 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Surface and interfacial adsorption of antibody molecules could cause structural unfolding and desorbed molecules could trigger solution aggregation, resulting in the compromise of physical stability. Although antibody adsorption is important and its relevance to many mechanistic processes has been proposed, few techniques can offer direct structural information about antibody adsorption under different conditions. The main aim of this study was to demonstrate the power of neutron reflection to unravel the amount and structural conformation of the adsorbed antibody layers at the air/water interface with and without surfactant, using a monoclonal antibody ‘COE-3′ as the model. By selecting isotopic contrasts from different ratios of H2O and D2O, the adsorbed amount, thickness and extent of the immersion of the antibody layer could be determined unambiguously. Upon mixing with the commonly-used non-ionic surfactant Polysorbate 80 (Tween 80), the surfactant in the mixed layer could be distinguished from antibody by using both hydrogenated and deuterated surfactants. Neutron reflection measurements from the co-adsorbed layers in null reflecting water revealed that, although the surfactant started to remove antibody from the surface at 1/100 critical micelle concentration (CMC) of the surfactant, complete removal was not achieved until above 1/10 CMC. The neutron study also revealed that antibody molecules retained their globular structure when either adsorbed by themselves or co-adsorbed with the surfactant under the conditions studied.
Abbreviations
| NR | = | Neutron reflection |
| Tween 80 | = | Polysorbate 80 |
| CMC | = | Critical micellar concentration |
| SLD | = | Scattering length density |
| NRW | = | Null reflecting water |
Introduction
Monoclonal antibodies (mAbs) represent an increasingly important class of therapeutic drugs in today's pharmaceutical pipeline.Citation1 High concentration liquid formulations are a prerequisite for low volume subcutaneous injections to meet the desired clinically efficacious dose. It is during the optimization of such formulations that careful attention must be paid to mitigating the formation of aggregates and particulates, which may arise via several destabilization processes.Citation2 Mechanisms leading to protein aggregation in bulk solution in the context of formulation have been widely reviewed.Citation3 Briefly, they include covalent changes such as oxidation and deamination, and physical changes arising from protein-protein interactions and surface adsorption-desorption.Citation4,5 The latter is encountered by mAbs exposed to air/liquid, solid/liquid and silicone oil/liquid interfaces as present during filling (pumping) and storage in primary packaging, such as glass vials with rubber stoppers and plastic/glass prefilled syringes.Citation6,7 Aggregation leading to particulate formation may be accelerated during manufacturing processes, in particular, fill-finish activities wherein the mAb is exposed to interfacial stresses.Citation8 Particulate limits are set by the pharmacopeias: for each unit dose referred to here, there must be less than 6000 particles > 10 μm and less than 600 particles > 25 μm (USP <787> and EP 2.9.19). The thorough characterization of the size distribution and nature of particulates remains an area of importance in the industry, and will inform our understanding of the potential capacity of protein particulates to elicit immunogenic responses.Citation9-11
In the biopharmaceutical industry, there is sufficient empirical data to link surface adsorption-desorption effects to particulate formation, although the underlying molecular origins remain sparsely defined. Early work used simple agitation to simulate high shear rates for different surface roughness conditions, as could be encountered during fill-finish.Citation12 More recent work by Shieh and Patel investigated how air/water surface pressure measurements could predict mAb aggregation as a consequence of interfacial adsorption-desorption; this, when applied as a screening tool, would benefit the assessment of mAb behavior upon filling into primary packaging or dilution into an intravenous bag and giving set.Citation13 An elegant experimental approach using a custom dilatational interfacial rheometer with simultaneous pressure and bubble shape measurements, has further enabled an understanding of aging of the adsorbed mAb film and consequent generation of aggregates.Citation14
Characterizing the precise molecular nature of adsorbed mAbs at an interface, however, requires state-of-the-art analytical techniques and data interpretation. Quartz crystal microbalance and total internal reflection fluorescence are useful benchtop methods, but lack the resolution to distinguish between individual adsorbed protein layers and the corresponding molecular orientation relative to the surface.Citation15,16 This becomes limiting when the intention is to define molecular changes to mAbs that undergo adsorption to an interface followed by partial unfolding revealing hydrophobic (core) residues. Consideration must then be given to pathways involving desorption, protein-protein self-assembly (oligomers), and the formation of soluble aggregates that nucleate sub-visible and visible particles. In this study, we apply neutron reflection to the problem of understanding mAb adsorption to an interface (reviewed in ref. Citation17). Neutron reflection has enabled molecular models of protein adsorption to be constructed,Citation18 has been used to determine the orientation of antibodies non-specifically adsorbed at the solid/liquid interface,Citation19 and has yielded the structural characterization of membrane proteins in bilayer models resting on a water-filled layer.Citation20
Current approaches to mitigate chemical and physical instabilities require the addition of buffers and excipients to mAb formulations.Citation21 Regarding protein adsorption and aggregation as discussed above, the industry generally uses non-ionic surfactants such as Polysorbate 20 and 80 (Tween® 20 and 80) to control shake-induced aggregation as would be experienced during drug product transportation, for instance. In general, polysorbates compete with the protein for an interface and adsorb to exposed hydrophobic patches on the protein surface. During formulation development, Tweens around 0.02–0.05% (w/v) are often found to minimize particulate formation during shaking studies designed to expose the protein to air/liquid interfaces and accelerate aggregation through surface adsorption/desorption. Low concentrations of Tween 20 (0.0025%, below the critical micelle concentration (CMC) of ca. 0.01%) may also confer some prevention of IgG1 aggregation when shaken.Citation22 A fundamental understanding of the competition between polysorbate and mAb at the air/water interface is directly relevant to our understanding of the behavior of drug substance (formulated mAb containing surfactant) in prefilled syringes. For example, Polysorbate 20, when added at concentrations above or below its CMC, attenuates mAb particle formation in agitated syringes harboring an air bubble; this action was further correlated with polysorbate-mediated inhibition of ‘gelation’ of the adsorbed mAb interfacial film.Citation23
The main aim of this study was to demonstrate the power of neutron reflection to unravel the amount and structural conformation of the adsorbed antibody layers at the air/water interface with and without surfactant. With the help of isotopic contrasts achieved by varying the ratio of H2O and D2O, we show that the antibody molecules in the adsorbed surface layers could be well represented by a uniform layer or Gaussian layer distributions, indicating no unfolding leading to the formation of polymer-like distributions along the surface normal direction. With the combined use of deuterated and hydrogenous Polysorbate 80 (Tween 80) surfactants (denoted as d-Surf and h-Surf, respectively), we have measured the surface composition from co-adsorbed surfactant and antibody, with the concentration of antibody fixed at 50 ppm, showing the concentration range above which mAb adsorption is inhibited. This study has demonstrated the potential of neutron reflection in helping to investigate how mixing of surfactant or addition of any other excipient affects the amount and structural conformation of an antibody adsorbed at the air/water interface.
