?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Antibody-drug conjugates (ADCs) are promising biotherapeutic agents for the treatment of cancer. The careful monitoring of critical quality attributes is important for ADCs' development, manufacturing and production. In this work, the effect of the presence of a trisulfide bond in the monoclonal antibody (mAb) conjugated to DM4 cytotoxic payload through a disulfide-bond linker sulfo-SPDB (sSPDB) was investigated. Three lots of antibody containing variable levels of trisulfide bonds were used. The identity and levels of trisulfide bonds were determined by liquid chromatography/ mass spectrometry (MS)/MS analysis. The antibodies were conjugated to sSPDB-DM4 to generate ADCs. Further analysis indicated that the drug-to-antibody ratio (DAR) value, a critical quality attribute, slightly increased for the conjugates made from antibody containing higher levels of trisulfide bond. Also, higher fragmentation levels were observed in the conjugates with more trisulfide bond. Detailed characterization by MS revealed that a small amount of DM4 payload was directly attached to inter-chain cysteine residues by disulfide or trisulfide bonds. Overall, our investigation indicated that the trisulfide bond present in the mAb could react with DM4 during the conjugation process. Therefore, the presence of trisulfide bonds in the antibody moiety should be carefully monitored and well controlled during the development of a maytansinoid ADC.
Abbreviations
| mAb | = | monoclonal antibodies |
| ADC | = | Antibody-drug conjugate |
| CQAs | = | critical quality contributes |
| ESI-MS | = | electrospray ionization mass spectrometry |
| DAR | = | drug-to-antibody ratios |
| SMCC | = | succinimidyl trans-4-(maleimidylmethyl) cyclohexane-1-carboxylate |
| sulfo-SPDB | = | N-succinimidyl 4-(2-pyridyldithio)-2-sulfobutanoate |
| DM4 | = | N(2′)-deacetyl-N(2′)-(4-mercapto-4-methyl-1-oxopentyl)-maytansine |
| CE | = | capillary electrophoresis |
Introduction
Antibody-drug conjugates (ADCs) are composed of monoclonal antibodies (mAbs) coupled to potent cytotoxic agents through various linker technologies. These molecules take advantage of the biologic specificity of mAbs and the high potency of chemotherapeutic agents. The results of such combinations are more targeted and efficacious novel biotherapeutic products.Citation1-6 By combining the target-specific capabilities of antibodies with the tumor-killing ability of cytotoxic molecules, ADCs allow sensitive discrimination between healthy and tumor tissue and work as “magic bullets” for targeted cancer therapy.Citation4 So far, 3 ADCs have been approved by the US Food and Drug Administration, and 2 of these are currently marketed: brentuximab vedotinCitation7 (Adcetris®) and ado-trastuzumab emtansineCitation8,9 (T-DM1, Kadcyla®). Many more ADCs are currently under development and in clinical trials, representing a new wave of cancer drug development.
A well-selected linker between the antibody and cytotoxic agent (payload) is a crucial aspect for the success of an ADC.Citation10-14 Appropriate linker technology will ensure the general stability of ADCs during in vivo circulation and efficient drug release once internalized.Citation4,10,15 Linkers are generally categorized into 2 groups: cleavable and non-cleavable. For example, ado-trastuzumab emtansine contains a non-cleavable linker, succinimidyl trans-4-(maleimidylmethyl) cyclohexane-1-carboxylate (SMCC). The maleimide moiety of SMCC links to the free sulfhydryl group of DM1, the cytotoxic agent, through a non-cleavable thioether bond.Citation8 In contrast, brentuximab vedotin contains a peptide linker that can be cleaved by proteases.Citation7,16,17 Other cleavable linkers include acid-labile linkers and thiol chemistry linkers, which form a disulfide bond between linker and cytotoxic agent.Citation4,18 The mechanism of release of the payloads is dependent on linker chemistry. For ado-trastuzumab emtansine, the cytotoxic drug is released after degradation of the ADC in lysosomes,Citation19,20 while for brentuximab vedotin, the cytotoxic drug is released by cathepsin.Citation7,17 Both cleavable and non-cleavable linkers should be stable in the blood stream because in vivo instability of an ADC may affect the off-target toxicity.
