 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hereditary hemorrhagic telangiectasia (HHT), a genetic vascular disorder associated with epistaxis and hepatic shunts, is responsible for high-output cardiac failure in rare cases. Bevacizumab, which targets vascular endothelial growth factor, was shown to decrease both cardiac index (CI) and epistaxis duration in HHT patients with severe liver involvement. The relationship between its serum concentration and change in both CI and epistaxis duration was investigated to design the bevacizumab maintenance dosing regimen of future therapeutic studies. Twenty-five HHT patients with dyspnea and high CI were included in a prospective non-comparative study. They received bevacizumab at a dose of 5 mg/kg per infusion every 14 days for a total of 6 injections. The relationships between bevacizumab serum concentration and both CI and epistaxis duration were described using transit compartments and direct inhibition pharmacokinetic-pharmacodynamic models. The performances of different maintenance regimens were evaluated using simulation. Infusions every 3, 2 and one months were predicted to maintain 41%, 45% and 50% of patients with CI <4 L/min/m2 at 24 months, respectively. The fraction of patients with <20 min epistaxis per month was predicted to be 34%, 43% and 60%, with infusion every 3, 2 or one months, respectively. Simulations of the effects of different maintenance dosing regimens predict that monthly 5 mg/kg infusions of bevacizumab should allow sustained control of both cardiac index and epistaxis.
Abbreviations
| HHT | = | hereditary hemorrhagic telangectasia |
| CI | = | cardiac index |
| AVM | = | arterovenous malformations |
| VEGF | = | vascular endothelial growth factor |
| TGF-β | = | transforming growth factor β |
| IgG | = | immunoglobulin G |
| AUC | = | area under the concentration vs time curve |
| PK-PD | = | pharmacokinetic-pharmacodynamic |
| ELISA | = | enzyme-linked immunosorbent assay |
| TMDD | = | target-mediated drug disposition |
| IIV | = | interindividual variability |
Introduction
Hereditary hemorrhagic telangiectasia (HHT), also known as the Rendu-Osler-Weber syndrome, is an inherited vascular dysplasia characterized by recurrent epistaxis, cutaneous telangiectasia and visceral arteriovenous malformations (AVM) that affect many organs, including the lungs, gastrointestinal tract, liver and brain.Citation1 In rare cases, hepatic involvement is associated with symptomatic liver shunting leading, in most cases, to elevated cardiac index and high-output cardiac failure. It is a disorder of unbalanced angiogenesis.Citation2 Patients show increased plasma concentrations and tissue expression of vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β).Citation3 TGF-β stimulates the production of VEGF, which plays a key role in angiogenesis.
We recently published the results of a prospective Phase 2 study on the efficacy of bevacizumab, a recombinant humanized monoclonal IgG1 antibody that inhibits the biologic activity of human VEGF, on reduction of both cardiac index (CI) and epistaxis duration.Citation4 Patients were given 5 mg/kg bevacizumab infusions every other week for a total of 6 infusions. During the study, bevacizumab serum concentrations were measured and bevacizumab area under the concentration vs time curve (AUC) until evaluation times was estimated. In this work, the decrease in CI and improvement of epistaxis at 3 and 6 months was not shown to be related to global exposure to bevacizumab.
The aim of this study was to develop a pharmacokinetic-pharmacodynamic (PK-PD) model in order to assess the individual concentration-effect relationship of bevacizumab and to identify the best maintenance dosing regimen for future studies in HHT patients.
Results
Twenty-five patients were included (). One patient received only 2 infusions of bevacizumab. A total of 317 bevacizumab serum concentration and 96 CI measurements were available. Records of daily epistaxis duration were available for all patients.