Neutron reflection and isotopic contrast variation
A schematic representation of the relationship between the optical geometry of the neutron beam setup and the surface adsorbed antibody layer is shown in . Note that the actual beam incidence angles are much lower than what is schematically depicted here. Specular neutron reflectivity, R, is usually plotted as a function of momentum transfer or wave vector, κ, perpendicular to the reflecting surface where(1)
(1)
Figure 1. Schematic representation of (a) the optical geometry of the incoming and exiting neutron beam with respect to the adsorbed antibody layer and (b) the co-adsorption of antibody and surfactant where surfactant molecules could be hydrogenated (h-Surf) and ethoxylate head deuterated (d-Surf) in the case of polysorbate (Tween®) 80.
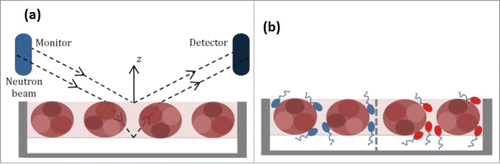
As solvent molecules can also become mixed with adsorbed antibody layers, the change in layer composition, which is determined by resolution of the scattering length density (SLD) along the surface normal direction, is more appropriately linked to R. Because of the difference in the scattering length between hydrogen and deuterium, isotopic substitution can be used to change the SLD if the antibody layer is mixed with water. In this work, neutron reflectivity profiles were first measured from antibody adsorption at the air/ null reflecting water (NRW) interface, where NRW consists of 8.1% D2O and 91.9% H2O by volume, with SLD = 0. Because the water is invisible under this isotopic contrast, the specular reflectivity only contains the information about the adsorbed protein layer, allowing a precise determination of its interfacial adsorbed amount and the thickness.Citation26 It is possible that the antibody layer is neither fully immersed nor fully afloat. When the immersed part of the layer is contrast matched to water, it becomes invisible and only the remaining part of the layer exposed to the air is seen by neutron reflection. This condition is met when the SLD is equal to 2.58 × 10−6 Å−2 (close to the equal ratio of H2O and D2O, termed CM2.58). When measured in D2O, the signal contains information about the entire interfacial layer including the association of water. Although the structural interpretation in this case is more complex, its combined analysis with the other 2 profiles measured in NRW and CM2.58 offers significant benefit to highlight the adsorbed antibody layer differently. Thus, the isotopic contrast of water shown as the faint background within the model protein layers, as depicted in , could be varied by adjusting the ratio of H2O to D2O in a given neutron reflection measurement.
Under the co-adsorption of surfactant and antibody (), it is possible to resolve the different adsorbed amount from the 2 surface active species because the use of d- and h-Surf under a given surfactant concentration gives us 2 different reflectivity profiles. Changes of scattering length for surfactant or water produce different SLDs for a given interfacial structure so that different reflectivity profiles can be produced. These reflectivity profiles together enable us to determine the composition of an interfacial system and in this work this technical feature is explored to determine the co-adsorption region of the antibody-surfactant system with increasing surfactant concentration.
The reflectivity profiles were analyzed using Motofit,Citation27 which uses an optical matrix formalism as described by Born and WolfCitation28 to fit Abeles layer models to the interfacial structure. Briefly, the fitting process consists of a procedure where an interfacial model is first assumed and the reflectivity is then calculated by fitting the structural parameters of the interface to the measured one. The structural parameters are then refined in a repetitive process to achieve the best fit. To account for the structural changes along the surface normal direction, the interfacial layer is often divided in a series of sublayers, each of them being described by thickness (τi), scattering length density (ρi) and roughness, where appropriate.
For the antibody adsorption systems, a uniform layer model was often appropriate, where the surface adsorbed amount or surface concentration (Γp, measured in mg/m2) of the antibody can be expressed as(2)
(2) where MW is the molecular weight of the antibody (in g/mol), Σbp is its scattering length (in Å), τp the thickness obtained from the fit (in Å) and ρp the scattering length density (in Å−2), respectively.Citation26 The constant of 6.023 is related to the conversion of the Avogadro's number (NA) and the unit difference between Angstrom (Å) and meter (m). The area per molecule (Ap, in Å2) can be obtained using:
(3)
(3)
For the measurements in D2O, the immersed part of the antibody layer must be fitted by taking into account the contributions from the solvent for space filling as well, with the total of the antibody volume fraction (ϕp) and solvent volume fraction (ϕw) being equal to unity. To ensure the SLD contributions consistent to the interfacial composition, we used the following equations:Citation29,30(4)
(4)
In the case of the antibody-surfactant systems in NRW, a single-layer model involving scattering length density (ρ) and thickness (τ), was used to solve the following simultaneous equations involving d-Surf and h-Surf:(5)
(5)
(6)
(6) where it was assumed that Γsd = Γsh or Asd = Ash at a given surfactant concentration. Solving these equations allowed us to calculate precisely the surface concentrations of surfactant and antibody in the mixed interfacial layer.
Results
Surface tension measurements
The surface tension changes of both hydrogenated and ethoxylate head deuterated Polysorbate 80 (denoted as h-Surf and d-Surf) were first measured, with the dynamic tension profiles measured for h-Surf at representative concentrations shown in . It can be seen that, while the surface tension decreases with increasing surfactant concentration, the time dependent change after the initial period occurs very slowly. As the concentration increases, the fast initial surface tension reduction becomes more pronounced, but the second stage of relaxation takes much longer. Even after the first 8000 seconds (over some 2 hr), the true equilibration might still have not been reached. For example, at the highest h-Surf concentration of 0.3 mM studied, the surface tension decreased steadily from 4000 to 8000 seconds and the net change was about 2 mN/m. The change during this slow process reflected minor structural rearrangements relating to the adjustment of the adsorbed layer structures. As the ethoxylate head groups were produced via polymerization, they are composed of a range of sizes that may have subtle differences in surface activity.