Conventionally, conjugation of the linker to the mAb was achieved through reactive amino acid groups such as lysine (e.g., ado-trastuzumab emtansine) or free thiols of cysteines from reduction of inter-chain disulfide bonds (e.g., brentuximab vedotin). In the latter case, the inter-chain cysteine was first reduced by tris(2-carboxyethyl)phosphine (TCEP), and the free cysteine therefore could conjugate to linker to generate the ADC. However, both methods will generate heterogeneous ADCs. Lysine-based conjugation generally results in 0 to 8 or more payloads per molecule, while the conjugation sites can be more than 20 per half antibody.Citation21 Cysteine conjugation, which occurs through reduction of inter-chain disulfide, results in 0, 2, 4, 6, to 8 linker-payloads per molecule, although the number of sites of conjugation is limited to 8. Therefore, the drug-to-antibody ratio (DAR) value is calculated as the average number of payload per antibody molecule. The average DAR value is a critical quality attribute (CQA) for ADCs, and it should be well controlled during the conjugation process and must be consistent between batches.Citation22,23
It is very important to identify and monitor CQAs during the ADC process development, manufacturing and lot release. Disulfide bonds are critical for antibody structures, stability and biologic function. Each subclass of IgG molecules has well-defined homogenous disulfide bond structures; however, free cysteine residues, scrambled disulfide bonds and the presence of trisulfide bonds and thioether bonds have also been widely reported.Citation24-26 More recently, trisulfide bonds have been reported in all 4 subclasses of human IgG molecules.Citation27-31 In a recent study of ADC development using inter-chain cysteine-directed linker chemistry as described above, trisulfide bonds have been reported to affect the reduction step by TCEP during the antibody-drug conjugation process and affect average DAR value.Citation30,32 This occurred because a fraction of TCEP reacted with trisulfide bonds to form a thiophosphine and a disulfide, therefore creating less free thiols available in the antibody for conjugation. This study underscored the importance of monitoring and controlling the trisulfide bonds during the process development of cysteine-linked ADCs.
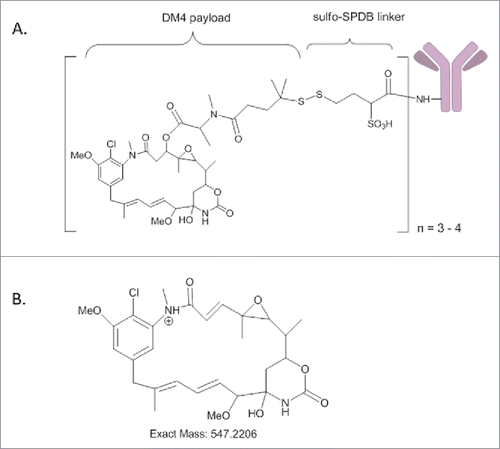
Pharmaceutical companies continue to develop linkers and cytotoxic agents to expand their arsenal of ADCs. Recently, a novel sulfo-SPDB (sSPDB-DM4) conjugation method for ADC manufacturing was developed (). Compared to ado-trastuzumab emtansine, this new technology uses lysine-based conjugation between the linker and antibody while using thiol chemistry (a cleavable disulfide bond instead of a non-cleavable thioether) between the linker (sSPDB) and cytotoxic agent (DM4). One example is recently developed mirvetuximab soravtansine (M9346A-sSPDB-DM4 conjugate), exhibiting potent anti-tumor activity against folate receptor-α-expressing tumors.Citation18
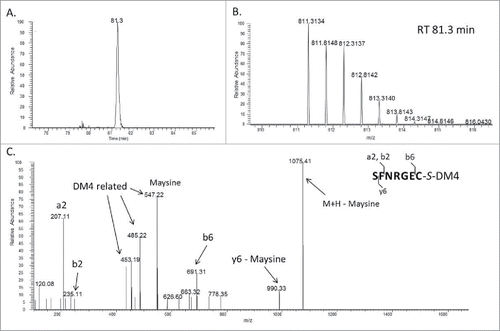
Figure 1. (A) Schematic diagram of antibody linked with DM4 cytotoxic agent through sulfo-SPDB; (B) Structure of the maysine fragment ion derived from DM4.
