ABSTRACT
Antibodies carry out a plethora of functions through their crystallizable fragment (Fc) regions, which can be naturally tuned by the adoption of several isotypes and post-translational modifications. Protein engineering enables further Fc function modulations through modifications of the interactions between the Fc and its functional partners, including FcγR, FcRn, complement complex, and additions of auxiliary functional units. Due to the many functions embedded within the confinement of an Fc, a suitable balance must be maintained for a therapeutic antibody to be effective and safe. The outcome of any Fc engineering depends on the interplay among all the effector molecules involved. In this report, we assessed the effects of Fc multiplication (or tandem Fc) on antibody functions. Using IgG1 as a test case, we found that, depending on the specifically designed linker, Fc multiplication led to differentially folded, stable molecules with unique pharmacokinetic profiles. Interestingly, the variants with 3 copies of Fc improved in vitro opsonophagocytic killing activity and displayed significantly improved protective efficacies in a Klebsiella pneumoniae mouse therapeutic model despite faster clearance compared with its IgG1 counterpart. There was no adverse effect observed or pro-inflammatory cytokine release when the Fc variants were administered to animals. We further elucidated that enhanced binding to various effector molecules by IgG-3Fc created a “sink” leading to the rapid clearance of the 3Fc variants, and identified the increased FcRn binding as one strategy to facilitate “sink” escape. These findings reveal new opportunities for novel Fc engineering to further expand our abilities to manipulate and improve antibody therapeutics.
Introduction
Antibody engineering of crystallizable fragment (Fc) domains has enabled the development of effective and safe antibody therapeutics, and the field continuously extends boundaries to add new dimensions to antibody functions through novel engineering approaches.Citation1 Not only can unwanted antibody effector functions be eliminated through mutations on selected residues within the Fc region without affecting the overall pharmacokinetics (PK) profiles,Citation2,3 but antibody half-life and effector functions can also be enhanced through targeted mutations within the Fc.Citation4,5 Furthermore, functions that are normally carried by a whole IgG molecule can be conferred on a Fc alone.Citation6 Thus, Fc-fusion therapeutics development has also gained significant momentum from Fc engineering approaches.Citation7 Isotype selectionsCitation8 or modifications through mutations or grafting,Citation9,10 affinity changes toward different FcγR, and glycosylation modificationsCitation11 have all been considered as a means to modify the Fc domain and antibody functions. With the increasing recognition of Fc functions in novel therapeutic conditions such as immune-modulation therapy,Citation12 it is a safe assumption that there will be more intense investigation into new ways to manipulate and apply Fc functions.
Intriguingly, a seemingly obvious option for Fc function modification has not been attempted to any depth. Fc multiplication appears to be a straightforward approach to enhance many of the Fc functions through the predicted avidity effect. Fc oligomerization as a result of antibody aggregation or through engagement of multimeric antigen can contribute to enhanced and sometimes undesirable effector function and may serve to dissuade such an attempt.Citation13 However, Fc arrangement within a tandemly linked Fcs might be topologically different than that within antibody aggregations resulting from antigen engagements. Given the potential benefit of a tandem Fc, this approach warrants further evaluation of both efficacy and safety. Hybrid Fc has been attempted previously, but this platform is different because it is designed to improve antibody-dependent cell-mediated cytotoxicity (ADCC) by endowing a conventional IgG1 with enhanced binding to a selected FcR (CD89).Citation10 Several reports from one group examined the consequences of Fc multiplication. An anti-CD20 antibody displayed relatively poor ADCC activity against CD20-expressing cancer cells. However, by adding an extra, and in particular 2 extra, Fcs to the original IgG1 antibody, both ADCCCitation14 and antibody-dependent cellular phagocytosisCitation15 activity improved significantly. Similar enhancement in ADCC was observed when tumor necrosis factor (TNF) receptor II was fused with a tandem Fc and used in treating TNF-expressing cells.Citation16 Although encouraging in vitro results were obtained, there was no in-depth biochemical or biophysical analysis performed, or in vivo studies reported by the same or other groups, leaving critical questions unanswered and a potentially promising direction unexplored.
To explore such a platform in depth, we generated tandem IgG1 Fc molecules by adding one or 2 extra copies of Fcs and compared their biochemical and biophysical characteristics, and PK profile to their parental IgG1 molecules. We further evaluated functional activities of these modified antibodies both in vitro and in vivo. Interestingly, we observed significantly improved protections by the antibodies with 3 Fc tandem repeats in a Klebsiella pneumoniae infection model. Importantly, the tandem Fc variants of huIgG1, the most potent human IgG isotype capable of mediating effector functions in mouse models,Citation17 did not induce any abnormal cytokine release or other adverse effect. Our data suggests a novel Fc engineering technology for enhancing effector functions safely, and could open new opportunities for development of novel antibody therapeutics.
Results
Expression profiles and purification of tandem Fc variants
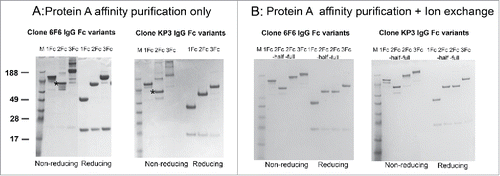
Design features of the various Fc variants are shown in and described in the materials and methods section. pOE expression vectors each carrying IgG with one, 2 or 3 Fc repeats were transfected into the 293X cells. The yields were estimated using a bilayer interferometry-based approach as described previously.Citation18 No significant differences were observed among the various Fc variants compared with the parental IgG in expression. Initial analysis with a protein A-based, one-step affinity purification revealed that IgG-1Fc and IgG-3Fc each had a dominant band corresponding to the expected molecular weight of 150 and 250 kDa, respectively. IgG-2Fc, however, had a dominant band that was half the size (∼100 kDa) of the expected molecular weight. There was one minor band with the expected molecular weight of 200 kDa (). There were aggregations to various degrees depending on the antibody and Fc repeats. However, the predicted molecular formats remained dominant except for the 2Fc constructs. All the Fc variants can be purified to near homogeneity (> 95% purity) with an additional, ion exchange-based step as described in the method section ().