Table 1. Characteristics of the patients at baseline
As previously reported, distribution and elimination half-lives of bevacizumab were 1.4 and 21.5 days, respectively.Citation4 In the studied population as a whole, decrease in CI and epistaxis duration at 3 and 6 months were not related to bevacizumab exposure as estimated by its cumulated AUC. However, descriptions of individual changes in CI () and epistaxis duration () were obtained using the PK-PD models. The pharmacokinetic model and the CI sub-model adequately described observed data as shown by goodness-of-fit plots of predicted population and individual values vs observations (Fig. S1). Plots of normalized prediction distribution error vs predicted values confirmed the adequacy of pharmacokinetic and both PK-PD sub-models with observed data (Fig. S2). The interindividual variability for epistaxis-duration risks λ0 was high (207%), but precisely estimated (r.s.e. = 26%). This reflects the high variability in epistaxis-duration risk observed between patients. Mean estimated minimum bevacizumab-induced CI (CImin) was 3.92 L/min/m2 (). The concentrations leading to 50% of maximal effect of bevacizumab on CI and epistaxis were 3.72 mg/L and 5.48 mg/L, respectively. None of the covariates tested (age, weight, body surface area or body mass index) explained the inter-subject variability of the estimated pharmacokinetic or PK-PD parameters. Concentrations of VEGF could not be integrated into the PK-PD model. No relationship was observed between bevacizumab pharmacokinetic parameters and the occurrence of adverse events.
Table 2. Estimated pharmacokinetic and pharmacokinetic-pharmacodynamic parameters
Figure 1. Observed and model-predicted cardiac index (CI) over time in patients. Closed circles are observed CI in patients treated with 6 injections de 5 mg/kg bevacizumab every other week. Continuous line and shaded areas represent median and 5th, 25th, 75th and 95th percentiles of model-predicted CI.
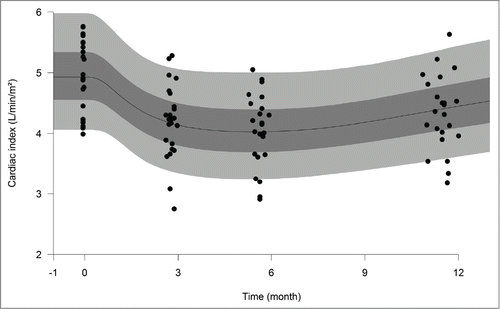
Figure 2. Observed frequencies and model-predicted probabilities of epistaxis daily durations. White, gray and dark gray bars are ‘no epistaxis’, ‘less than or equal to 10 min’ and ‘11 to 20 minutes’ mean daily epistaxis observed frequencies, respectively. Solid, dashed and long-dashed lines are the model predicted probabilities for ‘no epistaxis’, ‘less than or equal to 10 min’ and ‘less than or equal to 20 min’ daily epistaxis, respectively.
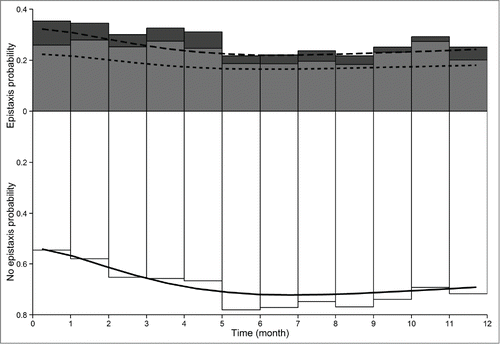
Simulation of bevacizumab concentrations, CI and epistaxis duration in 5,000 virtual patients that were treated with maintenance dosing regimens (DR) consisting of repeated injections (DR2, DR3 or DR4) led to sustained effects while an induction treatment every year (DR1) did not lead to a long term control of symptoms (). An infusion of 5 mg/kg bevacizumab every month (DR4) gave the best results (). The superiority of this regimen was particularly marked for epistaxis, with 28, 34, 43 and 60% of patients with less than 20 min of daily epistaxis at 24 month for DR1, DR2, DR3 and DR4, respectively. However, in terms of cardiac index, DR2, 3 and 4 were similarly effective since they were able to maintain 41%, 45% and 50% of patients with a CI < 4 L/min/m2 at 24 months, respectively (), compared with 21% with DR1.