Considering that thiol chemistry was used in our sSPDB-DM4 linker-drug technology, we investigated whether a high level of trisulfide bonds in an IgG antibody would affect this conjugation process. Trisulfide bonds are formed in Chinese hamster ovary cell-expressed mAbs likely due to the presence of hydrogen sulfide in the cell culture.Citation28 Cell culture process is very critical during the process development of biotherapeutics, and needs to be optimized to develop robust growth conditions that yield desired and consistent product quality. MAbs usually have very low levels of trisulfide bonds, but occasionally can contain high levels of trisulfide bonds as a function of the manufacturing conditions.Citation33
In this study, we investigated the effect of the presence of trisulfide bonds in the antibody on the quality attributes of the ADCs. Antibodies with the same protein sequence but with different levels of trisulfide bonds were generated, and their trisulfide bond levels were determined by peptide mapping. Antibodies with low and high levels of trisulfide bond were conjugated to DM4 through sSPDB linker to generate ADCs, and their quality attributes were monitored using physicochemical methods and bioassays. This work described for the first time the effect of trisulfide bonds present in an antibody on the quality of lysine-conjugated ADCs.
Results
Confirmation of trisulfide bonds by peptide mapping (LC/MS/MS) analysis
Three lots of mAb-A antibody samples were produced using different manufacturing conditions. Lys-C digestion under non-reducing conditions was performed to check the levels of trisulfide bonds (). As shown in the , an extra peak was detected at a retention time (RT) of 14.6 min in the peptide map in lot III of mAb-A (). The molecular mass of this peptide was accurately determined, which was 31.9714 Da higher than the mass of a peptide eluting at RT = 12.8 min (). The calculated mass (1261.5079 Da) of the peptide at RT = 12.8 min matched the theoretical mass of the native inter-chain disulfide bond that links the heavy and light chains (SFNRGEC-SCDK) (), and the mass increase of 31.9714 Da indicated the insertion of a sulfur.
Figure 2. (A) LC-UV (214 nm) peptide mapping profiles of the non-reduced Lys-C digests of mAb-A lots I and III. (B) Zoomed-in LC-UV (214 nm) profiles of Lys-C digested peptide maps lots I and III. Both A) and B) show a new peak detected at RT 14.6 min in the chromatogram of mAb-A lot III. (C) Mass spectra of the LC peaks at RT 12.8 min and 14.6 min, corresponding to the peptides linked by disulfide bond (top) and trisulfide bond (bottom) respectively at the inter-chain locations of the light and heavy chains.
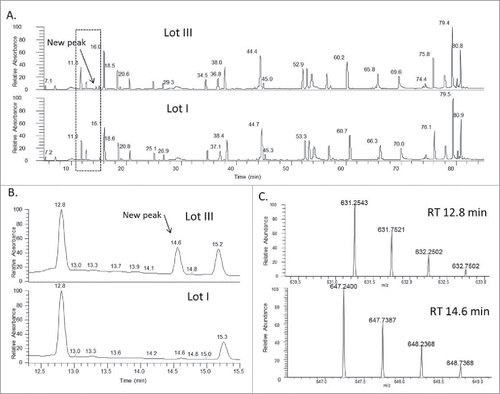
Tandem mass spectrometry (MS/MS) was performed to identify the new peak (). The MS/MS spectrum of the disulfide peptide (RT = 12.8 min) was compared with the tandem mass spectrum of the new peak (RT = 14.6 min). Most fragment ions were present in both spectra, which indicated that the 2 peptides have very similar amino acid sequences. Several fragments in the MS/MS spectrum of the new peptide showed 31.97 Da increase relative to the mass values of the fragment ions in the tandem mass spectrum of the disulfide linked peptide, which could be due to the presence of an extra sulfur atom. For example, m/z 844.31 corresponds to disulfide-linked peptide fragment ion SFNRGEC-SH (i.e., P1+SH in ; S denotes sulfur and S denotes serine), while m/z 876.28 corresponds to trisulfide-linked peptide fragment ion SFNRGEC-SSH (i.e., P1+SSH in ). Trisulfide bonds so far have mainly been reported at the inter-chain locations between the heavy and light chains (i.e., between the 2 Cys in the light-chain peptide SFNRGEC and heavy-chain peptide SCDK), which is consistent with what was observed in our study.Citation28 Overall, the full MS and MS/MS spectra confirmed that the new peak was due to a trisulfide bond in which a sulfur atom was inserted into the inter-chain disulfide linkage in the mAb. The level of trisulfide bonds in 3 antibody lots were further determined to be 1.6%, 18.2%, and 31.7%, respectively, by calculating the ratio of the extracted ion chromatogram (XIC) peak intensity of trisulfide-linked peptide versus the total intensities of both disulfide and trisulfide linked peptides ().