Figure 1. Design of the Fc tandem molecules. (A) Schematic representation of IgG1, IgG-2Fc and IgG-3Fc molecules. The IgG-2Fc contains 2 copies of Fc and has an expected molecular weight (MW) of 200 kDa, IgG-3Fc contains 3 copies of Fc and has an expected MW of 250 kDa. (B) The connecting region between Fcs include 2 (GGGGS) repeats and a full hinge region. (C) Schematic representation of the alternatively folded IgG-2Fc-half molecule with a MW of 100 kDa.
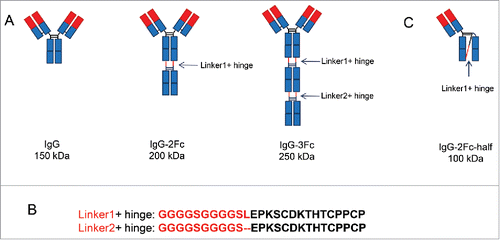
Figure 2. Expression and purification of IgG tandem Fc variants. (A) Mammalian cell-expressed IgG1, IgG-2Fc and IgG-3Fc proteins from 2 clones, anti-LPS 6F6 and anti-MrA KP3, were captured by protein A beads and analyzed by SDS-PAGE under reducing and non-reducing conditions. (B) The IgG-2Fc-half, IgG-2Fc and IgG-3Fc fractions were separated by an additional ion-exchange chromatography step and further purified. The purity and size of each IgG Fc variants were confirmed by a SDS-PAGE analysis. M, molecular weight (MW) marker. Numbers on the left indicate the MW in kDa. “*” denotes the location of the IgG-2Fc-half molecules under non-reducing condition.
Tandem Fc variants binding to FcγR, C1q, FcRn, and antigens
The apparent binding affinities to various effector molecules by the Fc variants can serve as important indicators for the appropriate folding of the Fcs and to predict antibody functions. Both 2Fc and 3Fc variants showed significantly improved bindings to low affinity receptors including FcγRIIa, IIIa, IIb and FcRn, suggesting the appropriate Fc presentation in the context of 2Fc and 3Fc carrying IgG molecules (). 2Fc and 3Fc variants also showed enhanced binding to C1q in the ELISA assays. Importantly, 2Fc half molecule could bind to the FcγRs, although at reduced level compared with the parental IgG1 molecule, further confirming the appropriate intramolecular folding of the Fc region that is critical for binding to FcγRs.
Figure 3. Binding profiles of IgG Fc variants to Fc receptors, C1q and antigens. (A) ELISA evaluation of 6F6 IgG Fc variants binding to Fc receptors including FcγRI, FcγRIIa, FcγRIIIa(158F), FcγRIIb,FcRn and C1q. (B) ELISA evaluation of binding activity of anti-MrkA KP3 and anti-LPS 6F6 IgG Fc variants to their respective antigens.
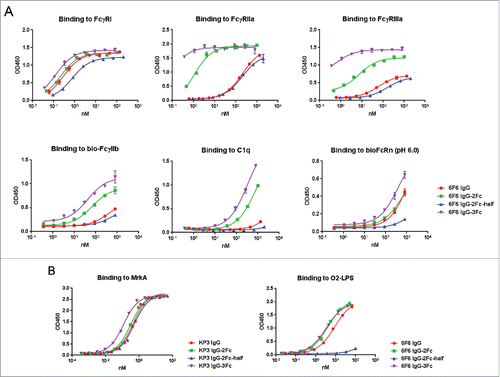
There was little effect on antigen bindings by Fc multiplication, with the exception of 6F6–2Fc half molecule (). 6F6 was isolated as an IgM, anti-lipopolysaccharide (LPS) antibody. It is likely that an avidity effect is required for robust antigen binding. KP3 was isolated through phage display and has an excellent KD (< 1 nM, data not shown). KP3–2Fc half molecule displayed similar apparent binding affinities as the other Fc variants ().
Stability assessment of the tandem Fc variants
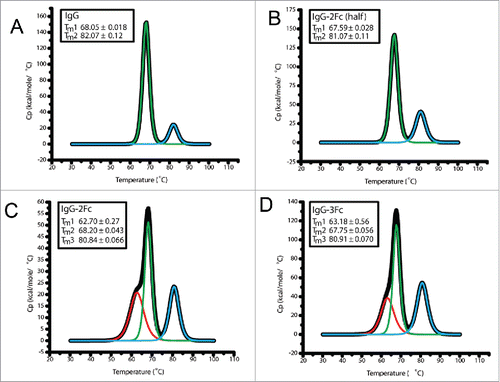
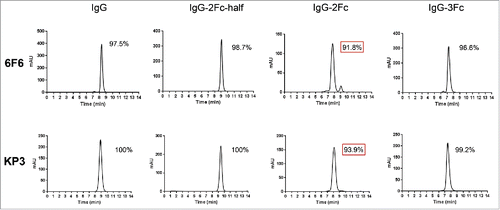
Stability is of critical importance to biotherapeutics. We conducted several developability assessments. The expression yields comparable to the parental IgG1 suggest that the folding and intracellular transportation was not significantly affected by the Fc multiplication even though the percentages of aggregations are higher than the parental IgG (). Purified Fc variants remain stable over an extended period of storage (one year) under 4°C. The percentages of monomeric 3Fc and 2Fc-half variants were over 96% for clone 6F6, and over 99% for clone KP3, and were comparable to those of their parental IgG1 molecules (). The purity of 2Fc full molecules was reduced to near or below 93%, suggesting potential developability issues. We further examined the temperature-induced unfolding of the Fc tandem molecules using differential scanning calorimetry (DSC). The DSC file of the 2Fc-half was the same as the IgG1, with the first transition Tm at 68°C and second transition Tm at 80°C. There was a minor, additional peak with slightly reduced melting temperature for the 2Fc and 3Fc variants at 62°C (). This may represent the unfolding of the extra Fcs, and the minor peak is more pronounced in the IgG-2Fc variant than in the 3Fc variant, which is in line with the observation from the long-term storage experiment. The overall thermostability was not affected, as all the melting temperatures fell within the normal ranges.Citation19
Figure 4. Size-exclusion high performance liquid chromatography (SE-HPLC) analysis of the purity of monomeric IgG Fc variants after prolonged storage. 6F6 (upper panels) and KP3 (lower panels) IgG variants including IgG1, IgG-2Fc-half, IgG-2Fc and IgG-3Fc were stored at 4°C for one year and analyzed by a SE-HPLC. Monomer percentage of each of the Fc variants is shown in each graph. Monomer percentages experiencing significant decrease (> 3%) are highlighted with a red box.