Figure 3. Simulations of bevacizumab concentrations and effects obtained with 4 different maintenance dosing regimen over 3 years. DR1 consists in 6 injections of 5 mg/kg bevacizumab, every over week, every year (3 upper panels). DR2, DR3 and DR4 consist in 6 injections of 5 mg/kg bevacizumab, every over week, followed by injections of 5 mg/kg every 3, 2 and 1 months, respectively (lower panels). Solid lines and shaded areas are median and 5th-95th percentiles of simulated bevacizumab concentrations (left panels), simulated cardiac index (center panel) and simulated epistaxis duration (right panel).
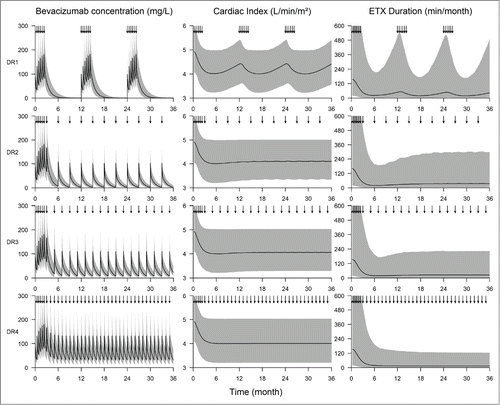
Figure 4. Simulation of proportion of responders at 24th and 30th month according to bevacizumab maintenance dosing regimen. DR1 consists in 6 injections of 5 mg/kg bevacizumab, every other week, every year. DR2, DR3 and DR4 consist in 6 injections of 5 mg/kg bevacizumab, every other week, followed by injections of 5 mg/kg every 3, 2 and 1 months, respectively. Each bar is divided into 4 shades of gray according to quartiles of the initial values of cardiac index and of the initial epistaxis duration. Clearest gray is the first quartile (mildest symptoms) and darkest is the fourth (highest severity of symptoms).
Discussion
Bevacizumab, an anti-VEGF monoclonal antibody, administered at a dose of 5 mg/kg every other week for 6 injections, was shown to decrease CI and to decrease epistaxis duration in HHT patients with severe liver involvement.Citation4 To better adjust bevacizumab dose to this indication and to design a maintenance regimen, we developed a dose-response model describing both bevacizumab pharmacokinetics and the relationships between its serum concentrations and HHT symptoms. Using this mathematical model and parameters estimated in HHT patients, we simulated over 3 years the effect of 4 dosing regimens on CI and epistaxis daily duration.
Bevacizumab pharmacokinetic parameters estimated in 25 HHT patients were similar to those reported for cancer patients.Citation5,6 Therefore, HHT patients were not suboptimally or over-dosed compared to cancer patients when the dosing regimen used in the latter condition was applied. We observed no relationship between bevacizumab pharmacokinetics and adverse drug reactions.
There was a delay between treatment initiation and improvement in pharmacodynamic endpoints (CI and epistaxis daily duration), and the effect of bevacizumab on these endpoints was sustained beyond the induction phase. Both delay and sustained effect were taken into account by using a pragmatic approach to describe the relationships between bevacizumab serum concentrations and the endpoints, i.e., the inclusion of transit effect-compartments in the PK-PD model as previously done to describe neutropenia induced by chemotherapy.Citation7 The measurement of serum VEGF did not allow construction of a more mechanistic model.
No influence of global exposure to bevacizumab, as assessed by AUC, on the decrease in CI or improvement of epistaxis at 3 and 6 months was observed in our previous study, when all the patients were analyzed together.Citation4 This can be explained by several factors: (a) the delay between exposure to bevacizumab and response; (b) the analysis of endpoints at given times despite their complex changes with time; and (c) the important interindividual variability in disease activity of HHT patients and in their sensitivity to bevacizumab, which are assessed by the interindividual variability in the estimated pharmacodynamic parameters Cke0, Eke0 and λ0 ().