Figure 3. (A) MS/MS spectrum of the inter-chain disulfide bonded peptides. (B) MS/MS spectrum of the inter-chain trisulfide bonded peptides. (Note: P1 and P2 refer to the 2 peptides; Sulfur atom (S) is italic to differentiate from serine (S); Fragment ion “y1/P2” represents y1 of P1 linked with P2 and so on)
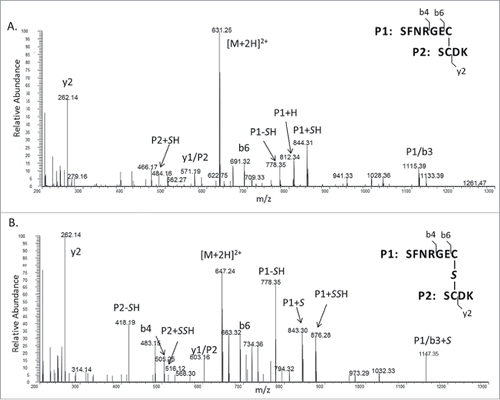
Table 1. Summary of the trisulfide levels and analytical results of selected quality attributes of different lots of mAb-A and their corresponding ADC.
The effect of trisulfide bond on quality attributes of ADCs
The samples from the 3 lots of mAb-A were then conjugated with DM4 using sSPDB linker to generate ADCs to investigate the effect of trisulfide bonds on the conjugation process. UV absorption spectroscopy is a routine method for determining the DAR value by measuring the concentrations of payload and antibody. UV measurements showed that the DAR value increased from 3.3 to 3.7 in the conjugate when the level of trisulfide bond increased from 1.6% to 31.7% in the mAb (). We also checked the level of antibody fragmentation in the conjugate by non-reducing capillary electrophoresis sodium dodecyl sulfate (CE-SDS NR). As shown in , the fragment level increased from 5.6% to 21.4% in the conjugate, while fragment levels increased from 3.3% to 11.5% in the mAb before conjugation. Consequently, the difference of protein fragmentation between antibody and conjugate increased from 2.3% to 9.9% when the trisulfide bond levels increased from 1.6% to 31.7% in the mAb. These results suggested that trisulfide bonds present in the antibody might lead to more protein fragmentation in the conjugate.
The effect of trisulfide bonds on conjugation with DM4
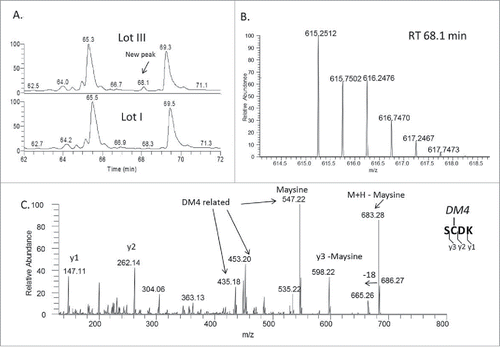
To explore the reason for the increased DAR and protein fragmentation, conjugates made from the 3 lots of mAb-A were digested using a non-reducing Lys-C peptide mapping method. When results of conjugates derived from low to high levels of trisulfide bonds in the antibody were compared, the UV chromatograms (214 nm) of their Lys-C digested peptides were very similar (); however, an extra peak was detected at a retention time of 68.1 min in the peptide map of the lot III conjugate, which had a high level of trisulfide bond in the antibody (). The mass of the peptide matched the theoretical mass of a heavy chain peptide SCDK linked to DM4 through a disulfide bond (S[C-DM4]DK) (). Several fragments in the MS/MS spectrum confirmed the presence of DM4 and the peptide sequence. For example, m/z 547.22 is the signature fragment ion of DM4 that corresponds to the structure of maysine (). This fragment ion was generated by cleavage of the ester bond in DM4, as shown in the published results.Citation18,34 The other fragment ions, such as m/z 262.14 corresponding to the y2 fragment, originated from the peptide. This observation indicated that DM4 was directly conjugated to the side chain of cysteine. We further searched for conjugated peptide that was linked to DM4 via trisulfide. An additional peak in the extracted ion chromatogram (XIC) was detected at a retention time of 81.3 min (). The mass of the peptide matched the theoretical mass of a light chain peptide conjugated with DM4 through an extra sulfur (SFNRGEC-S-DM4) (). Several fragment ions in the MS/MS spectrum of the new peptide confirmed the peptide sequence and presence of DM4 (). For example, m/z 453.19, 485.22 and 547.22 are all fragment ions of DM4, and m/z 691.31 is the b6 fragment ion of the peptide. Overall, these results confirmed the detection of peptides with inter-chain cysteine residues directly linked with DM4 by both disulfide and trisulfide bonds.