Pharmacokinetic profiles of the Tandem Fc variants
The PK profiles of all 4 Fc variants of KP3 were evaluated in C57BL/6 mice ( and ). Following a single intravenous (IV) administration of 10 mg/kg KP3–1Fc, a peak concentration of 110 µg/mL was observed 2 hour post-dose. Thereafter, serum concentration decreased in a bi-exponential manner with a clearance of 94 mL/day/kg and a terminal half-life of 2.2 d. For KP3–2Fc-half, the overall serum concentration-time profiles (i.e., AUClast) were comparable to that of KP3–1Fc, although more variable concentration data (especially oN-terminal phase) did not allow accurate estimation of its half-life and clearance. KP3–2Fc-full displayed a peak concentration similar to that of KP3–2Fc-half but was cleared rapidly with AUClast ∼3-fold lower compared with KP3–2Fc-half or wild type KP3 antibody. Conjugating another Fc to KP3–2Fc-full to form KP3–3Fc further increased clearance >2-fold and reduced overall exposure nearly 3-fold compared with KP3–2Fc-full.
Figure 6. Pharmacokinetic profiles of KP3 IgG Fc variants in C57BL/6 mice. The serum levels for each of the IgG variants were determined by an ELISA. Each data point represents the average IgG concentration in sera from 3 mice for different time points up to 7 d.
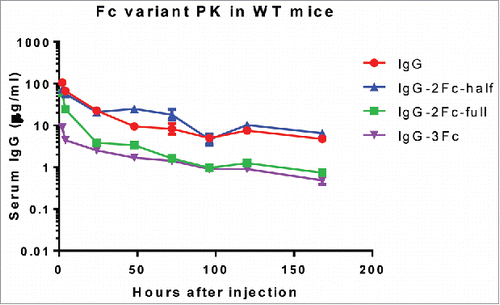
Table 1. Pharmacokinetic parameters of KP3 Fc variants following 10 mg/kg IV in mice. ND: not determined because of data fluctuation; Cmax = maximal observed concentration; AUClast = area under the concentration time curve up to the last measurable concentration; AUC∞ = Area under the curve from time zero to infinity; T1/2 = terminal phase elimination half- life; CL = systemic clearance; IV = intravenous injection.
In vitro and in vivo activity of the tandem Fc variants
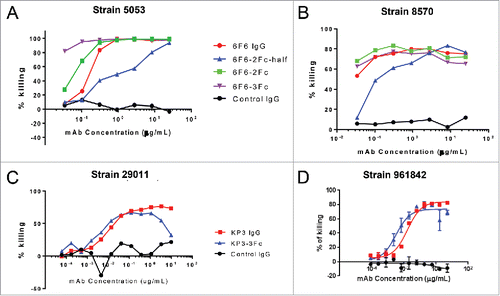
Opsonophagocytic killing (OPK) is a major mechanism of host protection against bacterial infection, and the in vitro OPK activity of an antibody can serve to predict its in vivo efficacy.Citation20 The in vitro activity of IgG variants was evaluated by OPK assays against different Klebsiella pneumoniae strains. The anti-LPS clone 6F6 IgG 2Fc and 3Fc variants improved the percentage of killing in the 5053 stains (), and maintained similar OPK activities as the parental IgG1 in the 8570 strain (). The 6F6-IgG-2Fc-half antibody showed a reduced OPK activity in both strains, which may reflect the diminished binding affinity to the antigen in the monovalent format (). The anti-MrkA KP3- IgG-3Fc maintained a similar OPK activity as the parental antibody against the 29011 strain (), while KP3-IgG-3Fc enhanced OPK activity against the 96184 strain slightly at low concentrations (). The KP3-IgG-3Fc molecules displayed a hook effect at higher concentrations for an unknown reason.
Figure 7. Opsonophagocytic killing (OPK) activity of IgG Fc variants against different Klebsiella pneumoniae (KP) strains. The OKP activity of 6F6 IgG variants were tested against KP 5053 (A) and 8570 (B) strains. The activity of KP3 IgG variants were tested against KP 29011 (C) and 961842 strains (D). The percentage of killing was determined for different IgG Fc variants against an isotype control IgG.
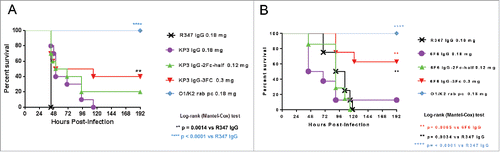
The in vivo activities of IgG-2Fc-half and IgG-3Fc variants were evaluated in a Klebsiella lethal pneumonia model and compared with their parental IgGs. Due to the low production and relatively poor stability of 2Fc-full molecules, they were not pursued further in the in vivo studies. The KP3-IgG-3Fc increased the survival rate to 40%, while the parental KP3-IgG1 did not provide any protection (). The 6F6-IgG-3Fc improved the survival rate to 70%, compared with the 10% survival rate by the parental antibody (). The IgG-2Fc-half antibodies of neither clones provided significant protection in this model similar to their parental IgGs ().
Figure 8. In vivo protective activity of IgG Fc variants. The in vivo activities of KP3 IgG Fc variants (A) and 6F6 IgG Fc variants (B) were evaluated in acute pneumonia mouse models. C57BL/6 mice were inoculated with a multi-drug resistant isolate intra-nasally. IgG Fc variants were administered one hour post bacterial infection. Mouse survival was monitored daily up to day 7 post challenge. For the evaluation of the KP3 variants, 5.22×107 CFU of KP strain 29011 were inoculated intra-nasally to the mice one hour before the IV injections of the KP3 Fc variants. For the evaluation of the 6F6 variants, 1.32×104 CFU of KP strain 3048570 were inoculated intra-nasally to the mice one hour before the IV injections of the 6F6 Fc variants. R347 is a human IgG1 isotype (negative) control. A rabbit polyclonal antibody generated against a wild type KP strain was included as a positive control. There were 10 animals in each group in (A) and 8 animals in each group in (B).