Assuming that models developed to describe the concentration-effect relationship of bevacizumab over a one-year follow-up could be applied to a maintenance treatment, we tested different dosing regimen to design future maintenance clinical trials. A repetition of the induction regimen (5 mg/kg every other week for 6 injections) every year (DR1) was able to control CI and epistaxis at 30 months, but not at 24 months. A maintenance regimen consisting of single injections of 5 mg/kg every month (DR4) was the most effective of the tested regimen on epistaxis and CI control. However, according to the chosen endpoints, injections every 3 months (DR2) performed reasonably well. This last regimen may therefore prove to be effective in the whole population of patients. On the other hand, bimonthly (DR3) or monthly infusions may be useful in patients with worrying epistaxis. No immune response against bevacizumab was described when used in its oncological indications. However, immune response against therapeutic proteins is uncommon in cancer patients. In HHT, long-term bevacizumab treatment could be a risk of immune response against bevacizumab, especially when infusions are spaced out (i.e., DR2). Nevertheless, these dosing regimens must be tested in prospective studies.
Methods
Patients
This pharmacokinetic and PK-PD study was nested in a completed clinical trial (clinicaltrials.gov identifier: NCT00843440). Details regarding trial design, patient characteristics, treatment plan, and outcomes were published previously.Citation4 Briefly, patients with clinically confirmed HHT with severe liver involvement, dyspnea (≥class II according to the New York Heart Association) and high CI on echocardiography (defined as >3.9 L/min/m2 in men and >3.6 L/min/m2 in women) were included in a prospective, open-label, non-comparative study conducted in a single institution after nationwide recruitment. Patients received bevacizumab intravenously at a dose of 5 mg/kg per injection every 14 days for a total of 6 injections.
This study was approved by the local research ethics committee and by the French Medical Products Agency (AFSSaPS, now ANSM). Oral and written informed consents were obtained from all patients in accordance with national regulations.
Clinical measurements
Cardiac output and CI were calculated before and 3, 6 and 12 months after the first injection, CI being a normalization of cardiac output measure by body surface area. Daily duration and number of episodes of epistaxis were daily-recorded by patients, before inclusion and over 12 months after treatment initiation. Adverse events were graded 1 to 4 according to the National Cancer Institute common toxicity criteria (NCICT-CAE) version 3.0.Citation4
Biological measurements
Bevacizumab serum concentrations were measured in blood samples collected 5 hours and 24 hours after the first infusion, then, right before the beginning and 2 hours after the end of each infusion. Bevacizumab serum concentrations were measured using a validated enzyme-linked immunosorbent assay (ELISA).Citation8 Limit of detection and lower limit of quantitation were 0.033 mg/L and 5 mg/L, respectively.
Total VEGF concentrations were measured in plasma using a solid phase sandwich ELISA kit (Quantikine® kit of R&D Systems Europe, Ltd., Abingdon, UK). Limit of detection and lower limit of quantification were 9 μg/L and 31.2 μg/L, respectively.
Pharmacokinetic-Pharmacodymamics analysis
Data management and graphical evaluation were done using R (version 3.0.1), Vienna, Austria.Citation9 Pharmacokinetic and PK-PD analyses were performed by nonlinear mixed-effects modeling, using Monolix (version 4.2.2), Lixsoft Orsay, France.Citation10 All models were evaluated using goodness-of-fit and residual plots and objective function values. Discrimination between hierarchical models was based on the likelihood ratio test, for which a change in objective function value > 3.84 was considered statistically significant (α < 0.05).
Bevacizumab pharmacokinetic model
One- and two-compartment models with first-order elimination from the central compartment were tested to describe bevacizumab serum concentrations over time. A two-compartment model gave the best description of the observed concentrations (EquationEquation 1(1)
(1) ):
(1)
(1) where C1, is the concentration in the central volume of distribution V1, k10 is systemic elimination rate, C2 is the concentration in the peripheral compartment of distribution and k12 and k21 are distribution rates between the 2 compartments.
Relationship between bevacizumab and VEGF concentrations
With the aim of developing a mechanistic PK-PD model, we first analyzed the relationship between bevacizumab serum concentration and plasma VEGF using a target-mediated drug disposition (TMDD) model.Citation11 Because the assay measured both free VEGF and VEGF-bevacizumab complexes, an apparent increase in plasma VEGF concentrations during bevacizumab treatment was observed, as previously reported.Citation12 In addition, several patients had low baseline VEGF concentrations, and extrapolated plasma concentrations of free VEGF (calculated because the assay measured both VEGF and bevacizumab-VEGF complex) during treatment were not related to cardiac indexes and epistaxis durations. Then, it was not possible to link plasma VEGF concentrations to these 2 pharmacodynamic parameters.
