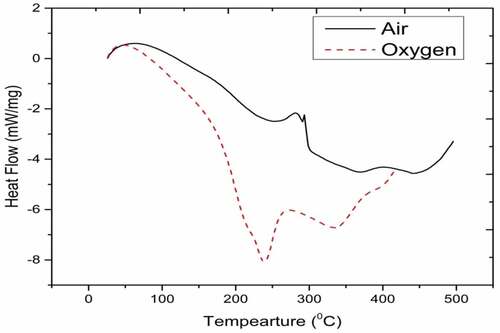
shows DSC curve for HOME in air and oxygen with reaction ranging from 118.46 to 438°C and 81.1 to 335°C, respectively. Peak combustion temperature occurs at 293 and 400.5°C in air, whereas in oxygen it occurs at 239.3°C with high enthalpy of −1375 J/g. Similar to diesel the end point of fuel preparatory phase for HOME reduces from 118.46°C to 81.1°C in air and oxygen. Combustion occurs in HOME at high temperature due to wide range of carbon number C14-C20 (Mohammed et al. Citation2018). Oxidative atmosphere for biodiesel favours the combustion reaction. Presence of weaker double bond and oxygen content in fuel structure helps the combustion reaction to proceed efficiently, which is justified from the high enthalpy of 1375 J/g (Vinay Atgur et al. Citation2022). In engine, HOME led short delay in time due to high Cetane number.
Short premixed heat release rate occurred in biodiesel combustion. Continuation of combustion process enhanced diffusion burning phase (Vinay Atgur, Desai, and Nageswara Rao Citation2022). Transesterification reaction of biodiesel made more ignitable triglycerides.
The DSC thermogram of B-20 blend in air and oxygen in indicates occurrence of combustion exothermic reaction in the initial stage at 33.1°C in air and 41°C in oxygen. Peak combustion temperature and enthalpy occurs at 274°C and 102.4 J/g in air, whereas in oxygen these are 226°C and 120.6 J/g, respectively (Vinay Atgur et al. Citation2022). DSC curves confirm that molecules of biodiesel and diesel are mixed homogeneously at 20%. In the initial phase, diesel combustion takes place following the combustion of biodiesel (Vinay Atgur, Desai, and Nageswara Rao Citation2022). B-20 blend combustion profile is identical to diesel. Enhancing the mixing rate of biodiesel required increasing of temperature for cracking reaction. More heat required to crack the molecule. Hence, mixing of biodiesel makes the fuel more stable. The peak combustion temperature of B-20 blend is reduced. The reaction starts in the early stage releasing heat to the atmosphere. provides the reaction region, peak combustion temperature heat flow and enthalpy for diesel, HOME and B-20 blend in air and oxygen.
Table 3.. Reaction region, peak temperature, heat flow and enthalpy in air and oxygen.
Little quantity (3 to 7 ml) of fuel required in sample testing, whereas engine testing required more quantity (250 ml to 1 litre) of fuel and hence the combustion occurs above 1000 K (Atgur et al. Citation2022b; Vinay Atgur, Desai, and Nageswara Rao Citation2021; Mohammed et al. Citation2018; Vinay Atgur, Desai, and Nageswara Rao Citation2022).