Figure 2. Surface tension profiles measured from (a) h-Surf over a range of surfactant concentration plotted against time (plots from top to bottom represent separately: 0.0001, 0.0003, 0.001, 0.003, 0.01, 0.03, 0.1 and 0.3 mM), (b) both h-Surf and d-Surf taking the readings at 8000 seconds, the longest time measured, (c) the same plots as in (a) but containing 50 ppm of COE-3 and (d) h-Surf with and without 50 ppm of COE-3, taking the readings at 8000 seconds. The continuous lines in (b) and (d) were drawn to indicate the occurrence of the CMC around 0.012 µM.
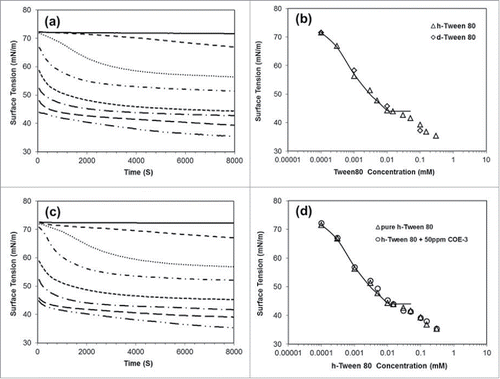
For convenience, we have, however, taken the surface tension readings at 8000 seconds as the equilibrated values. compares these values measured from the 2 differently labeled surfactants. It can be seen that, although the surfactants were made separately, the equilibrated values overlap well over the experimental errors. The continuous line was drawn to highlight the kink that is very close to 0.012 mM, the widely cited CMC of this surfactant.Citation31,32 Note that the surface tension continues to fall, although the rate of decrease slows down substantially. This is very typical of polymer-like surfactants with very low CMCs, implying that, as the total surfactant concentration increases above their CMCs, additional monomers become available to reduce the surface tension further.
The surface tension of the mAb alone (denoted as COE-3) was then measured. It was found that the adsorption of the mAb had little influence on surface tension reduction when its concentration was varied from 10 to 500 ppm (0.5 mg/ml) (data not shown). Given that each measurement of surface tension needed about 15 ml of the sample and 50 ml for the subsequent neutron reflection, we chose to fix the mAb concentration at 50 ppm throughout this study to minimize the amount of mAb used. shows that the presence of 50 ppm of COE-3 did not measurably alter the dynamic surface tension over the entire surfactant concentration range, and even the equilibrated surface tension readings at the 8000 second interval show an almost exact overlap with the data measured without the mAb (). This outcome reveals that the presence of the mAb did not influence the surface tension much, but, as shown below, neutron reflection revealed the co-adsorption of the mAb molecules over a wide range of surfactant concentrations studied.
Antibody adsorption on the surface of water
At the air/NRW interface, the adsorbed antibody layer is the only component that contributes to the specular neutron reflection as the water surface is made invisible to neutrons. The reflectivity measured thus offers useful information about the adsorbed layer thickness and composition without any complication arising from the solvent. shows a set of reflectivity profiles measured at several representative antibody concentrations, plotted as log [R] versus log[κ] for better visualization. Because most surface changes tend to occur over the first 30–40 min, neutron reflectivity measurements were taken after the first hour of surface equilibration (mainly during the 2–4 hour period to avoid further complications relating to possible surface sample aging). It can be seen from that an increase in the bulk antibody concentration can steadily increase the level of the reflectivity profile, but its shape appears not to change much, implying that, while the surface adsorbed amount increases, the thickness of the antibody adsorbed layer remains almost constant.
Figure 3. Neutron reflectivity profiles measured on the surface of null reflecting water from COE-3 adsorption at 4 representative concentrations of 5, 25, 50 and 100 ppm under the His buffer of pH 5.5 (25 mM ionic strength). The continuous lines denote the best uniform layer fits with thickness and SLD given in . The scatter of the data indicates the statistical errors in each reflectivity profile measured.
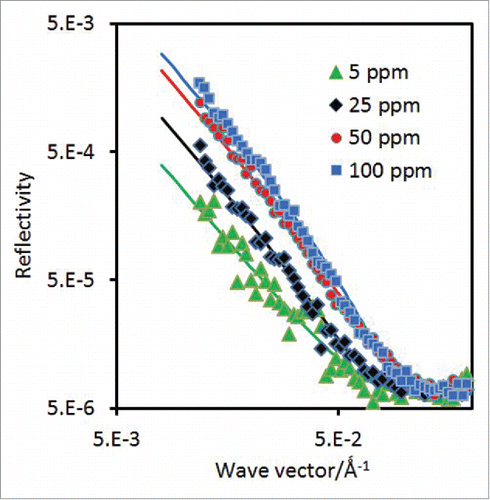
As outlined previously, the most common approach for the analysis of measured neutron reflectivity data are to adopt the model fitting based on the optical matrix formula.Citation27,28 The continuous lines shown in represent the best uniform layer fitting to each of the reflectivity profiles measured under different antibody concentrations, with the structural parameters listed in . It can be seen from that the fits generated from the uniform layer model do reproduce the measured reflectivity profiles well within the experimental error, adding confidence to the structural parameters obtained from the analysis.
Table 1. Structural parameters obtained from the best uniform layer fits to the adsorbed COE-3 layers on the surface of NRW water at pH 5.5. The numbers in brackets were obtained from the kinematic approach using Equ (Equation8(8)
(8) ) by assuming Gaussian distribution. Note the 3 repeats at 50 ppm were to check the reproducibility of the measurements from independently prepared solutions.
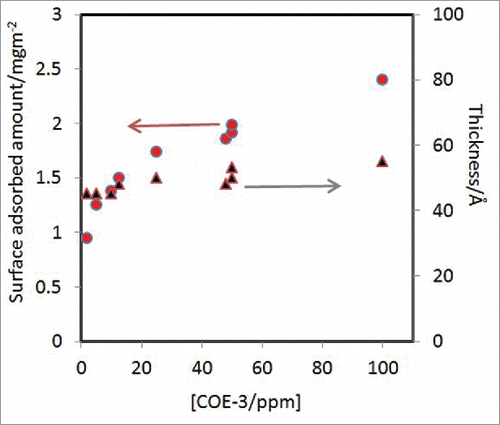
The changes of layer thickness and adsorbed amount obtained from the best uniform layer fitting are shown in . Over the concentration range studied (2 to 100 ppm), the surface adsorbed amount shows a steady increase from 1 to 2.5 mg/m2, but the thickness of the adsorbed layers changes very little. Layer thicknesses could be well fitted within 45 to 55 Å over the concentration range studied. The errors as shown in after the thickness values indicated the range of the values that could give acceptable fits to the measured reflectivity under each antibody concentration. This structural feature clearly signifies the retaining of globular framework of the Fab (fragment antigen-binding) and Fc (fragment crystallisation) segments at the interface, as any unfolding of the globular structure would lead to polymer-like structural inhomogeneity along the surface normal direction that must be taken into account by a multilayer model.Citation33
Figure 4. Changes in the amount (o) and layer thickness (▴) from COE-3 adsorption plotted as a function of COE-3 solution concentration. The 3 data points measured at 48 and 50 ppm indicate the error range of the data.