Relationship between bevacizumab pharmacokinetics and adverse events
Estimated bevacizumab pharmacokinetic parameters of patients with at least one adverse event of grade > 1 or 2 were compared with those of other patients using Wilcoxon signed rank test.
Cardiac index sub-model
According to the cardiac index data, the effect of bevacizumab on CI was delayed and a long duration of this effect was observed. Different model were tested to quantify the influence of bevacizumab concentrations on CI over time. Direct response and indirect response models with inhibition or stimulation of CI “production” or ”elimination” led to biases, i.e., unsatisfactory fitting of data. An effect-compartment model was good at describing the long lasting effect of bevacizumab,Citation13 but not the delay in the appearance of this effect. The delay effect of bevacizumab concentration on CI was taken into account with chained effect-compartments, also called transit compartments, as previously used to describe chemotherapy induced myelosuppression.Citation7 Two to 5 transit effect-compartments model were tested. A transit effect compartment model with 4 compartments and a direct inhibition of CI gave the best description of the long lasting effect of bevacizumab concentrations on CI (EquationEquation 2(2)
(2) ):
(2)
(2) where CCe1 to CCe4 are the transit CI effect-compartment concentrations, Cke0 is bevacizumab transit CI effect-compartment constant, C1 is the bevacizumab concentration in the central volume of the pharmacokinetic model, CI(t) is CI as a function of time, CI0 and CImin are baseline and minimal CI, respectively (L/min/m2), and CC50 is concentration of bevacizumab in the final CI effect-compartment concentration, leading to 50% of maximal effect i.e.,
.
Epistaxis sub-model
Epistaxis episodes are spontaneous and recurrent in HHT patients. As a consequence, the daily epistaxis duration is highly variable from day to day and cannot be described with a conventional quantitative model. The objective of the pharmacodynamic model was therefore to describe the probability (risk) of occurrence of the different daily-epistaxis duration classes. Daily epistaxis duration was categorized by 10 minute classes (dETX): “0” was for no epistaxis, “1” was for less than or equal to 10 min per day, “2” was for 11 to 20 min per day, etc., and “7” was for an epistaxis duration of more than 1 hour per day. Probabilities of occurrence of the different duration classes were verified to be stable before treatment. Because mean and variance of duration classes were similar, data were modeled using a Poisson distribution with parameter λ. The best description of λ variation over time (λ(t)) was obtained using a sigmoid inhibitory function and 4 transit effect compartments. The relationship between λ and concentration in the final epistaxis-effect-compartment (ECe4) (EquationEquation 3(3)
(3) ) was:
(3)
(3) where ECe1 to ECe4 are transit epistaxis effect-compartment concentrations, Eke0 is bevacizumab transit epistaxis effect-compartment constant, C1 is the bevacizumab concentration in the central volume of the pharmacokinetic model, λ(t) and λ0 are Poisson distribution parameters of epistaxis durations over time and at baseline, respectively, and EC50 is the final epistaxis effect-compartment concentration, leading to a λ(t) of λ0/2.
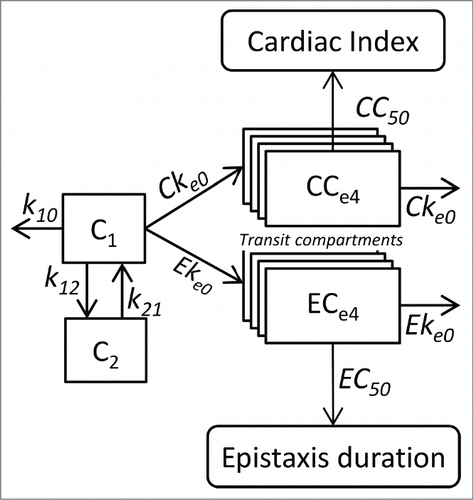
Pharmacokinetic and pharmacokinetic-pharmacodynamic models are schematically described in .