3.2 Thermogravitometry-Derivative thermogravitometry (TG-DTG) analysis
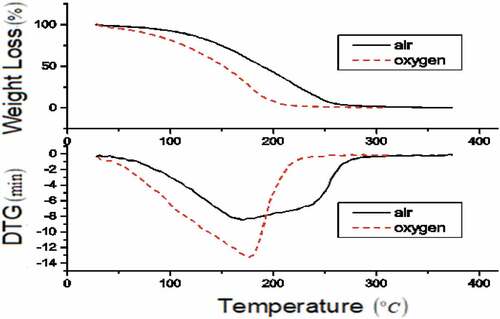
TG-DTG curves in are for diesel in synthetic air and oxygen. Thermal decomposition process involves two phases. In the initial phase oxidation of lighter compounds in diesel fuel takes place (Chabane et al. Citation2018). In the air phase-1 occurs from 30 to 100°C with 10% mass loss, whereas it occurs at 66°C in oxygen atmosphere. Second phase decomposition in air occurs from 100 to 280°C with 90% mass loss and at 340°C complete degradation taking place. In oxygen atmosphere phase-2 decomposition occurs from 100 to 210°C with 90% mass loss and at 290°C with complete mass loss. Broader thermal decomposition profile for diesel is due to many organic compounds in composition (Chabane et al. Citation2018). Peak decomposition occurs for diesel in air and oxygen atmosphere at 184.5°C and 146.9°C, respectively. Uniform DTG curve at 300°C and 230°C in air and oxygen atmosphere indicates insignificant temperature reactivity. Remaining 0.5% mass loss corresponds to the non-burning soot particle.
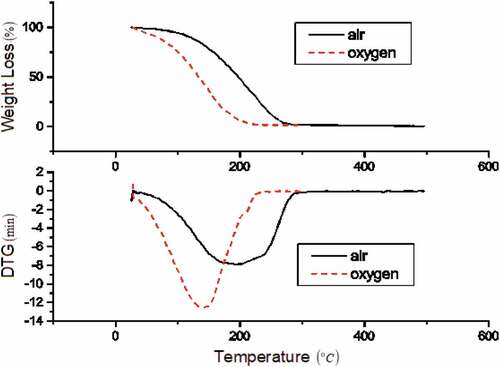
The TG-DTG profile in is for HOME. Decomposition takes place at three phases of reaction. But, the temperature profile differs due to fatty acids (Nicolau et al. Citation2018). HOME is stable up to 130°C in air and oxygen. Phase-1 decomposition (means low temperature oxidation) for HOME, corresponds to the water content removal. Low temperature volatiles like alcohols used in transterification (Almazrouei and Janajreh Citation2019). In air, phase-1 occurs from 30 to 198.45°C, whereas in oxygen, it occurs from 30 to 163.69°C with 10% mass loss. Phase-2 and phase-3 decomposition are important for HOME over 198.4 to 300°C in air and 163.69 to 250°C in oxygen. Majority of fatty acids present in HOME. Linoleic acid, oleic acid and palmitic acid decompose with boiling temperature of 230°C, 268°C and 310°C (Peer et al. Citation2017; Loo et al. Citation2015). Fatty acids such as linolenic, behenic and stearic acids decompose above 300°C. Third phase of decomposition (related to the rest 2% mass through products of the fuel oxidation process) is nothing but the impurities (like degradation of residuals from previous phases 1 and 2) of aldehydes, ketones, hydroperoxides and carboxylic acids (Silva et al. Citation2014; Jain and Sharma Citation2011). 90% mass loss occurs at 313.46°C in air and at 288.69°C in oxygen. Maximum decomposition occurs in DTG profile at 271.4°C in air, whereas in oxygen at 171.4°C and 230.9°C. Inactive reaction occurred in air and oxygen above 350°C and 300°C, respectively.