In contrast to the analysis using the optical matrix based layer fitting as described above, a more direct analysis method is based on the kinematic approach where the neutron reflectivity R measured from NRW can be related to the layer structural parameters from the following Equation:Citation24(7)
(7)
(8)
(8) where hpp(κ) is termed antibody's self-partial structure factor, and by assuming that the adsorbed layer takes the Gaussian distribution the layer thickness σ, defined as the full width at the height of 1/e, and area per molecule A, the same as previously defined, can be obtained from the linear plotting of ln[hpp(κ)] vs. κ2 as shown in Equ. (Equation8
(8)
(8) ). In contrast to the values of τ based on the uniform layer model, the corresponding σ values should be smaller by about 10%.
shows the plots of ln[hpp(κ)] vs. κ2 converted from reflectivity profiles as shown in , but the new plots enabled us to interpret the data by fitting the straight lines using Equ 8. It can be seen from that the measured data appear to become more scattered, and this is certainly the case for the 2 lower concentrations, but the best linear fits give the layer thicknesses and adsorbed amount directly. As shown in , the values in brackets from the kinematic approach broadly match those from the optical matrix model fitting well. For the surface adsorbed amount, the range of variations falls in ± 0.2 mg/m2. In contrast, the layer thickness from the low concentration of 5 ppm of COE-3 has large experimental error, and, as a result, there appears to be some inconsistency, but such a discrepancy is well within the experimental error range. As the mAb concentration increases, better consistency is observed, that is, the values in σ from the Gaussian model are slightly lower than those from the corresponding uniform layer fits. This exercise shows that consistent structural parameters could be obtained about adsorbed amount and layer thickness in spite of different data analysis approaches adopted.
Figure 5. Plots of ln[hpp(κ)] vs. κ2 converted from reflectivity profiles measured at 5, 25, 50 and 100 ppm at pH 5.5 and an ionic strength of 25 mM (His buffer). The straight lines denote the best fits using Equ 8 with the values of σ, A and Γ listed in brackets in . The extent of scatter indicates the statistical errors in each data set.
![Figure 5. Plots of ln[hpp(κ)] vs. κ2 converted from reflectivity profiles measured at 5, 25, 50 and 100 ppm at pH 5.5 and an ionic strength of 25 mM (His buffer). The straight lines denote the best fits using Equ 8 with the values of σ, A and Γ listed in brackets in Table 2. The extent of scatter indicates the statistical errors in each data set.](/cms/asset/428937ed-6f24-4c42-a2fa-308d49e5c6ab/kmab_a_1276141_f0005_c.gif)
It should be noted that like other proteins, COE-3 has labile hydrogen atoms associated with polar groups such as -NH2, -OH and -NH-, which will undertake H/D exchanges with the bulk solvent. As most labile hydrogens can freely access water, the exchanges tend to be complete. Thus, the exact scattering length (Σb) and scattering length density (SLD or ρ) of the antibody in a given mixture of H2O and D2O, such as NRW can be calculated and the values are listed in . From these values, the surface adsorbed amount and the equivalent area per molecule were calculated from Equ. (2–3) by taking into account the appropriate H/D exchanges.
Table 2. The scattering lengths (Σb), scattering length densities (ρ), volumes and molecular weights (MW) of antibody COE-3 and hydrogenous and deuterated Tween 80 surfactants (h-Surf and d-Surf) used for the model fitting.
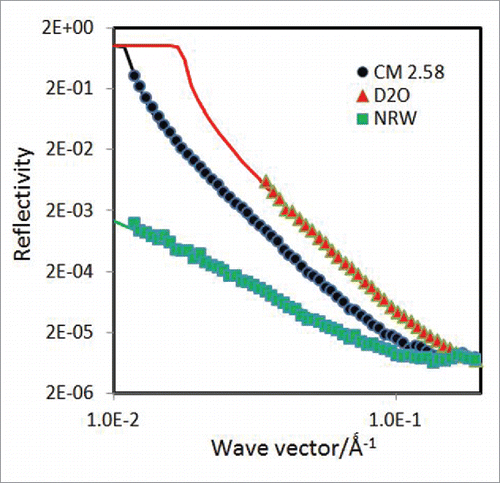
When the SLD of the solvent increases from 0 to 6.35 × 10−6 Å−2, that for the antibody increases from 2.05 to 3.36 × 10−6 Å−2 (). At SLD = 2.58 × 10−6 Å−2, the 2 SLD values become identical, implying that when the antibody is immersed in water under this contrast, it becomes invisible to the specular neutron reflection. The reflectivity in this solvent contrast can, however, inform about the part of the layer that stays out of the water surface.Citation34 shows the reflectivity measured at 50 ppm of the antibody concentration together with the best uniform layer fit, giving a thickness of 12 ± 2 Å and SLD of 0.7 × 10−6 Å−2. The SLD gives the same volume fraction of antibody as that calculated from the SLD value obtained in NRW, consistent with the uniform layer structure. For comparison, the parallel measurements in NRW and D2O under the same antibody concentration of 50 ppm are also plotted in . As already described above, the reflectivity profile from NRW could be well represented by a uniform layer of τ = 50 ± 5 Å and SLD = (0.56 ± 0.03) × 10−6 Å−2, giving ϕp = 0.27 ± 0.05. The fitting to the corresponding D2O profile must reflect the structural constraints obtained from the NRW and CM2.58 (contrast matched to mAb) profiles, consisting of a top layer of 12 Å on the air side and a bottom layer of 38 Å immersed in water. The corresponding SLDs for the 2 layers on the surface of D2O are 0.9 and 5.6 × 10−6 Å−2, and the acceptable fitting led to the same thickness and SLD for the top layer as converted from the 2 other water contrasts, but the bottom layer was represented by the best fitted thickness of 35 ± 3 Å and SLD of 5.85 × 10−6 Å−2. The fitting from the D2O contrast shows a highly consistent outcome that reinforces the overall structural features, but the minor discrepancies from the immersed bottom layer might be caused by a slightly lower amount of antibody adsorbed from the D2O profile.