Figure 5. Pharmacokinetic and pharmacokinetic-pharmacodynamic models relating cardiac index and epistaxis duration with bevacizumab concentrations. Bevacizumab pharmacokinetics are described using a 2-compartment model with linear elimination. Effects of bevacizumab are described using transit effect-compartments. The concentrations in the last effect-compartments influence cardiac index and risk of epistaxis through sigmoid Emax models. C1 and C2 are concentrations in the central and peripheral compartments, respectively. k10 is elimination rate and k12 and k21 are distribution rates. CCe1 to CCe4 are transit effect-compartment concentrations. Cke0 is transit rate constant. CI0 and CImin are initial and minimal cardiac index, respectively. CC50 is CCe4 concentration leading to a cardiac index of (CI0+CImin)/2. ECe1 to ECe4 are transit effect-compartment concentrations. Eke0 is the transit rate constant. λ0 is the Poisson distribution parameter of the epistaxis duration classes. EC50 is ECe4 concentration leading to λ = λ/2.
Interindividual variability, residual error models and model evaluation
The interindividual variability (IIV) of parameters was described using a log-normal distribution. IIV variability estimation was accepted if its r.s.e. was < 40%. The final model included IIV for all pharmacokinetic parameters, except distribution parameter k12. Inclusion of IIV for Cke0 reduced the objective function significantly (by 200 points). No IIV was estimated for CC50 and EC50. A covariate analysis was performed to identify individual factors explaining IIV.Citation14,15 A proportional error model gave the best description of residuals of bevacizumab concentrations and CI over time. For epistaxis, which was described using a Poisson distribution model, no error model was needed.
The pharmacokinetic model and the CI sub-model were evaluated using goodness-of-fit plots of the predicted population values vs observations and predicted individual values vs observations. The performance of these 2 models were further evaluated by monitoring deviations from normality of the normalized prediction distribution error.Citation16
In silico evaluation of maintenance dosing regimens
The pharmacokinetic and PK-PD models, together with the population estimated parameters, were used to predict the performances of different maintenance regimens regarding both CI and epistaxis control, as a basis for future studies in HHT patients.Citation17,18
Building of the virtual patient cohort
The CI and epistaxis sub-models were used to relate CI and daily epistaxis duration probabilities to bevacizumab concentration. Both models were based on data obtained after a one-year follow-up, but the assumption was made that they are valid to describe maintenance treatment over 3 years. The pharmacokinetic and PK-PD parameters of 5,000 virtual patients were generated from distributions and covariance matrix of the real patient population ().
Dosing regimen simulations
Four different 36 months maintenance dosing regimens were tested in the 5,000 virtual patients. An induction dosing regimen of 6 injections of 5 mg/kg bevacizumab, every other week, repeated every year was noted as DR1. Regimens DR2, DR3 and DR4 were constituted by an induction of 6 injections of 5 mg/kg bevacizumab, every other week, followed by single injections of 5 mg/kg every 3, 2 and 1 month, respectively. Because the main purpose of this simulation experiment was to characterize the PK profiles of bevacizumab obtained with 4 different dosing regimens and their influence on CI and epistaxis, no trial protocol deviations were incorporated, i.e., all enrolled subjects were assumed to be fully compliant to the assigned dosing regimen, without dropouts or losses to follow up. Monolix (version 4.2.2, Lixsoft, Orsay, France)Citation10 was used to perform the simulation.
Evaluation of the different dosing regimens
The long-term performance of the different maintenance dosing regimens was assessed in terms of CI and mean epistaxis duration (in min/month). This ‘mean epistaxis duration’ () was calculated using the λ parameter of the Poisson distribution of the daily epistaxis duration classes with the following formula:
. Because the highest fluctuations in CI and epistaxis risk with time were observed with DR1, dosing regimens were compared at 24 months (the ‘worst time’ for DR1, at which the bevacizumab effects on CI and epistaxis were minimal), and at 30 months (the “best time” for DR1, at which bevacizumab effect was maximal).