Figure 4. LC-UV (214 nm) peptide mapping profiles of the non-reduced Lys-C digests of ADCs manufactured using lots I and III mAb-A.
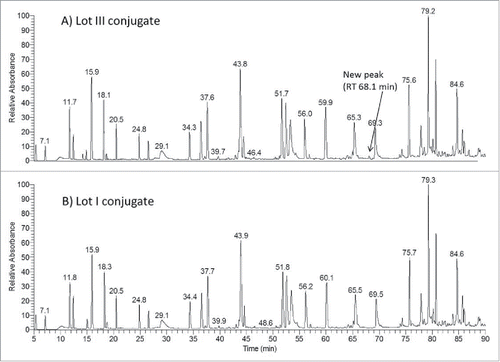
Figure 5. (A) Zoomed-in LC-UV (214 nm) profile of Lys-C digested lot III ADC showing a new peak at 68.1 min. (B) Mass spectrum of the peak at 68.1 min that corresponds to DM4 connected directly (without a linker) through a disulfide bond to the side chain of cysteine in the peptide SCDK; (C) MS/MS spectrum of the SCDK peptide disulfide-linked with DM4.
Discussion
Biopharmaceutical companies are increasingly interested in the development of ADCs for novel cancer therapies. By February 2016, more than 50 ADCsCitation35,36 were in clinical studies, and ∼75% of ADCs in development used a maytansinoid or an auristatin type of payload.Citation36 CQAs of ADCs should be carefully monitored and well controlled during ADC development. Due to the increased complexity of ADCs compared with unconjugated antibodies, orthogonal analytical approaches are required for full characterization of ADCs. State-of-the-art MS-based approaches, including top-down, middle-down and bottom-up, combined with the power of chromatographic or electrophoretic techniques, have been successfully used for identification of CQAs such as drug distribution, DAR value, site of conjugation and chemical liability in ADCs.Citation37,38 Here, similar analytical methods were used to evaluate the CQAs of a lysine-conjugated ADC.
Trisulfide bonds can be present in recombinant mAbs. In this work, we used 3 lots of a mAb that contained different levels of trisulfide bonds and investigated the effect of trisulfide bonds on the structure of ADCs. When these antibodies were conjugated using our thiol chemistry-based sSPDB-DM4 linker and payload technology, it was found that the trisulfide bond could be a soft spot for conjugation, resulting in DM4 linked directly by disulfide and trisulfide bonds with cysteine at inter-chain locations (i.e., between heavy and light chain).
The DAR value was measured by UV absorption spectrometry and found to increase from 3.3 to 3.7 when the antibody contained high levels of trisulfide bond. The antibody fragment levels were also measured by CE-SDS NR, and it was found that the fragment levels were also significantly higher for conjugate made from the antibody lot with a high level of trisulfide. The observation of DM4 conjugated directly to inter-chain cysteine residues by both disulfide and trisulfide bonds explained the increased DAR values and levels of protein fragmentation. Since the DAR measurement is dependent on the drug load, the covalent linkage between disulfide bond-DM4 and trisulfide bond-DM4 increased the drug load in the antibody, and thus increased the DAR value. The increased protein fragmentation was likely due to the observed dissociation of the trisulfide bond during conjugation. For example, the level of trisulfide bonds in the lot III antibody decreased from 31.7% (before conjugation) to 18.5% (after conjugation), which indicated partial dissociation of the trisulfide bond during the conjugation process (). These results suggest that the trisulfide bond was not stable during the conjugation process due to the interaction with DM4.
In conclusion, our results, presented here for the first time for a lysine-conjugated ADC, clearly indicated that a high level of trisulfide bond in the mAb could have significant effects on the ADC conjugation process when using thiol chemistry.