Figure 6. Reflectivity profiles measured from the adsorption of 50 ppm COE-3 on the surface of NRW, CM2.58 (contrast matched to mAb) and D2O at pH 5.5 (His buffer, ionic strength of 25 mM). The continuous line through the NRW profile represents the uniform layer with τ = 50 Å and ρ = 0.56 × 10−6 Å−2; part of the layer that stays out of the surface of the CM2.58 contrast water was measurable, with τ = 12 Å and ρ = 0.71 × 10−6 Å−2, showing the same volume fraction as that from the NRW profile. The D2O profile was fitted with τ1 = 12 Å and ρ1 = 0.90 × 10−6 Å−2 for the surface layer exposed to air and τ2 = 35 Å and ρ2 = 5.85 × 10−6 Å−2 for the remaining layer immersed in water; apart from the slightly higher SLD for the immersed layer, the fitting was entirely consistent with the other 2 contrasts.
Co-adsorption of antibody and surfactant
It is widely considered that the addition of non-ionic surfactants in mAb solutions can prevent the antibody molecules from undergoing surface adsorption, thereby minimizing surface-induced structural unfolding. While this is widely accepted, there is a lack of direct experimental evidence to demonstrate that surfactant adsorption can indeed help ‘protect’ an antibody from undergoing surface and interfacial processes associated with structural changes. For a specific surfactant, it is also important to know when and how it dominates surface adsorption in a given mixture and if there is a concentration range over which co-adsorption occurs. The aim of the following neutron reflection experiment was to reveal that the respective surface composition of a mixture of surfactant and antibody can be determined from the appropriate use of isotopic contrasts.
As evident from , the h-Surf has a scattering length of 1.16 × 10−3 Å, while with 20 deuterated ethoxylate units, the d-Surf has a scattering length of 9.49 × 10−3 Å, giving their respective SLD of 0.63 and 5.13 × 10−6 Å−2 (in NRW). This means that while a strong neutron reflectivity will be detected from the d-Surf over most of the concentration range studied, the signal from the h-Surf will be weak and difficult to detect. However, the signal from specular neutron reflection is dictated by the adsorbed amount and following Equ (Equation2(2)
(2) ), the adsorbed amount Γ is proportional to layer thickness × SLD, i.e., τρ. This works well for the adsorption of the antibody alone; as the adsorbed amount increases, the level of reflectivity rises and so is the product of τρ, although it is ρ that increases with τ varying little in this case.
At a fixed antibody concentration, similar changes are expected when a surfactant is added. Following the relations as shown in Equ (Equation5(5)
(5) ) and (Equation6
(6)
(6) ), the product of τρ will respond to the co-adsorption from both surfactant and antibody with 2 noticeable effects. Note that the actual amount of antibody adsorbed varies with surfactant adsorption because it is a competitive process. When surfactant adsorption starts to become dominant, the product of τρ will deviate from that of the antibody alone due to the different adsorbed amount, and the actual signal difference was also linked to bsd and bsh (the scattering length for d-Surf and h-Surf, respectively). The products of τρ measured from co-adsorption of h-Surf and d-Surf with the concentration of antibody fixed at 50 ppm are shown in . As before, the solution pH was fixed at 5.5 with His buffer and the same ionic strength fixed at 25 mM. Over the lowest surfactant concentration range from 1/1000 to 1/100 CMC, the products of τρ remain the same as that of the antibody alone, showing little co-adsorption of the surfactant. As the surfactant concentration increases from 1/100 to 1/50 CMC, (τρ)sd begins to increase while (τρ)sh begins to decrease, indicating the early co-adsorption of the surfactant. From 1/50 to 1/20 CMC, a noticeable decline is observed from (τρ)sh while (τρ)sd rises sharply, indicating that surfactant adsorption is becoming dominant. From 1/10 CMC onwards, (τρ)sh undergoes a further, slight decline before plateau but (τρ)sd keeps rising sharply; the difference reflects the contributing effects from bsd and bsh. Thus, without solving the combined Equs (Equation5
(5)
(5) ) and (Equation6
(6)
(6) ), clearly shows how surface composition varies with increasing surfactant concentration, with COE-3 completely removed from the surface at surfactant concentrations above 1/10 CMC. It should be noted that the product of τρ was measured over a narrow low momentum transfer range. Thus, the reflectivity data could be measured relatively quickly.
Figure 7. Plots of thickness × SLD (τρ/10−6 Å−2) vs. the concentration of surfactant [expressed as the fraction of CMC] for both h-Surf and d-Surf, with the concentration of COE-3 fixed at 50 ppm. The product from the binary mixture of d-Surf and COE-3 is marked in blue diamonds ((τρ)sd) and that from the mixture of h-Surf and COE-3 in red squares ((τρ)sh). The τρ data from d-Surf alone are shown in green triangles.