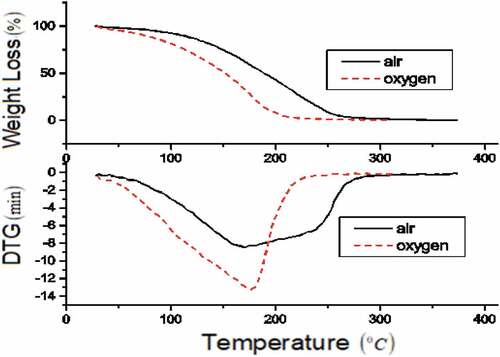
The TG-DTG profile of B-20 blend is in . Blend thermogram is similar to diesel indicating two phases of decomposition reaction. Mixed blend of HOME and diesel molecules exhibited homogenous at 20% (Jain and Sharma Citation2012). Blend decomposed at high offset temperature (Soto et al. Citation2018; Oliveira and Dweck Citation2018). Blend decomposition temperature increased the biodiesel content (Candeia et al. Citation2007). Blends thermal decomposition occurred in air from 58 to 305°C and in oxygen from 34°C to 269°C. In phase-1, lighter fractions of Naphthenes, olefins, paraffins and aromatics in diesel starts decomposition followed by evaporation and combustion of methyl esters in HOME and finally led to carbonisation (Santos et al. Citation2016). From DTG profile of the blend, maximum decomposition in air is at 196.7°C, whereas in oxygen it is at 173.1°C. Degradation reaction becomes inactive in air at 210°C, whereas in oxygen at 290°C. From the TG-DTG curve, oxidative atmosphere combustion process shifted to lower temperature side and reaction occurs intensively. provides the onset, offset temperature, reaction region, and maximum decomposition temperature and weight loss for diesel, HOME and B-20 blend.
Table 4. Onset, offset temperature, reaction region, maximum decomposition temperature and weight loss in air and oxygen atmosphere.
3.3 Kinetic analysis
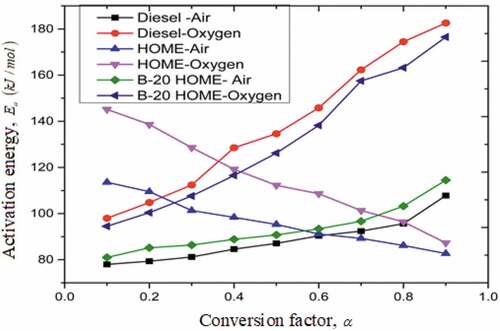
The activation energy of diesel, HOME and B-20 blend in is for the conversion factor varying from 0.1 to 0.9, which is the least energy, required crossing the barrier for initiation of chemical reaction (Bezerra et al. Citation2017). HOME starts at the activation energy of 113.6 kJ/mol in air and 145.2 kJ/mol in oxygen. Gradual decay in activation energy observed due to progression of decomposition and reached to 82.7 and 87.3 kJ/mol in air and oxygen, respectively. The trend in diesel is reverse. That is, kinetic energy during degradation increased gradually up to the activation energy of 107.8 kJ/mol in air and 182.6 kJ/mol in oxygen (Farias et al. Citation2011). Initially the activation energy of B-20 blend in air is 81 kJ/mol, whereas in oxygen it is 94.5 kJ/mol. The activation energy increased due to progression of decomposition. For the blend up to conversion factor of 0.5, activation energy is intermediate of HOME and diesel with negative slope in oxygen. Positive slope of the activation energy above 0.6 conversion factor is due to high compounds in the diesel (C12- C18), thereby affecting the thermal stability of the blend (Conconi and Manoel Citation2013). In blends, presence of diesel decreased the activation energy in the initial phase of decomposition. Consequently, affects the ignition performance of blends (Chouhan, Singh, and Sarma Citation2013). Average kinetic energy in air and oxygen for diesel is 88.50 and 138.17 kJ/mol; for HOME is 96.39 and 115.28 kJ/mol; finally for B-20 blend is 93.34 and 131.18 kJ/mol. Presence of HOME in B-20 blend influenced combustion phenomenon (Volli and Purkait Citation2014). High Cetane number (low activation energy) followed by shorter ignition delay. From Table-1, HOME exhibits high Cetane number.
Details of ignition temperature, burnout temperature, and maximum DTG are in . Compared to HOME, diesel and B-20 blend have less devolatisation stage and HOME degradation represents in three stages (Ren et al. Citation2014; Vinay Atgur et al. Citation2021). Combustion characteristics of diesel, HOME and B-20 HOME in air and oxidative atmosphere are in showing combustion index of volatilisation and combustion stages. Combustion index differed in air and oxidation environment due to high degradation temperature of combustion (Niu, Han, and Lu Citation2011). Low mass degradation in later stage is due to low combustion index. HOME has significant mass degradation after third stage leading to high intensity of combustion in-turn hard burning.