3Fc variant PK modulations through mutations within the Fc domains
We hypothesized that the rapid clearance of the 3Fc variants may be due to their increased binding to FcγRs, therefore creating a sink effect. We introduced TM mutations to each of the 3 Fcs within the 3Fc variant to greatly reduce the effector molecule bindings.Citation2 The 3Fc-TM variant displayed diminished binding to various effector molecules as expected (), but adopted a PK profile in the huFcRn transgenic mice resembling that of a conventional IgG1, thus supporting the “sink” hypothesis ( A and ). However, under various therapeutic conditions the effector functions will be necessary and 3Fc-TM format will not be amenable for this purpose. We therefore tested if serum clearance can be reduced without eliminating the effector functions. We introduced YTE mutations to each of the Fcs and confirmed their enhanced binding to the human FcRn, agreeing with previous studiesCitation4(). YTE mutations partially reduced the 3Fc bindings to other effector molecules, also in agreement with a previous report.Citation21 Interestingly, the 3Fc-YTE variant adopted a PK profile in the huFcRn transgenic mice resembling that of a conventional IgG1 until day 4 ( and ). The data points after day 4 displayed a precipitous drop to fall below the level of detection, leading to a poor overall serum half-life. We confirmed that 3Fc-YTE induced anti-drug activity (ADA) that likely contributed to this phenomenon. This ADA was not seen with the original 3Fc variant or with 3Fc-YTE at early time points (). Therefore, the fast clearance after day 4 for 3Fc-YTE is an ADA-associated event rather than an intrinsic feature of the molecule, and further tests in non-human primate could be performed to further address this question. These data suggest that PK profiles of the 3Fc variants can be improved effectively through sink removal or escape.
Figure 9. Binding profiles of IgG Fc variants with Fc point mutations to FcγRs, C1q and antigens. The 2 isoforms of FcγRIIIa (158F and 158V) are indicated.
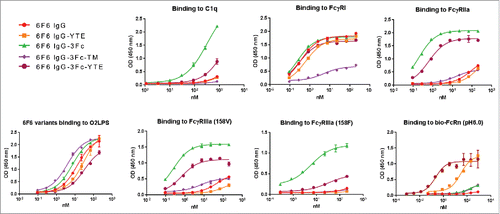
Figure 10. Pharmacokinetic profiles of 6F6 IgG Fc variants in human FcRn transgenic mice. (A) The serum levels for each of the IgG variants were determined by an ELISA. Each data point represents the average IgG concentrations in sera from 3 mice for up to 9 d. (B) 3Fc-YTE induced significant anti-antibody response in mice whereas 3Fc did not. In a direct ELISA, 6F6–3Fc and 6F6–3Fc-YTE were coated to an ELISA plate directly and subjected to detection with sera from mice injected with 6F6–3Fc or 6F6–3Fc-YTE, respectively. The sera of representative mice from both groups collected at day 4 (upper panel) and day 9 (lower panel) were used in the ELISA.
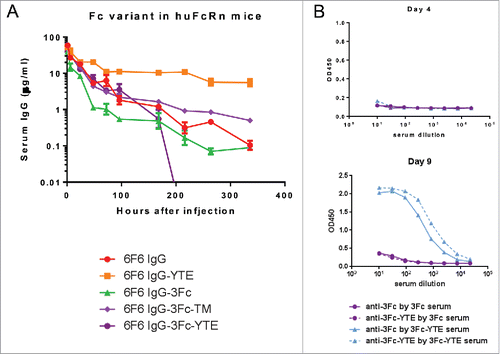
Table 2. Pharmacokinetic parameters of KP3 Fc mutant variants following 2 mg/kg IV in huFcRn transgenic mice. The parameter symbols used are the same as in .
Lack of Fc variant-induced cytokine release in mice
Cytokine release induced by immune complex through FcγRs cross-linking can be pathogenic. There is legitimate concern that Fcs arranged in tandem can cause such an effect inadvertently. We did not observe any adverse effect on animals that received IgG-2Fc and 3Fc in the course of PK or pharmacodynamics studies. Furthermore, we failed to detect any abnormal release of cytokines that are associated with inflammatory responses, including interferon (IFN)-γ, interleukin (IL)-1b, IL-2, IL-4, IL-5, IL10, IL-12, KC/GRO and TNF (data not shown). It appears that multiplication of Fc in the context of our molecules is not sufficient to induce nonspecific release of pro-inflammatory stimuli. We used human IgGs in murine models in the current studies. Human IgG1 has been demonstrated to be the most potent human IgG isotype in mediating effector functions in murine models,Citation17 therefore this observation is relevant.
Discussion
The Fc domain of an IgG is a proven target for protein engineering to optimize antibody functions and achieve better therapeutic effect. Many strategies have been attempted with success, but tandem Fc modifications have not been investigated to any depth despite apparent advantages, such as enhanced effector molecule/cell engagement for improved therapeutic effect against cancer or infections. In this study, we engineered 2 distinct anti-Klebsiella pneumoniae antibodies by tandem Fc modifications, and compared their biochemical and biophysical properties, PK profiles, and in vitro and in vivo functions with the parental IgG1. Our data show that tandem Fc modifications resulted in antibodies with good developability and distinct PK profiles. More importantly, antibodies with 3Fc displayed superior in vivo efficacy against bacterial infections without inducing adverse effects. Our data also suggested strategies to further improve this platform to achieve superior therapeutic efficacies.