Disclosure of Potential Conflicts of Interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Gilles Paintaud is involved in clinical studies sponsored by Genzyme, Novartis and Roche Pharma; his research team has received financings from Abbott Pharma, Chugai, Janssen, LFB (Laboratoire Français des Biotechnologies), Pierre-Fabre Laboratories, Wyeth and Merck Serono.
Authorship Contributions
Nicolas Azzopardi performed research, collected, analyzed, and interpreted data, and wrote the manuscript. Sophie Dupuis-Girod designed, funded and coordinated the clinical study, reviewed the manuscript. David Ternant collected and interpreted data, participated in the writing of the manuscript. Anne-Emmanuelle Fargeton managed patients, organized follow up, collected data and reviewed the manuscript. Isabelle Ginon performed all the Echocardiography studies and reviewed the manuscript. Frédéric Faure performed nose examination in all patients and reviewed the manuscript. Evelyne Decullier performed the study design, collected data and reviewed the manuscript. Adeline Roux performed the study design, collected data and reviewed the manuscript. Marie-France Carette recruited patients and reviewed the manuscript. Brigitte Gilbert-Dussardier recruited patients and reviewed the manuscript. Pierre-Yves Hatron recruited patients and reviewed the manuscript. Pascal Lacombe recruited patients and reviewed the manuscript. Vanessa Leguy-Seguin recruited patients and reviewed the manuscript. Sophie Rivière recruited patients and reviewed the manuscript. Romain Corre recruited patients and reviewed the manuscript. Sabine Bailly performed biological dosages and reviewed the manuscript. Gilles Paintaud designed, funded and coordinated the present PK-PD study, interpreted data, and supervised manuscript writing.
Supplemental_Material.zip
Download Zip (1.5 MB)Acknowledgments
We thank all the patients who participated in this study, the association of patients, and all the members of the French HHT Network. No one received compensation for the contributions.
Funding
The present study was supported by the French Higher Education and Research ministry under the program “Investissements d’avenir” Grant Agreement: LabEx MAbImprove ANR-10-LABX-53-01.
Supplementary Material
Supplemental data for this article can be accessed on the publisher's website.
Additional information
Notes on contributors
Nicolas Azzopardi
Nicolas Azzopardi performed research, collected, analyzed, and interpreted data, and wrote the manuscript.
Sophie Dupuis-Girod
Sophie Dupuis-Girod designed, funded and coordinated the clinical study, reviewed the manuscript.
David Ternant
David Ternant collected and interpreted data, participated in the writing of the manuscript.
Anne-Emmanuelle Fargeton
Anne-Emmanuelle Fargeton managed patients, organized follow up, collected data and reviewed the manuscript.
Isabelle Ginon
Isabelle Ginon performed all the Echocardiography studies and reviewed the manuscript.
Frédéric Faure
Frédéric Faure performed nose examination in all patients and reviewed the manuscript.
Evelyne Decullier
Evelyne Decullier performed the study design, collecting data and reviewed the manuscript.
Adeline Roux
Adeline Roux performed the study design, collecting data and reviewed the manuscript.
Marie-France Carette
Marie-France Carette recruited patients and reviewed the manuscript.
Brigitte Gilbert-Dussardier
Brigitte Gilbert-Dussardier recruited patients and reviewed the manuscript.
Pierre-Yves Hatron
Pierre-Yves Hatron recruited patients and reviewed the manuscript.
Pascal Lacombe
Pascal Lacombe recruited patients and reviewed the manuscript.
Vanessa Leguy-Seguin
Vanessa Leguy-Seguin recruited patients and reviewed the manuscript.
Sophie Rivière
Sophie Rivière recruited patients and reviewed the manuscript.
Romain Corre
Romain Corre recruited patients and reviewed the manuscript.
Sabine Bailly
Sabine Bailly performed biological dosages and reviewed the manuscript.