![Figure 7. Plots of thickness × SLD (τρ/10−6 Å−2) vs. the concentration of surfactant [expressed as the fraction of CMC] for both h-Surf and d-Surf, with the concentration of COE-3 fixed at 50 ppm. The product from the binary mixture of d-Surf and COE-3 is marked in blue diamonds ((τρ)sd) and that from the mixture of h-Surf and COE-3 in red squares ((τρ)sh). The τρ data from d-Surf alone are shown in green triangles.](/cms/asset/0e83429d-9128-4ad6-8c0a-bd7b9f5a9cd6/kmab_a_1276141_f0007_c.gif)
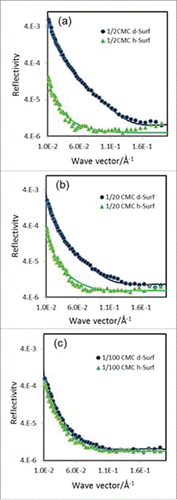
To further exemplify the changes in surface composition and layer thickness as a result of surfactant co-adsorption, shows 3 sets of reflectivity profiles measured at surfactant concentrations of 1/100, 1/20 and 1/2 CMC using both d-Surf and h-Surf in NRW at pH 5.5 and ionic strength 25 mM. Unlike the reflectivity profiles corresponding to the extractions of τρ shown in , the reflectivity profiles shown in were measured across the full κ range so that small differences between the different labels could be revealed. At 1/100 CMC of surfactant (), COE-3 adsorption is dominant and surfactant co-adsorption is minor due to its very low concentration. Nevertheless, the difference between the 2 reflectivity profiles is already obvious. Data analysis revealed that while the total layer thickness remained at τ = 48 ± 4 Å, the values for Ap = 17000 ± 3000 Å2 (Γ = 1.4 ± 0.2 mg.m−2) and As = 2500 ± 400 Å2 indicated the early desorption of COE-3 from the surface even at this very low surfactant concentration. At 1/50 CMC of surfactant, the total layer thickness still remained at τ = 48 ± 4 Å, but Ap = 19000 ± 3000 Å2 and As = 1600 ± 400 Å2 indicated further desorption of COE-3 (Section 3, Supporting Information). At 1/20 CMC of surfactant (), the gap between the 2 reflectivity profiles becomes wider, indicating the more dominant contribution from the adsorbed surfactant. The total layer thickness was slightly reduced to τ = 44 ± 4 Å with a concomitant fall in Ap = 35000 ± 4000 Å2 (Γ = 0.7 ± 0.3 mg.m−2) and As = 300 ± 30 Å2. This trend continues on further increase in surfactant concentration to 1/10 CMC, with the total layer thickness remaining at τ = 44 ± 4 Å, but Ap = 53000 ± 4000 Å2 (Γ = 0.4 ± 0.3 mg.m−2) and As = 260 ± 30 Å2. At a surfactant concentration of 1/5 CMC, the total layer thickness declines to 35 Å and Ap falls into the error margin, with As = 170 ± 15 Å2. The decline of the layer thickness is consistent with the desorption of COE-3 from the surface. Thus, from a surfactant concentration of 1/2 CMC onwards (), the layer thickness remains almost constant at 35 ± 3 Å and As = 150 ± 15 Å2. Hence, although at the very low surfactant concentration of 1/100 CMC the surfactant begins to desorb COE-3, the detailed analysis of these reflectivity pairs (including those in Supporting Information) depicts a clear picture of progressive replacement of COE-3 from the surface by the surfactant over a wide surfactant concentration range. Complete replacement could well occur when surfactant concentration is above 1/10 CMC.
Figure 8. Neutron reflectivity profiles measured from the co-adsorption of COE-3 and surfactant from NRW at pH 5.5 (His buffer, ionic strength 25 mM) with the COE-3 concentration fixed at 50 ppm but with varying concentrations of d-Surf and h-Surf at 1/2 CMC (a, top); at 1/20 CMC (b, middle) and 1/100 CMC (c, bottom).
Discussion
Our study demonstrates that neutron reflection in combination with deuterium labeling is an effective method for revealing the amount and physical state of antibody adsorbed at the air/water interface with and without surfactant co-adsorption. From a nearly constant layer thickness and uniform layer model (or Gaussian distribution), it can be inferred that the adsorbed COE-3 molcules retained their globular structure with no indication of unfolding. All mAb layer thicknesses were around 50 ± 5 Å under the conditions studied. These values are close to the dimensions of Fab and Fc,Citation36-38 showing that Fab and Fc fragments are rather well aligned in the adsorbed layers and that they do not stack up with fragments above or below each other. As shown in , the increase in bulk mAb concentration leads to the rise of surface packing density. The slight thickness increase indicates the orientation adjustment in response to the increasing packing density.
Subsequent studies of COE-3 co-adsorption with Polysorbate 80 revealed that the surfactant at 1/100 CMC started to desorb the mAb molecules, but that co-adsorption was retained over a wide surfactant concentration range and complete desorption of the mAb did not occur until the surfactant concentration reached 1/10 CMC. Under the solution conditions studied, there was no sign of surfactant-induced surface unfolding of the mAb molecules.
Surface tension measurements revealed that COE-3 alone did not reduce surface tension, even at concentrations up to 0.5 mg/ml (500 ppm), nor did COE-3 much influence the surface tension when mixed with Polysorbate 80. However, the neutron reflection measurements indicate that COE-3 alone adsorbed steadily over the concentration range studied () and, more importantly, co-adsorption also occurred over a wide Polysorbate 80 concentration range. Thus, COE-3 is not only surface active, but its surface adsorbed amount is quite high. Even at the lowest concentration of 2 ppm studied, its adsorbed amount is about 1 mg/m2 and by 50 ppm, the adsorbed amount increases to 2 mg/m2. indicates a clear trend wherein the amount of surface adsorbed COE-3 increases notably with its solution concentration, while the concomitant change in the layer thickness is much less pronounced.
In contrast, the layer thicknesses over the co-adsorption region remained relatively constant ( and the related text describing about the surfactant concentration range for co-adsorption to occur), again suggesting the dominant influence of the mAb present at the surface. Once mAb molecules are desorbed from the surface, the layer thicknesses decreases markedly, consistent with the typical surfactant layers adsorbed.
In summary, neutron reflection could play an active role in unravelling the surface and interfacial composition and structure for different antibodies, different interfaces and different solution conditions, particularly when another surface active ingredient is involved. The current work forms a useful basis for further neutron reflection experiments investigating mAb adsorption at concentrations well above 100 ppm and the effects of surfactant co-adsorption. As illustrated in , surface adsorption tends to plateau above 100 ppm, but any changes as a result of mAb instability and interaction with surfactant can be unravelled by neutron reflection with appropriate contrast variations.
Materials and methods
Hydrogenous Polysorbate (Tween®) 80 surfactant (denoted as h-Surf) was purchased from Sigma-Aldrich and was used as supplied. Its critical micellar aggregation in water was found to be 0.012 mM from surface tension measurements, consistent with the value reported from literature.Citation31,32 The deuterated Polysorbate 80 (20 ethoxylate groups deuterated only, denoted as d-Surf) was synthesized by reacting the sorbitan ester, sorbitan monooleate with deuterated ethylene oxide following the standard procedures.Citation35 Its CMC was also checked by surface tension measurements and the comparison with h-Surf is given in .
The antibody, denoted as COE-3, was expressed in Chinese hamster ovary cells and purified using industry-standard methods. The solution behavior of COE-3 under different mAb concentrations and ionic strengths has been studied by Roberts et al.Citation36 It was a full length IgG1 with sequence molecular weight equal to 144.8 kDa and supplied as a stock solution of 46.4 mg/ml in histidine (‘His buffer’ composed of histidine and histidine hydrochloride) at pH 6 with an ionic strength of 25 mM; 7 % w/v sucrose was also added to stabilize the antibody. The stock sample was stored under −80 °C, and, when needed, it was thawed and diluted directly into histidine buffer at pH 5.5, also at the ionic strength of 25 mM. It was diluted into subphases of different ratios of H2O and D2O. As the concentrations studied in this work were very low, typically below 1 mg/ml (1000 ppm), its dilutions into D2O meant that the levels of mixing of H2O were very low, with the exact amount noted and taken into account during neutron data analysis. The exact sequences of the light and heavy chains are given under Section 2.3 of the Supporting Information, allowing the relevant physical properties such as scattering lengths to be calculated under different solvent isotopic contrasts.