As the first step of gauging the effect of Fc multiplication, we added either one or 2 extra copies of Fc to the original IgG molecule. To assess the functional effect of Fc multiplication, we chose the human IgG1 isotype for its dominant effector functions both in human and mouse. The linker is composed of 2 repeats of (GGGGS), which has been used in numerous studies.Citation22 The whole hinge including the upper hinge region, which has been shown to play important functional roles,Citation23 is included to maintain the effector functions and flexibility (). Stable, correctly-folded molecules were expressed and purified as expected, except for the IgG-2Fc format for both antibodies tested. It appears that 2Fc antibodies preferred intra-molecule Fc interactions resulting in the production of predominantly IgG-2Fc-half molecules. This result is in contrast to a previous study in which there was no report of the production of half molecules.Citation14 Therefore, this appears to be a linker-dependent event, and suggests ways to improve the generation of either 2Fc or 2Fc-half molecules depending on the needs. Both IgG-2Fc-half molecules displayed excellent stability, suggesting the suitability of such a platform for further development for various purposes. Similarly, both IgG-3Fc molecules displayed good developabilities, which is important for potential drug development ().
The IgG-2Fc and IgG-3Fc molecules showed significantly improved binding to FcγRs, FcRn and C1q. The improvement is most obvious toward the low affinity FcγRs, C1q, and FcRn. Avidity effects appeared to play an important role, as the 3Fc molecule showed incremental improvement in bindings compared with the 2Fc molecule. Supporting the hypothesis that stronger binding would lead to better effector functions, both KP3, an anti-MrkA antibody, and 6F6, an anti-LPS antibody, displayed better OPK activity when additional Fcs were added to their original IgG1 molecule. The improvement, however, was more pronounced for 6F6. This may be due to the fact that KP3 is a high affinity antibody that possesses potent OPK activity even in its original format, whereas 6F6 has very poor affinity, as evidenced by the loss of binding by the 2Fc-half format, presumably due to its monovalent binding moiety ().
Importantly, there was a correlation between the in vitro OPK and in vivo protective activity. The marginally improved OPK activity by KP3–3Fc resulted in appreciable improvement in the survival after Klebsiella pneumoniae challenge, whereas, in the context of 6F6–3Fc, more significant improvement was seen in both in vitro and in vivo studies. For both antibodies, the otherwise non-protective antibodies were transformed into protective ones through Fc multiplication. It appears that the adoption of multiple Fcs confers better protection through at least one mechanism, which is the enhancement of binding and effector function through the potential avidity effect. It is not completely clear if this mechanism alone is solely responsible for the enhanced in vivo protection. Another intriguing hypothesis is that 3Fc molecule may offer better accessibility to effector cells through its longer Fc domains compared with a traditional IgG molecule. Whether this mechanism played any role in the current study remains unknown.
It is apparent from this study that the degree of enhancement in the in vitro and in vivo activity is dependent on the target and antibody, even though a similar mechanism of action (MOA) was mainly involved in the in vivo protective activity. Tempting as it is to use the Fc multiplication as a universal mechanism to boost antibody effector functions, we could observe wide variations of enhancing effect as we expand to include more targets for different disease indications in future studies. Correspondingly, the design of the Fc multiplications should be adapted to various targets and antibodies.
It has been well recognized that the PK profiles of antibodies depend on a multitude of factors.Citation24 The effect of Fc multiplications on the antibody PK profiles is a critical issue for the development of such a potential drug format, which in turn may serve as an excellent platform to study the interrelationships among the many factors involved in determining the PK profiles. Both 2Fc and 3Fc formats led to the rapid clearance of the antibodies, despite their apparent enhancement in FcRn bindings at pH6.0 but not at pH7.4 (data not included for negative binding at pH7.4). Among the many possible contributing factors, we identified the “sink” as the major mechanism leading to a rapid serum clearance. Our finding is supported by a previous study showing an improved PK profile through elimination of FcγR binding by an IgG2 antibody.Citation25 Further, we showed that this “sink” effect can be alleviated through either removal of FcγR binding or enhancement of FcRn binding. The adoption of either strategy will be dependent on the MOA of antibody therapeutics. Although YTE was used as a proof of concept study, other FcRn binding enhancing mutations can be used without negatively impacting the effector functions if it is desired.Citation5,26 We used 2 mouse strains to perform the PK studies. The first is a wild-type mouse strain C57BL/6, which was used to develop our Klebsiella pneumonia infection model. The model was designed to help us understand the in vivo protective data. A human FcRn transgenic mouse was used to gauge the likely PK profiles when the 3Fc platform is used in human. Future work in non-human primates should help to further elucidate the PK profiles of the Fc variants. Despite the very rapid clearance in mouse, anti-Klebsiella pneumoniae antibodies with 3Fc platform displayed significantly improved protective effect in a therapeutic model. Disease models carrying human FcRn will be developed to assess the in vivo activity of our improved Fc variants such as 3Fc-YTE. We did observe an ADA activity when 3Fc and YTE platforms were combined; however, this experiment was performed against a mouse genetic background using fully human antibody sequences. Furthermore, huFcRn binding enhancing mutations with fewer and different residues involved, such as the LS mutationsCitation5 or others, can be adopted by the 3Fc platform to help avoid ADA. We anticipate that this should be manageable if it becomes an issue in therapeutics development.
Due to the modular features of the Fc, a tandem Fc provides significant flexibility in Fc modifications. The multitude of Fc engineering approaches can be applied to all or selected Fcs within the context of the 3Fc, resulting in a large number of combinations of various Fcs mediating different effector functions. This will enable the ability to cater to different therapeutic needs that would be difficult with a conventional IgG.
It has been reported that a higher drug-to-antibody ratio (DAR) confers superior anti-cancer activity by a site-specific antibody-drug conjugate (ADC) format.Citation27 However, despite the various strategies, the DAR will be limited due to the restricted conjugation sites that are appropriate and the effect of hydrophobicity of the payload on the ADC, leading to poor PK profiles. The IgG-3Fc, especially the 3TM variant described in this study, offers a potentially convenient solution to these problems providing additional conjugation sites, thereby increasing the possible DAR, without severely reducing the developability of an ADC.