Table 5. Ignition, burnout temperature, DTGmax and Tmax of Diesel, HOME and B-20 HOME in air and oxidative atmosphere.
Table 6. Combustion characteristics of Diesel, HOME and B-20 HOME in air and oxidative atmosphere.
4. Concluding remarks
Thermal behaviour of diesel, HOME and its B-20 blend is examined in this paper performing TG-DSC analysis in air and oxygen. Decomposition of carbonados mineral occurred in a single step in air and two steps in oxygen. In oxygen environment, DSC curves are indicative of decreasing trend in the reaction region and maximum enthalpy of transaction. Peak temperature of combustion reduces by 20% in oxygen. B-20 HOME exhibits maximum enthalpy and reduced peak temperature of combustion indicating its suitability for combustion applications. Thermal stability is in the following order HOME > B-20 HOME > Diesel. High range of offset temperature for B-20 blend HOME, indicate more duration of combustion process when compared to diesel. Decomposition reaction region decreased for all samples in oxygen and degradation completes at lower temperature when compared to air atmosphere. Presence of diesel in blend lowers activation energy in the earlier phase and subsequently increases the ignition performance due to release of low molecular weight compounds in diesel and its interaction with other fuels in the blend. Presence of biodiesel influences the combustion performance. Thermal degradation for HOME takes place in three stages whereas two stages for diesel and B-20 blend. Therefore, HOME represents better combustion index. In engine, it led to hard burning justifying high intensity of combustion. Environmental studies on fuel samples are useful for minimising emissions.
Abbreviations
B-20 Biodiesel 20%, Diesel 80%.
HOME Honge oil methyl ester
DSC Differential Scanning Calorimetry
TG-DTG Thermogravitometry-Derivative Thermogravitometry
Acknowledgements
The authors would like to acknowledge greatly to the Visvesvarayya Technological University, Belgaum and Sophisticated Analytic instrumentation facility centre, Indian Institute of Technology, Madras for providing testing facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Vinay Atgur
Vinay Atgur is Associate Professor in Mechanical Engineering Department, KL Deemed to be University, Vaddeswaram.
G. Manavendra
G. Manavendra is Professor, Department of Mechanical Engineering at BIET, Davangere.
G.P. Desai
G.P. Desai is Professor, Department of Chemical Engineering, BIET, Davangere.
N. R. Banapurmath
N.R. Banapurmath is Head Centre of Excellence in Material Science, Professor, Mechanical Engineering KLE Technological University, Hubballi.
Chandramouli Vadlamudi
Chandramouli Vadlamudi is Aerospace integration Engineers at Aerosapien Technologies, USA.
Sanjay Krishnappa
Sanjay Krishnappa is Aerospace integration Engineers at Aerosapien Technologies, USA.
B. Nageswara Rao
B. Nageshwar Rao is Professor, Mechanical Engineering Department at KL Demmed to be University, Vaddeswaram.
References
- Abiola, O., J. Zhu, K. Nishida, X. Wang, and Z. Huang. 2013. “Characterization of Spray and Combustion Processes of Biodiesel Fuel Injected by Diesel Engine Common Rail System.” Fuel 104: 838–846. doi:10.1016/j.fuel.2012.05.014.
- Almazrouei, M., and I. Janajreh. 2019. “Thermogravimetric Study of the Combustion Characteristics of Biodiesel and Petroleum Diesel.” Journal of Thermal Analysis and Calorimetry 136 (2): 925–935. doi:10.1007/s10973-018-7717-6.
- Al-Samaraae, R. R., A. E. Atabani, G. Uguz, G. Kumar, O. Arpa, A. Ayanoglu, and H. Farouk. 2017. “Perspective of Safflower (Carthamus Tinctorius) as a Potential Biodiesel Feedstock in Turkey: Characterization, Engine Performance and Emissions Analyses of butanol–biodiesel–diesel Blends.” Biofuels 7269 (November): 1–17. doi:10.1080/17597269.2017.1398956.