D2O (99% D), histidine and histidine hydrochloride were purchased from Sigma-Aldrich and also used as supplied. H2O was processed using an Elgastat PURELAB water purification system. Scattering length and SLD for basic elements are given in Table SI1 from which the scattering length and SLD for H2O, D2O and any different ratios of them can be calculated. These values for the surfactants and antibody are given in .
Surface tension measurements
Surface tension measurements were made using a Krüss K11 tensiometer. The du Noüy ring was freshly flamed before each surface tension measurement. The solution dishes and other glassware used were freshly cleaned by soaking them in dilute Decon solution, followed by copious water rinsing. All the measurements were performed at the room temperature of 22 ± 2 °C. The surface tension was recorded by raising the sample dish up to touch the ring and once in contact, the balance automatically adjusted the height so that the maximal pulling force was achieved. Each measurement was followed for up to 2–3 hour to monitor the time-dependent change. All the experiments were repeated at least 3 times, to ensure the reproducibility of the measurements.
Neutron reflectivity measurements
Neutron reflection measurements were performed using both the SURF reflectometer at ISIS Neutron Facility, Rutherford Appleton Laboratory, STFC, UK and the FIGARO reflectometer at the Institut Laue-Langevin (Grenoble, France). The neutron optical system in SURF provided the neutron wavelength range, typically between 0.5 and 7 Å. With the help of a supermirror setup, neutron reflectivity can be measured at the 3 incidence angles of 0.35, 0.8 and 1.5°, covering a momentum transfer range (κz) from about 0.01 to 0.5 Å. In contrast, the FIGARO instrument gave the neutron wavelength range between 2 and 30 Å and the data could be acquired at 2 incident angles of 0.62° and 3.8°, giving a momentum transfer range from about 0.005 to 0.4 Å−1. Both instruments were calibrated by taking the reflection measurements from a clean D2O surface. All the measurements were performed at the room temperature of 20 ± 3 °C.
Key structural constants needed for undertaking the model analysis using the above equations are listed in , with further information about the elementary scattering lengths and means to calculate the scattering lengths and SLDs of the surfactants (h-Surf and d-Surf) and COE-3 under different water contrasts given in Sections 1 and 2 of the Supporting Information.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supporting_Information.docx
Download MS Word (21.5 KB)Acknowledgments
RH and FP were supported by funding from MedImmune Ltd. We thank neutron beam times awarded to undertake this work at the Institut Laue Langevin, Grenoble and ISIS Neutron Facility, Chilton, Didcot, under the support of STFC.
References
- Ecker DM, Jones SD. The therapeutic monoclonal antibody market. mAbs 2015; 7(1):9-14 PMID:25529996; http://dx.doi.org/10.4161/19420862.2015.989042
- Ripple DC, Dimitrova MN. Protein particles: What we know and what we do not know. J Pharm. Sci. 2012; 101(10):3568-79; PMID:22736521; http://dx.doi.org/10.1002/jps.23242
- Lowe D, Dudgeon K, Rouet R, et al. Aggregation, stability and formulation of human antibody therapeutics, in Advances in Protein Chemistry and Structural Biology. 2011, Vol 84, pp 41-61, Ed. Donev R; PMID:21846562; http://dx.doi.org/10.1016/B978-0-12-386483-3.00004-5
- Roberts D, Keeling R, Tracka M, van der Walle CF, Uddin S, Warwicker J, Curtis R. Specific ion and buffer effects on protein-protein interactions of a monoclonal antibody. Mol Pharm 2015; 12(1):179-93; PMID:25389571; http://dx.doi.org/10.1021/mp500533c
- Pinholt C, Hartvig RA, Medlicott NJ, Jorgensen L. The importance of interfaces in protein drug delivery - why is protein adsorption of interest in pharmaceutical formulations? Expert Opin Drug Deliv 2011; 8:949-64; PMID:21557707; http://dx.doi.org/10.1517/17425247.2011.577062
- Badkar A, Wolf A, Bohack L, Kolhe P. Development of biotechnology products in pre-filled syringes: technical considerations and approaches. AAPS PharmSciTech 2011; 12(2):564-72; PMID:21538214; http://dx.doi.org/10.1208/s12249-011-9617-y
- Vazquez-Rey M, Lang DA. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng 2011; 108(7):1494-508; PMID:21480193; http://dx.doi.org/10.1002/bit.23155
- Bee JS, Randolf TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of surfaces and leachables on the stability of biopharmaceuticals. J Pharm Sci 2011, 100(10):4158-70; PMID:21523787; http://dx.doi.org/10.1002/jps.22597
- Bee JS, Goletz TJ, Ragheb JA. The future of protein particle characterization and understanding its potential to diminish the immunogenicity of biopharmaceuticals: A shared perspective. J. Pharm. Sci. 2012; 101:3580-5; PMID: 22736570; http://dx.doi.org/10.1002/jps.23247
- Johnson R, Jiskoot W. Models for evaluation of relative immunogenic potential of protein particles in biopharmaceutical protein formulations. J. Pharm. Sci. 2012; 101:3586-92; PMID: 22736238; http://dx.doi.org/10.1002/jps.23248
- Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci 2011; 100:354-87; PMID:20740683; http://dx.doi.org/10.1002/jps.22276
- Biddlecombe JG, Smith G, Uddin S, Mulot S, Spencer D, Gee C, Fish BC, Bracewell DG. Factors influencing antibody stability at solid-liquid interfaces in a high shear environment. Biotechnol Prog 2009; 25(5):1499-507; PMID:19585551; http://dx.doi.org/10.1002/btpr.211