The IgG-2Fc-half is an interesting molecular format that offers several unique opportunities. This molecule can be readily produced in large quantity and has good stability. It maintains the binding affinity to FcγRs, FcRn and C1q, and has a PK profile comparable to that of the parental IgG1 despite its significantly reduced molecular weight. This is very useful when monovalent antigen binding is desired, and effort had been made to generate such a format, albeit a knob-in-hole strategy was used.Citation28 Our approach essentially eliminated the unwanted mismatches between different heavy chains and should prove to be more efficient. Additionally, the IgG-2Fc-half platform provides a convenient platform to generate ADCs with DAR = 1, which may be useful in reducing toxicity and improving developability.
Finally, it is important to note that we did not observe any abnormal cytokine release or other adverse effects after the injection of Fc variants to mice in the current studies. Further testing in non- human primate will be necessary to address any concerns that might be associated with the Fc variants before therapeutics development for human use. The lack of adverse effect in the current study represents a promising first step in a new direction.
Materials and method
Reagents, antibodies and Klebsiella pneumoniae strains
All human FcγRs and FcRn were expressed and purified in Medimmune and C1q (Cat# A400) was purchased from Quidel (San Diego, CA). Monoclonal antibody (mAb) KP3 is an anti-MrkA antibody and was described in a previous study.Citation29 An anti-LPS mAb 6F6 was discovered in the same study as KP3 using a hybridoma platform after further hybridoma screening and selection for OPK killing positive clones. It originally had an IgM isotype and was reformatted into a huIgG1 in experiments in this study. Klebsiella pneumoniae strains and animal challenge models were as described.Citation29
Tandem Fc antibody design, construction and expression
Two tandem Fc constructs, IgG1–2Fc and IgG1–3Fc, were designed and constructed on the backbone of an in-house IgG1 expression vector pOE.Citation18 To make the IgG-2Fc plasmid, a DNA fragment containing GSlinker1-hinge-CH2-CH3 was generated by PCR and linked to the C-terminus of the CH3 in the pOE vector to yield 2 copies of Fc (). A HindIII site was introduced at the 3′ end of GSlinker1 for cloning purpose, adding an extra leucine (L) to the (G4S)2 linker (). For construction of IgG-3Fc, a 1.4 Kb DNA fragment containing CH2-CH3-GSlinker2-hinge-CH2-CH3 with a HindIII site at the 5′end and a NotI site at the 3′end was synthesized (GeneArt) and cloned into the IgG-2Fc vector using the HindIII/NotI sites, resulting in 3 copies of Fc (). The amino acid sequences of the complete linker1 and linker2 are shown in . The variable domains of 2 antibodies: KP3 (against MrkA) and 6F6 (against LPS) were cloned into the IgG-2Fc and IgG-3Fc vectors, respectively. The resulting plasmids were verified by sequencing and used in transfection of 293X cells (Invitrogen, Carlsbad, CA) for transient IgG production as described previously.Citation18 The expression level of all IgG variants was evaluated in a ForteBio Octet QK384 instrument using protein A sensors.
Purification of IgG Fc variants
For purification of IgG Fc variants, the antibodies were each captured from the cell culture medium by application to MabSelect SuRe resin (GE Healthcare, Uppsala, Sweden). Resins were packed in Vantage L columns from EMD Millipore (Billerica, Massachusetts). Each antibody was loaded to a less than 20 g/L capacity and then washed with 1X phosphate-buffered saline (PBS), pH 7.2. IgG was eluted using a low pH acetate buffer (50 mM acetate, pH 3.5) directly into 3 column volumes of neutralizing Tris buffer (50 mM, pH 8.0) to minimize aggregate formation. The product was analyzed by size-exclusion high performance liquid chromatography (SE-HPLC) to determine the amount of aggregation.
Mixed-mode chromatography was designed to separate the monomers from the aggregated species and to concentrate the product. Each product from the previous step was spiked with phosphate to 5 mM and loaded onto CHT resin (Bio-Rad, Hercules, California) to a capacity of less than 15 g/L. The protein was eluted over a steep 20 CV gradient from 10 mM phosphate, 10 mM sodium chloride pH 7.4 to 300 mM phosphate and 10 mM sodium chloride pH 7.4. During the elution 0.5 column volume fractions of concentrated material were collected. All purification steps were controlled by Unicorn 5.2 software on an Akta Explorer from GE Healthcare (Uppsala, Sweden).
The CHT gradient fractions were analyzed by SE-HPLC and pooled to yield a product that was greater than 95% monomeric. The CHT product was formulated into 1X PBS, pH 7.2 by dialysis using a Slide-A-Lyzer cassette (ThermoFisher Scientific, Waltham, MA).
Thermostability measurement by differential scanning calorimetry
Differential scanning calorimetry experiments were performed using a Microcal VP-DSC scanning microcalorimeter (Microcal) with an auto-sampler. All samples were in 1X PBS buffer and heated from 30°C to 100°C at a scan rate of 60°C/hr. Samples were assayed at a concentration of 5 μM in 400 μL. Signal from experiments using only buffer were subtracted as the baseline. The software Origin (version 7) was used for data analysis to determine apparent melting temperatures, Tm.Citation19
To further confirm the stability of the 4 Fc variants including IgG1, IgG1–2Fc, IgG1–2Fc-half, and IgG1–3Fc, purified proteins that had been stored at 4°C in PBS for one year were subjected to a SE-HPLC analysis to check for formation of aggregation or degradation. The samples were analyzed on a SE-HPLC equipment Model 1290 Infinity from Agilent. SE-HPLC is performed using a TSKgel G3000SWXL column (Tosoh Bioscience LLC, Montgomeryville, PA) in a buffer containing 100 mM sodium phosphate (pH 6.8) at a flow rate of 1 ml/min.
ELISAs to measure Fc variant binding to antigen and various FcγRs
An ELISA was performed as described with several modifications.Citation18 Antigens including LPS and MrkA were coated on a Nunc 96-well ELISA plate at 2 µg/ml in PBS at 4°C overnight for direct antigen binding tests. Various human FcγRs, FcRn, and C1q were coated similarly for Fc variant binding test. For FcRn binding by the Fc variants, 5 µg/ml streptavidin was coated first onto ELISA plates, followed by the incubation of the plates with biotin-labeled FcRn. Fc variants were serially diluted and added to the coated plates. For the antigen binding assay, goat anti-huFc-HRP (Jackson ImmunoResearch Lab, # 109–001–008) was used as the secondary antibody. For Fc engagement assay goat anti-huFab-HRP (Jackson ImmunoResearch Lab, # 109–035–097) was used as the secondary antibody. For FcRn engagement assay, all blocking and washing buffer had a pH of either 6 or 7.2, whereas for all other ELISA assays the buffers had a pH of 7.2.