- Andrade, R. D. A., E. Pozzebom, E. A. Faria, F. D. Filho, P. A. Z. Suarez, and A. G. S. D. Prad. 2012. “Thermal Behavior of diesel/biodiesel Blends of Biodiesel Obtained from Buriti Oil.” Acta Scientiarum. Technology 34 (2): 243–248. doi:10.4025/actascitechnol.v34i2.12797.
- Atgur, V., G. Manavendra, N. R. Banapurmath, B. N. Rao, A. A. Rajhi, T. M. Y. Khan, C. Vadlamudi, S. Krishnappa, A. M. Sajjan, and R. Venkatesh. 2022a. “Essence of Thermal Analysis to Assess Biodiesel Combustion Performance.” Energies 15 (18): 6622. doi:10.3390/en15186622.
- Atgur, V., G. Manavendra, G. P. Desai, B. N. Nageswara Rao, I. M. Rizwanul Fattah, B. A. Mohamed, N. Sinaga, and H. H. Masjuki. 2022b. “Thermogravimetric and Combustion Efficiency Analysis of Jatropha Curcas Biodiesel and Its Derivatives.” Biofuels 13 (9): 1069–1079. doi:10.1080/17597269.2022.2090136.
- Banapurmath, N. R., P. G. Tewari, and R. S. Hosmath. 2008. “Combustion and Emission Characteristics of a Direct Injection, compression-ignition Engine When Operated on Honge Oil, HOME and Blends of HOME and Diesel.” International Journal of Sustainable Engineering 1 (2): 80–93. doi:10.1080/19397030802221265.
- Bezerra, T., M. Gomes, A. Manoel, B. Dantas, E. Arruda, K. C. Arau, and G. De Bitu. 2017. “Blends of Diesel and Biodiesel of Cooking Oil Waste and Moringa (Moringa Oleı´ Fera Lam): Kinetic and Thermal Analysis and Monitoring during Storage.” Int J Energy Environ Eng 135–141. doi:10.1007/s40095-017-0232-x.
- Bică, M., C. Cernăianu, and D. Tutunea. 2009. “Thermogravimetric Analysis of Different Blends of Biodiesel of Rapeseed and Petrodiesel.” Bulletin of the Transilvania University of Brasov 2 (51): 2–5. http://193.254.231.99/jspui/bitstream/123456789/1350/1/Bica.pdf.
- Candeia, R. A., J. C. O. Freitas, M. A. F. Souza, M. M. Conceição, I. M. G. Santos, L. E. B. Soledade, and A. G. Souza. 2007. “Thermal and Rheological Behavior of Diesel and Methanol Biodiesel Blends.” Journal of Thermal Analysis and Calorimetry 87 (3): 653–656. doi:10.1007/s10973-006-7861-2.
- Chabane, S., M. Benziane, K. Khimeche, D. Trache, S. Didaoui, and N. Yagoubi. 2018. “Low-temperature Behavior of diesel/biodiesel Blends: Solid–liquid Phase Diagrams of Binary Mixtures Composed of Fatty Acid Ethyl Esters and Alkanes.” Journal of Thermal Analysis and Calorimetry 131 (2): 1615–1624. doi:10.1007/s10973-017-6614-8.
- Channappagoudra, M., K. Ramesh, and G. Manavendra. 2019. “Comparative Study of Standard Engine and Modified Engine with Different Piston Bowl Geometries Operated with B20 Fuel Blend.” Renewable Energy 133: 216–232. doi:10.1016/j.renene.2018.10.027.
- Chouhan, A. P. S., N. Singh, and A. K. Sarma. 2013. “A Comparative Analysis of Kinetic Parameters from TGDTA of Jatropha Curcas Oil, Biodiesel, Petroleum Diesel and B50 Using Different Methods.” Fuel 109: 217–224. doi:10.1016/j.fuel.2012.12.059.