Materials and methods
Materials
The 3 lots of IgG1 mAb-A and the ADCs were produced in-house at ImmunoGen. Lysyl Endopeptidase (Lys-C) was purchased from Wako Pure Chemical Industries, Ltd (125–02543). No-weigh format dithiothreitol (DTT) (LC144431) and trifluoroacetic acid (TFA) (A116_10×1mL) were purchased from Thermo Scientific. Guanidine hydrochloride (GuHCl) (50937), N-ethylmaleimide (NEM) (04259), 1M Tris hydrochloride solution stock (pH 8.0, T2694–1L) LC-MS grade water (39253) and acetonitrile (34967) were all purchased from Sigma-Aldrich.
Enzymatic digestion
For Lys-C digestion, an aliquot of 250 μL of mAb or ADC solution (∼500 μg) was first incubated with 10 mM NEM to cap free thiols of cysteine residues then denatured with 6 M GuHCl, 50 mM phosphate buffer (pH 6.5) for 3 hours at 37°C. The samples were then diluted with 50 mM Tris (pH 7.0) to 2 M GuHCl, and then Lys-C (1:25, w/w) was added to the protein solution and incubated for 16 hours at 37°C. Half of each sample was reduced by 20 mM DTT to confirm the elution positions of the peptides containing disulfide and trisulfide bonds.
LC-MS and LC-MS/MS for peptide mapping analysis
The LC-MS analysis was performed using a Xevo G2 XS QTOF mass spectrometer (Waters) and LC-MS/MS was performed with a Q-Exactive orbitrap mass spectrometer (Thermo Fisher Scientific) (both MS instruments were coupled with UHPLC). A CSH C18 UPLC column (2.1 × 100 mm, Waters) was used for peptide separation. An elution gradient with mobile phase A (0.05% TFA in 80:20 acetonitrile: water) and mobile phase B (0.05% TFA in 80:20 acetonitrile: water) was generated using the following program: 1) isocratic for 2 min at 0% B; 2) linear gradient from 0 to 20% B for 25 min; 3) linear gradient from 20 to 32% B for 43 min; 4) linear gradient from 32 to 50% B for 20 min; 5) linear gradient from 50 to 100% B for 1 min; and finally 6) isocratic at 100% B for 3 min. The flow rate of the column was maintained at 0.25 mL/min and column temperature was maintained at 40°C. For LC-MS/MS analysis, the Q-Exactive mass spectrometer was operated in data-dependent mode as follows: survey full-scan MS spectra (m/z 220–2000) were acquired with a mass resolution of 70,000 at m/z 400, followed by sequential MSCitation2 scans of top 10 ions, with a mass resolution of 17,500 at m/z 400.
Trisulfide and conjugation sites identification
Peptides or conjugates of interest were identified manually by searching their m/z values within the experimental full mass spectrum using either Masslynx or Xcalibur software packages. MS/MS data were analyzed manually using Xcalibur software for mass detection and data interpretation. Relative amounts of trisulfide modifications were calculated by manual integration of modified and unmodified peptide XIC peaks.
Concentration and DAR determination of ADCs by UV/VIS Spectrophotometry
The methodology relies on the principle of Beer's Law in which the absorbance of a solution of an analyte is directly proportional to its concentration. DM4 and the antibody have overlapping absorbance spectra in the UV wavelength range with different absorption profiles. The absorption of the ADC solution was measured at 2 wavelengths (252 and 280 nm), and, by using a system of 2 equations based on Beer's Law (Eqs. Equation1(1)
(1) and Equation2
(2)
(2) ), the molar concentrations of DM4 and of the antibody were determined. The DAR was calculated by dividing the molar concentration of DM4 to that of the antibody. Samples were diluted to 0.7 mg/mL with the formulation buffer in duplicate. The absorptions of the samples were measured using a 1 cm quartz cuvette in a Cary 50 UV-Vis spectrophotometer (Varian).
(1)
(1)
(2)
(2) where, A280 and A252 are the total UV absorbance measured at 280 nm and 252 nm, respectively; ϵAb,280 and ϵD,280 are the extinction coefficients of the mAb and DM4, respectively, at 280 nm; ϵAb,252 and ϵD,252 are the extinction coefficients of the mAb and DM4, respectively, at 252 nm; and CAb and CD are the molar concentrations of the mAb and DM4, respectively.