Gilles Paintaud
Gilles Paintaud designed, funded and coordinated the present PK-PD study, interpreted data, and supervised manuscript writing.
References
- Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91:66-7; PMID:10751092; http://dx.doi.org/10.1002/(SICI)1096-8628(20000306)91:1%3c66::AID-AJMG12%3e3.0.CO;2-P
- David L, Mallet C, Keramidas M, Lamandé N, Gasc J-MM, Dupuis-Girod S, Plauchu H, Feige J-JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res 2008; 102:914-22; PMID:18309101; http://dx.doi.org/10.1161/CIRCRESAHA.107.165530
- Sadick H, Riedel F, Naim R, Goessler U, Hörmann K, Hafner M, Lux A, Hormann K. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica 2005; 90:818-28; PMID:15951295
- Dupuis-Girod S, Ginon I, Saurin J-C, Marion D, Guillot E, Decullier E, Roux A, Carette M-F, Gilbert-Dussardier B, Hatron P-Y, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA 2012; 307:948-55; PMID:22396517; http://dx.doi.org/10.1001/jama.2012.250
- Lu J-F, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 2008; 62:779-86; PMID:18205003; http://dx.doi.org/10.1007/s00280-007-0664-8
- Li J, Gupta M, Jin D, Xin Y, Visich J, Allison DE. Characterization of the long-term pharmacokinetics of bevacizumab following last dose in patients with resected stage II and III carcinoma of the colon. Cancer Chemother Pharmacol 2013; 71:575-80; PMID:23228985; http://dx.doi.org/10.1007/s00280-012-2031-7
- Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 2002; 20:4713-21; PMID:12488418; http://dx.doi.org/10.1200/JCO.2002.02.140
- Ternant D, Cézé N, Lecomte T, Degenne D, Duveau A-C, Watier H, Dorval E, Paintaud G. An enzyme-linked immunosorbent assay to study bevacizumab pharmacokinetics. Ther Drug Monit 2010; 32:647-52; PMID:20720519; http://dx.doi.org/10.1097/FTD.0b013e3181ef582a
- R Core Team. Computational many-particle physics. Berlin, Heidelberg: Taylor & Francis; 2008.
- Bauer RJ, Guzy S, Ng C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J 2007; 9:E60-83; PMID:17408237; http://dx.doi.org/10.1208/aapsj0901007
- Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol 2006; 72:1-10; PMID:16469301; http://dx.doi.org/10.1016/j.bcp.2005.12.041
- Stefanini MO, Wu FTH, Mac Gabhann F, Popel AS. Increase of plasma VEGF after intravenous administration of bevacizumab is predicted by a pharmacokinetic model. Cancer Res 2010; 70:9886-94; PMID:21118974; http://dx.doi.org/10.1158/0008-5472.CAN-10-1419
- Bellissant E, Sébille V, Paintaud G. Methodological issues in pharmacokinetic-pharmacodynamic modelling. Clin Pharmacokinet 1998; 35:151-66; PMID:9739481; http://dx.doi.org/10.2165/00003088-199835020-00004
- Bonate PL. The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res 1999; 16:709-17; PMID:10350015; http://dx.doi.org/10.1023/A:1018828709196
- Maitre PO, Bührer M, Thomson D, Stanski DR. A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm 1991; 19:377-84; PMID:1920085; http://dx.doi.org/10.1007/BF01061662
- Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 2008; 90:154-66; PMID:18215437; http://dx.doi.org/10.1016/j.cmpb.2007.12.002
- Holford N, Ma SC, Ploeger BA. Clinical trial simulation: a review. Clin Pharmacol Ther 2010; 88:166-82; PMID:20613720; http://dx.doi.org/10.1038/clpt.2010.114
- Milligan PA, Brown MJ, Marchant B, Martin SW, van der Graaf PH, Benson N, Nucci G, Nichols DJ, Boyd RA, Mandema JW, et al. Model-based drug development: a rational approach to efficiently accelerate drug development. Clin Pharmacol Ther 2013; 93:502-14; PMID:23588322; http://dx.doi.org/10.1038/clpt.2013.54