Opsonophagocytic killing activity measurement
OPK activity was measured as described.Citation29 Briefly, log phase culture of luminescent Klebsiella pneumoniae strains (Lux) was diluted to ∼2×106 cells/ml. Bacteria, diluted baby rabbit serum providing complement (Cedarlane, 1:10), dimethylformamide (DMF) differentiated HL-60 cells, anti-MrkA or anti- LPS antibodies in various Fc formats were mixed in 96-well plates and incubated at 37°C for 2 hours with shaking (250 rpm). The relative light units (RLUs) were then measured using an Envision Multilabel plate reader (Perkin Elmer, Waltham, Massachusetts). The percentage of killing was determined by comparing RLUs derived from assays with no antibodies to RLUs obtained from anti- K. pneumoniae mAbs and a negative control mAb.
Pharmacokinetics study of the tandem Fc variants
Eight week old C57BL/6 mice were used first for the PK studies of the KP3 Fc variants. All experiments were conducted in accordance with MedImmune's Institutional Animal Care and Use Committee protocols and guidance. Four KP3 Fc variants were administered IV to mice at a dose level of 10 mg/kg (6 mice/group, divided into 2 sub-groups of 3 for bleeding in alternating fashion per regulation). The first data point was at 2 hour post IV injection. HuFcRn transgenic mice carrying fully human FcRnCitation30,31 were used for further PK comparison among 6F6 Fc variants to evaluate the potential effect of the TM and YTE mutations on the PK profiles of Fc variants in human. TM mutations (L234F/L235E/P331S) in the Fc eliminate mostly its FcγR and C1q bindings,Citation2 whereas YTE mutations (M252Y/S254T/T256E) increase its bindings to FcRn significantly, leading to extended serum half-life of human IgG1.Citation4 These mutations were introduced into the 6F6–3Fc construct, expressed and purified similarly to the regular KP3–3Fc as described above. The dose level was at 2 mg/kg. Blood samples were collected at various time points with the first one at 1 hour post IV injection. The serum concentrations of each Fc variant were determined using a direct binding ELISA assay. Briefly, 2 µg/ml purified MrkA (for KP3 variants) or LPS (for 6F6 variants) in PBS was coated to an ELISA plate. The plate was blocked with PBS + 3% dry milk. Serially diluted mouse sera collected from each time point were applied to the ELISA plate. On the same plate, known concentrations of Fc variants were spiked into mouse serum, serially diluted and used as the standard. Serum concentrations of Fc variants from different time points were calculated against the standard curves using the Prizm program. This ELISA format had a lower limit of quantification of ∼5 ng/ml for KP3 variants and ∼100 ng/ml for 6F6 variants.
Pharmacokinetic data analysis method
A sparse sampling non-compartmental model for a bolus IV administration was used for PK data analysis using Phoenix 64 WinNonlin 6.3 (Pharsight, Mountain View, CA). The maximum observed peak plasma concentration (Cmax) and the time at which it was observed (Tmax) were determined by inspection of the observed data using WinNonlin. The terminal elimination half-life (t½) was determined using the equation ln(2)/λz, where λz is the slope of the terminal portion of the natural-log concentration-time curve, determined by linear regression of at least the last 3 time points. The systemic exposure was determined by calculating the area under the plasma concentration versus time curve (AUClast) from the start of dosing to the time of last measurable concentration using the linear/log trapezoidal rule. Area under the plasma-concentration time curve from time 0 to infinity (AUC∞) was calculated as: AUClast + Clast/λz, where Clast is the last quantifiable concentration. Clearance was calculated by Dose/AUC∞, and steady-state volume of distribution (Vss) was calculated as: (AUMC∞·CL)/AUC∞, where AUMC∞ is the area under the first moment extrapolated to infinity. PK parameters were summarized statistically and presented as means.
In vivo bacterial challenge studies
C57BL/6 mice were received from Jackson laboratories and maintained in a special pathogen-free facility. K. pneumoniae strains were grown on agar plates overnight and diluted in saline at a suitable concentration. The inoculum titer was determined by plating a serial dilutions of bacteria onto agar plates before and post challenge. In acute pneumonia models, C57BL/6 mice were inoculated with appropriate numbers of colony-forming units (CFU) of multi-drug resistant isolates intra-nasally. Antibodies and controls were administered one hour post bacterial infection, mimicking a therapeutic setting. Mouse survival was monitored daily up to day 7 post challenges. Survival data of representative experiments was plotted and analyzed in Prism software.
Mouse serum cytokine level measurement
Mouse ProInflammatory 7-Plex Tissue Culture Kit (#15012B-2, MSD, Rockville, MD) was used to measure the cytokine levels, including IL-10, IL-6, IL-12p70, IFN-γ, IL-1β, KC/GRO, and TNF, in the sera collected from the mice injected with various Fc variants over time, following the protocol suggested by the manufacturer.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
We wish to thank M. Jack Borrok for his advice on PK studies and the Medimmune LAR staff for their assistance with in vivo experiments.
Funding
The study was solely funded by MedImmune.