- Christensen, E., R. L. Mccormick, and O. Con. 2014. “Long-term Storage Stability of Biodiesel and Biodiesel Blends.” Fuel Processing Technology 128: 339–348. doi:10.1016/j.fuproc.2014.07.045.
- Conceição, M. M., V. J. Fernandes, A. S. Araújo, M. F. Farias, I. M. G. Santos, and A. G. Souza. 2007. “Thermal and Oxidative Degradation of Castor Oil Biodiesel.” Energy and Fuels 21 (3): 1522–1527. doi:10.1021/ef0602224.
- Conconi, C. C., and P. Manoel. 2013. “Thermal Behavior of Renewable Diesel from Sugar Cane, Biodiesel, Fossil Diesel and Their Blends.” Fuel Processing Technology 114: 6–11. doi:10.1016/j.fuproc.2013.03.037.
- Dantas, M. B., A. R. Albuquerque, L. E. B. Soledade, N. Queiroz, S. M. Ary, I. M. G. Santos, A. L. Souza, E. Cavalcanti, A. K. Barro, and A. G. Souza. 2011. “Biodiesel from Soybean Oil, Castor Oil and Their Blends : Oxidative Stability by PDSC and Rancimat.” Journal of Thermal Analysis and Calorimetry 106 (2): 607–611. doi:10.1007/s10973-011-1410-3.
- Dwivedi, G., and M. P. Sharma. 2016. “Experimental Investigation on Thermal Stability of Pongamia Biodiesel by Thermogravimetric Analysis.” Egyptian Journal of Petroleum 25 (1): 33–38. doi:10.1016/j.ejpe.2015.06.008.
- Farias, R. M. C., M. M. Conceic, M. C. D. Silva, A. G. Souza, V. J. Fernandes, and A. G. Souza. 2011. “Evaluation of the Thermal Stability of Biodiesel Blends of Castor Oil and Passion Fruit.” Journal of Thermal Analysis and Calorimetry 106 (3): 651–655. doi:10.1007/s10973-011-1566-x.
- Febrero, L., E. Granada, C. Pérez, D. Patiño, and E. Arce. 2014. “Characterisation and Comparison of Biomass Ashes with Different Thermal Histories Using TG-DSC.” Journal of Thermal Analysis and Calorimetry 118 (2): 669–680. doi:10.1007/s10973-014-3717-3.
- Jain, S., and M. P. Sharma. 2011. “Thermal Stability of Biodiesel and Its Blends: A Review.” Renewable and Sustainable Energy Reviews 15 (1): 438–448. doi:10.1016/j.rser.2010.08.022.
- Jain, S., and M. P. Sharma. 2012. “Correlation Development between the Oxidation and Thermal Stability of Biodiesel.” Fuel 102: 354–358. doi:10.1016/j.fuel.2012.06.110.
- Jose, T. K., and K. Anand. 2016. “Effects of Biodiesel Composition on Its Long Term Storage Stability.” FUEL 177: 190–196. doi:10.1016/j.fuel.2016.03.007.
- Lai, J. Y. W., K. C. Lin, and A. Violi. 2011. “Biodiesel Combustion : Advances in Chemical Kinetic Modeling.” Progress in Energy and Combustion Science 37 (1): 1–14. doi:10.1016/j.pecs.2010.03.001.
- Li, X., B. Ma, L. Xu, Z. Hu, and X. Wang. 2006. “Thermogravimetric Analysis of the co-combustion of the Blends with High Ash Coal and Waste Tyres.” Thermochimica Acta 441 (1): 79–83. doi:10.1016/j.tca.2005.11.044.
- Loo, L., B. Maaten, A. Siirde, T. Pihu, and A. Konist. 2015. “Experimental Analysis of the Combustion Characteristics of Estonian Oil Shale in Air and oxy-fuel Atmospheres.” Fuel Processing Technology 134: 317–324. doi:10.1016/j.fuproc.2014.12.051.