- Shieh IC, Patel AR. Predicting the agitation-induced aggregation of monoclonal antibodies using surface tensiometry. Mol Pharm 2015; 12:3184-93; PMID:26198590; http://dx.doi.org/10.1021/acs.molpharmaceut.5b00089
- Lin GL, Pathak JA, Kim DH, Carlson M, Riguero V, Kim YJ, Buff JS, Fuller GG. Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions. Soft Matter 2016: 12(14):3293-302; PMID:26891116; http://dx.doi.org/10.1039/c5sm02830b
- Couston RG, Skoda MW, Uddin S, van der Walle CF. Adsorption behavior of a human monoclonal antibody at hydrophilic and hydrophobic surfaces. MAbs 2013; 5(1):126-39; PMID:23196810; http://dx.doi.org/10.4161/mabs.22522
- Kapp SJ, Larsson I, Van De Weert M, Cárdenas M, Jorgensen L. Competitive adsorption of monoclonal antibodies and nonionic surfactants at solid hydrophobic surfaces. J Pharm Sci 2015; 104(2):593-601; PMID:25446557; http://dx.doi.org/10.1002/jps.24265
- Lakey JH. Neutrons for biologists: a beginner's guide, or why you should consider using neutrons. J R Soc. Interface 2009; 6:S567-73; PMID:19656821; http://dx.doi.org/10.1098/rsif.2009.0156.focus
- Campana M, Hosking SL, Petkov JT, Tucker IM, Webster JR, Zarbakhsh A, Lu JR. Adsorption of bovine serum albumin (BSA) at the oil/water interface: a neutron reflection study. Langmuir 2015; 31(20):5614-22; PMID:25875917; http://dx.doi.org/10.1021/acs.langmuir.5b00646
- Xu H, Zhao XB, Grant C, Lu JR, Williams DE, Penfold J. Orientation of a monoclonal antibody adsorbed at the solid/solution interface: A combined study using atomic force microscopy and neutron reflectivity. Langmuir 2006; 22:6313-20; PMID:16800692; http://dx.doi.org/10.1021/la0532454
- Clifton LA, Holt SA, Hughes AV, Daulton EL, Arunmanee W, Heinrich F, Khalid S, Jefferies D, Charlton TR, Webster JR, et al. An accurate in vitro model of the E. coli envelope. Angew Chem Int Ed Engl 2015; 54(41):11952-5; PMID:26331292; http://dx.doi.org/10.1002/anie.201504287
- Ohtake S, Kita Y, Arakawa T. Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliver Rev 2011; 63:1053-73; PMID:21756953 ; http://dx.doi.org/10.1016/j.addr.2011.06.011
- Kiese S, Papppenberger A, Friess W, Mahler HC. Shaken, not stirred: Mechanical stress testing of an IgG1 antibody. J Pharm Sci 2008; 97:4347-66; PMID:18240293; http://dx.doi.org/10.1002/jps.21328
- Gerhardt A, Mcumber AC, Nguyen BH, Lewus R, Schwartz DK, Carpenter JF, Randolph TW. Surfactant Effects on Particle Generation in Antibody Formulations in Pre-filled Syringes. J Pharm Sci 2015; 104(12):4056-64; PMID:26413998; http://dx.doi.org/10.1002/jps.24654
- Lu JR, Lee EM, Thomas RK. The analysis and interpretation of specular neutron and x-ray reflection. Acta Cryst 1996; A52:11-41; http://dx.doi.org/10.1107/S0108767395011202
- Lu JR, Thomas RK. Neutron reflection from wet interfaces. J Chem Soc Faraday Trans 1998; 94:995-1018; http://dx.doi.org/10.1039/A707853F
- Lu JR, Thomas RK, Penfold J. Surfactant layers at the air-water interfaces: structure and composition. Adv Colloid Interface Sci 2000; 84:143-304; PMID:10696453; http://dx.doi.org/10.1016/S0001-8686(99)00019-6
- Nelson A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J Appl Crystallogr 2006; 39:273-76; http://dx.doi.org/10.1107/S0021889806005073
- Born M, Wolf E. 1970. Principles of Optics. Pergamon Press, Oxford.
- Green RJ, Su TJ, Joy H, Lu JR. Interaction of lysozyme and sodium dodecyl sulphate at the air-water interface. Langmuir 2000; 16:5797-805; http://dx.doi.org/10.1021/la000043t
- Lu JR, Su TJ, Thomas RK. Binding of surfactants onto pre-adsorbed layers of bovine serum albumin at the silica-water interface. J Phys Chem 1998; B102:10307-15; http://dx.doi.org/10.1021/jp983126f
- Chou DK, Krishnamurthy R, Randolph TW, Carpenter JF, Manning MC. Effects of Tween 20 and Tween 80 on the stability of Albutropin during agitation. J Pharm Sci 2005; 94(6):1368-81; PMID:15858848; http://dx.doi.org/10.1002/jps.20365
- le Maire M, Champeil P, Moller JV. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 2000; 1508:86-111; PMID:11090820; http://dx.doi.org/10.1016/S0304-4157(00)00010-1
- Lu JR, Thomas RK, Penfold J, Richards RW. The segment density distribution profiles of polyethylene oxide adsorbed at the air-water interface, as studied by neutron reflection. Polymer 1996; 37:109-14; http://dx.doi.org/10.1016/0032-3861(96)81605-3
- Lu JR, Su TJ, Thomas RK, Penfold J, Webster J. Structural conformation of lysozyme layers at the air-water interface studied by neutron reflection. J Chem Soc Faraday Trans 1998; 94:3279-87; http://dx.doi.org/10.1039/A805731A
- Tucker IM, Petkov JT, Penfold J, Thomas RK, Li PX, Cox AR, Hedges N, Webster JRP. Spontaneous surface selfassembly in protein-surfactant mixtures: interactions between hydrophobin and ethoxylated polysorbate surfactants. J. Phys. Chem. B 2014; 118:4867-75; PMID:24738908; http://dx.doi.org/10.1021/jp502413p
- Roberts D, Keeling R, Tracka M, van der Walle CF, Uddin S, Warwicker J, Curtis R. The role of electrostatics in protein-protein interactions of a monoclonal antibody. Mol Pharm 2014; 11(7):2475-89; PMID:24892385; http://dx.doi.org/10.1021/mp5002334
- Trakhanov S, Parkin S, Raffaï R, Milne R, Newhouse YM, Weisgraber KH, Rupp B. Structure of a monoclonal 2E8 Fab antibody fragment specific for the low-density lipoprotein-receptor binding region of apolipoprotein E refined at 1.9 Å. Acta Crystallogr D Biol Crystallogr. 1999; 55(Pt 1):122-8; PMID:10089402; http://dx.doi.org/10.1107/S090744499800938X
- Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 2000; 406(6793):267-73; PMID:10917521; http://dx.doi.org/10.1038/35018508