References
- Sondermann P, Szymkowski DE. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr Opin Immunol 2016; 40: 78-87; PMID:27038127; http://dx.doi.org/10.1016/j.coi.2016.03.005
- Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64: 700-4; PMID:18560159; http://dx.doi.org/10.1107/S0907444908007877
- Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol 2000; 200: 16-26; PMID:10716879; http://dx.doi.org/10.1006/cimm.2000.1617
- Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol 2002; 169: 5171-80; PMID:12391234; http://dx.doi.org/10.4049/jimmunol.169.9.5171
- Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010; 28: 157-9; PMID:20081867; http://dx.doi.org/10.1038/nbt.1601
- Ying T, Gong R, Ju TW, Prabakaran P, Dimitrov DS. Engineered Fc based antibody domains and fragments as novel scaffolds. Biochim Biophys Acta 2014; 1844: 1977-82; PMID:24792384; http://dx.doi.org/10.1016/j.bbapap.2014.04.018
- Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 2012; 4: 1015-28; PMID:22837174; http://dx.doi.org/10.1002/emmm.201201379
- Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol 2007; 25: 1369-72; PMID:18066027; http://dx.doi.org/10.1038/nbt1207-1369
- Brezski RJ, Georgiou G. Immunoglobulin isotype knowledge and application to Fc engineering. Curr Opin Immunol 2016; 40: 62-9; PMID:27003675; http://dx.doi.org/10.1016/j.coi.2016.03.002
- Borrok MJ, Luheshi NM, Beyaz N, Davies GC, Legg JW, Wu H, Dall'Acqua WF, Tsui P. Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcalphaRI (CD89) binding. mAbs 2015; 7: 743-51; PMID:25970007; http://dx.doi.org/10.1080/19420862.2015.1047570
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8: 34-47; PMID:18064051; http://dx.doi.org/10.1038/nri2206
- Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 Axis. Cancer Cell 2015; 28: 285-95; PMID:26373277; http://dx.doi.org/10.1016/j.ccell.2015.08.004
- Marsh CB, Wewers MD, Tan LC, Rovin BH. Fc(gamma) receptor cross-linking induces peripheral blood mononuclear cell monocyte chemoattractant protein-1 expression: role of lymphocyte Fc(gamma)RIII. J Immunol 1997; 158: 1078-84; PMID:9013945
- Nagashima H, Tezuka T, Tsuchida W, Maeda H, Kohroki J, Masuho Y. Tandemly repeated Fc domain augments binding avidities of antibodies for Fcgamma receptors, resulting in enhanced antibody-dependent cellular cytotoxicity. Mol Immunol 2008; 45: 2752-63; PMID:18353438; http://dx.doi.org/10.1016/j.molimm.2008.02.003
- Nagashima H, Ootsubo M, Fukazawa M, Motoi S, Konakahara S, Masuho Y. Enhanced antibody-dependent cellular phagocytosis by chimeric monoclonal antibodies with tandemly repeated Fc domains. J Biosci Bioeng 2011; 111: 391-6; PMID:21215693; http://dx.doi.org/10.1016/j.jbiosc.2010.12.007
- Nagashima H, Kaneko K, Yamanoi A, Motoi S, Konakahara S, Kohroki J, Masuho Y. TNF receptor II fusion protein with tandemly repeated Fc domains. J Biochem 2011; 149: 337-46; PMID:21278157; http://dx.doi.org/10.1093/jb/mvq149
- Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol 2012; 189: 3430-8; PMID:22956577; http://dx.doi.org/10.4049/jimmunol.1200356
- Xiao X, Chen Y, Mugabe S, Gao C, Tkaczyk C, Mazor Y, Pavlik P, Wu H, Dall'Acqua W, Chowdhury PS. A novel dual expression platform for high throughput functional screening of phage libraries in product like format. PloS one 2015; 10: e0140691; PMID:26468955; http://dx.doi.org/10.1371/journal.pone.0140691
- Ionescu RM, Vlasak J, Price C, Kirchmeier M. Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J Pharm Sci 2008; 97: 1414-26; PMID:17721938; http://dx.doi.org/10.1002/jps.21104
- DiGiandomenico A, Warrener P, Hamilton M, Guillard S, Ravn P, Minter R, Camara MM, Venkatraman V, Macgill RS, Lin J, et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J Exp Med 2012; 209: 1273-87; PMID:22734046; http://dx.doi.org/10.1084/jem.20120033
- Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 2014; 514: 642-5; PMID:25119033; http://dx.doi.org/10.1038/nature13612
- Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Del Rev 2013; 65: 1357-69; PMID:23026637; http://dx.doi.org/10.1016/j.addr.2012.09.039
- Dall'Acqua WF, Cook KE, Damschroder MM, Woods RM, Wu H. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J Immunol 2006; 177: 1129-38; PMID:16818770; http://dx.doi.org/10.4049/jimmunol.177.2.1129
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Therap 2008; 84: 548-58; PMID:18784655; http://dx.doi.org/10.1038/clpt.2008.170
- Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 2014; 65: 114-26; PMID:23872058; http://dx.doi.org/10.1016/j.ymeth.2013.06.035
- Borrok MJ, Wu Y, Beyaz N, Yu XQ, Oganesyan V, Dall'Acqua WF, Tsui P. pH-dependent binding engineering reveals an FcRn affinity threshold that governs IgG recycling. J Biol Chem 2015; 290: 4282-90; PMID:25538249; http://dx.doi.org/10.1074/jbc.M114.603712
- Strop P, Delaria K, Foletti D, Witt JM, Hasa-Moreno A, Poulsen K, Casas MG, Dorywalska M, Farias S, Pios A, et al. Site-specific conjugation improves therapeutic index of antibody drug conjugates with high drug loading. Nat Biotechnol 2015; 33: 694-6; PMID:26154005; http://dx.doi.org/10.1038/nbt.3274
- Salas J, Liu T, Lu Q, Kulman JD, Ashworth T, Kistanova E, Moore N, Pierce GF, Jiang H, Peters R. Enhanced Pharmacokinetics of Factor VIIa as a Monomeric Fc Fusion. Thromb Res 2015; 135: 970-6; PMID:25721936; http://dx.doi.org/10.1016/j.thromres.2014.12.018
- Wang Q, Chang CS, Pennini M, Pelletier M, Rajan S, Zha J, Chen Y, Cvitkovic R, Sadowska A, Heidbrink Thompson J, et al. Target-agnostic identification of functional monoclonal antibodies against klebsiella pneumoniae Multimeric MrkA Fimbrial Subunit. J Infect Dis 2016; 213: 1800-8; PMID:26768253; http://dx.doi.org/10.1093/infdis/jiw021
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 2003; 170: 3528-33; PMID:12646614; http://dx.doi.org/10.4049/jimmunol.170.7.3528
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 2003; 197: 315-22; PMID:12566415; http://dx.doi.org/10.1084/jem.20021829