- Ma, B.-G., X.-G. Li, L. Xu, K. Wang, and X.-G. Wang. 2006. “Investigation on Catalyzed Combustion of High Ash Coal by Thermogravimetric Analysis.” Thermochimica Acta 445 (1): 19–22. doi:10.1016/j.tca.2006.03.021.
- Manaf, I. S. A., N. H. Embong, S. N. M. Khazaai, M. H. A. Rahim, M. M. Yusoff, K. T. Lee, and G. P. Maniam. 2019. “A Review for Key Challenges of the Development of Biodiesel Industry.” Energy Conversion and Management 185 (November 2018): 508–517. doi:10.1016/j.enconman.2019.02.019.
- Mohammed, M. N., A. E. Atabani, G. Uguz, C. H. Lay, G. Kumar, and R. R. Al-Samaraae. 2018. “Characterization of Hemp (Cannabis Sativa L.) Biodiesel Blends with Euro Diesel, Butanol and Diethyl Ether Using FT-IR, UV–Vis, TGA and DSC Techniques.” Waste and Biomass Valorization 1–17. doi:10.1007/s12649-018-0340-8.
- Nicolau, C. L., A. N. V. Klein, C. A. A. Silva, A. R. Fiorucci, J. M. Stropa, E. O. Santos, K. C. S. Borges, et al. 2018. “Thermal Properties of the Blends of Methyl and Ethyl Esters Prepared from Babassu and Soybean Oils.” Journal of the Brazilian Chemical Society 29 (8): 1672–1679. doi:10.21577/0103-5053.20180040.
- Niu, S., K. Han, and C. Lu. 2011. “Characteristic of Coal Combustion in oxygen/carbon Dioxide Atmosphere and Nitric Oxide Release during This Process.” Energy Conversion and Management 52 (1): 532–537. doi:10.1016/j.enconman.2010.07.028.
- Oliveira, T. F., and J. Dweck. 2018. “Liquid Phase Oxidation Quantitative Analysis of biodiesel/diesel Blends by Differential TG and DTA.” Journal of Thermal Analysis and Calorimetry 134 (3): 1–11. doi:10.1007/s10973-018-7298-4.
- Peer, M. S., R. Kasimani, S. Rajamohan, and P. Ramakrishnan. 2017. “Experimental Evaluation on Oxidation Stability of biodiesel/diesel Blends with Alcohol Addition by Rancimat Instrument and FTIR Spectroscopy.” Journal of Mechanical Science and Technology 31 (1): 455–463. doi:10.1007/s12206-016-1248-5.
- Rajasekar, E., and S. Selvi. 2014. “Review of Combustion Characteristics of CI Engines Fueled with Biodiesel.” Renewable and Sustainable Energy Reviews 35 (x): 390–399. doi:10.1016/j.rser.2014.04.006.
- Ramalingam, S., S. Sudagar, R. Balamurugan, and R. Naveen. 2019. “Performance and Emission Behavior of Biofuel from Milk Scum: A Waste Product from Dairy Industry.” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. doi:10.1080/15567036.2019.1633441.
- Ramkumar, S., and V. Kirubakaran. 2016. “Biodiesel from Vegetable Oil as Alternate Fuel for C.I Engine and Feasibility Study of Thermal Cracking: A Critical Review.” Energy Conversion and Management 118: 155–169. doi:10.1016/j.enconman.2016.03.071.
- Ren, X., J. Meng, A. M. Moore, J. Chang, J. Gou, and S. Park. 2014. “Thermogravimetric Investigation on the Degradation Properties and Combustion Performance of bio-oils.” Bioresource Technology 152: 267–274. doi:10.1016/j.biortech.2013.11.028.
- Santos, A. G. D., V. P. S. Caldeira, L. D. Souza, D. S. Oliveira, A. S. Araujo, and G. E. Luz. 2016. “Study of the Thermal Stability by Thermogravimetry for Oil, Biodiesel and Blend (B10) of Different Oilseeds.” Journal of Thermal Analysis and Calorimetry 123 (3): 2021–2028. doi:10.1007/s10973-015-4943-z.
- Shancita, I., H. H. Masjuki, M. A. Kalam, S. S. Reham, A. M. Ruhul, and I. M. Monirul. 2016. “Evaluation of the Characteristics of non-oxidative Biodiesels: A FAME Composition, Thermogravimetric and IR Analysis.” RSC Advances 6 (10): 8198–8210. doi:10.1039/c5ra23963j.
- Silva, T. A., R. M. N. De Assunção, A. T. Vieira, M. F. De Oliveira, and A. C. F. Batista. 2014. “Methylic and Ethylic Biodiesels from Pequi Oil (Caryocar Brasiliense Camb.): Production and Thermogravimetric Studies.” Fuel 136: 10–18. doi:10.1016/j.fuel.2014.07.035.
- Soto, F., M. Alves, J. Carlos, O. Armas, P. Crnkovic, G. Rodrigues, L. Melo, and L. Melo. 2018. “The Determination of the Activation Energy of Diesel and Biodiesel Fuels and the Analysis of Engine Performance and Soot Emissions.” Fuel Processing Technology 174: 69–77. doi:10.1016/j.fuproc.2018.02.008.
- Srikanth, H. V., J. Venkatesh, S. Godiganur, B. Manne, S. Bharath Kumar, and S. Spurthy. 2020. “Combustion, Performance, and Emission Characteristics of dairy-washed Milk Scum Biodiesel in a Dual Cylinder Compression Ignition Engine, Energy Sources, Part A: Recovery.” Utilization, and Environmental Effects 42 (23): 2873–2890. doi:10.1080/15567036.2019.1618996.
- Vinay Atgur, G., M. G. P. Desai, and B. Nageswara Rao. 28 January 2022. “CFD Combustion Simulations and Experiments on the Blended Biodiesel Two-Phase Engine Flows.” Computational Fluid Dynamics (Edited Volume by Dr. Suvanjan Bhattacharyya), IntechOpen Book Series doi:10.5772/intechopen.102088.
- Vinay Atgur, G. M., G. P. Desai, and B. Nageswara Rao. 2021. “Thermal Characterisation of Dairy Washed Scum Methyl Ester and Its B-20 Blend for Combustion Applications.” International Journal of Ambient Energy. doi:10.1080/01430750.2021.1909651.
- Vinay Atgur, G. M., G. P. Desai, B. Nageswara Rao, and B. Nageswara Rao. 2021. “Experimental Investigation on Thermal Conductivity and Thermal Degradation of Honge Oil Methyl Ester with B-20 Blend.” Journal of Thermal Engineering 7 (7): 1604–1613. doi:10.18186/thermal.1026846.
- Vinay Atgur, G. M., B. Nageswara Rao, B. Nageswara Rao, and B. Nageswara Rao. 2022. “Thermogravitometry and Calorimetric Evaluation of Honge Oil Methyl Ester and Its B-20 Blend.” Cleaner Engineering and Technology 6: 100367. doi:10.1016/j.clet.2021.100367.
- Volli, V., and M. K. Purkait. 2014. “Physico-chemical Properties and Thermal Degradation Studies of Commercial Oils in Nitrogen Atmosphere.” Fuel 117: 1010–1019. doi:10.1016/j.fuel.2013.10.021.
- Vossoughi, S., and Y. M. El-Shoubary. 1990. “Kinetics of Liquid Hydrocarbon Combustion Using the DSC Technique.” Thermochimica Acta 157 (1): 37–44. doi:10.1016/0040-6031(90)80004.
- Zhao, H., Y. Cao, W. Orndorff, Y. H. Cheng, and W. P. Pan. 2012. “Thermal Behaviors of Soy Biodiesel.” Journal of Thermal Analysis and Calorimetry 109 (3): 1145–1150. doi:10.1007/s10973-012-2551-8.