Non-reducing capillary electrophoresis for protein fragmentation
CE-SDS was performed using an AB Sciex PA800 instrument with a PDA detector. Non-reduced samples were alkylated by adding 5 µL of 40 mM iodoacetamide (Pierce Biotechnology) to 20 µL of sample and 75 µL of 50 mM MES, 1% SDS sample denaturing buffer, which brought the final concentration to 1 mg/mL. The samples were heated at 70°C for 5 min. The samples were prepared with a 10 kDa internal standard (AB Sciex). A 50-cm I.D. bare fused-silica capillary trimmed to 30.2 cm was used for electrophoresis for a 50-minute analysis at 11 kV and data was analyzed using Empower software package (Waters).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol 2005; 5:543-9; PMID:16087399; http://dx.doi.org/10.1016/j.coph.2005.04.017
- Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 2005; 23:1137-46; PMID:16151407; http://dx.doi.org/10.1038/nbt1141
- Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res 2008; 41:98-107; PMID:17705444; http://dx.doi.org/10.1021/ar700108g
- Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs 2014; 6:34-45; PMID:24423619; http://dx.doi.org/10.4161/mabs.27022
- Gerber HP, Senter PD, Grewal IS. Antibody drug-conjugates targeting the tumor vasculature: Current and future developments. MAbs 2009; 1:247-53; PMID:20069754; http://dx.doi.org/10.4161/mabs.1.3.8515
- Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol 2009; 13:235-44; PMID:19414278; http://dx.doi.org/10.1016/j.cbpa.2009.03.023
- Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35). Clin Cancer Res 2011; 17:6428-36; PMID:22003070; http://dx.doi.org/10.1158/1078-0432.CCR-11-0488
- LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res 2011; 17:6437-47; PMID:22003071; http://dx.doi.org/10.1158/1078-0432.CCR-11-0762
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008; 68:9280-90; PMID:19010901; http://dx.doi.org/10.1158/0008-5472.CAN-08-1776
- Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J 2008; 14:154-69; PMID:18536555; http://dx.doi.org/10.1097/PPO.0b013e318172d704
- Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, Senter PD. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem 2008; 19:1960-3; PMID:18803412; http://dx.doi.org/10.1021/bc800289a
- Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S, de Sauvage FJ, Eaton D, Elkins K, Elliott JM, et al. Antibody-drug conjugates for the treatment of non-Hodgkin's lymphoma: target and linker-drug selection. Cancer Res 2009; 69:2358-64; PMID:19258515; http://dx.doi.org/10.1158/0008-5472.CAN-08-2250
- Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem 2010; 21:5-13; PMID:19769391; http://dx.doi.org/10.1021/bc9002019
- Zhao RY, Wilhelm SD, Audette C, Jones G, Leece BA, Lazar AC, Goldmacher VS, Singh R, Kovtun Y, Widdison WC, et al. Synthesis and evaluation of hydrophilic linkers for antibody-maytansinoid conjugates. J Med Chem 2011; 54:3606-23; PMID:21517041; http://dx.doi.org/10.1021/jm2002958
- Ravandi F. Gemtuzumab ozogamicin: one size does not fit all–the case for personalized therapy. J Clin Oncol 2011; 29:349-51; PMID:21172885; http://dx.doi.org/10.1200/JCO.2010.32.2693
- Gualberto A. Brentuximab Vedotin (SGN-35), an antibody-drug conjugate for the treatment of CD30-positive malignancies. Expert Opin Investig Drugs 2012; 21:205-16; PMID:22127011; http://dx.doi.org/10.1517/13543784.2011.641532
- Bradley AM, Devine M, DeRemer D. Brentuximab vedotin: an anti-CD30 antibody-drug conjugate. Am J Health Syst Pharm 2013; 70:589-97; PMID:23515511; http://dx.doi.org/10.2146/ajhp110608
- Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D, LaBelle A, Payne G, Lutz RJ, Pinkas J, et al. IMGN853, a Folate Receptor-alpha (FRalpha)-Targeting Antibody-Drug Conjugate, Exhibits Potent Targeted Antitumor Activity against FRalpha-Expressing Tumors. Mol Cancer Ther 2015; 14:1605-13; PMID:25904506; http://dx.doi.org/10.1158/1535-7163.MCT-14-1095
- Barginear MF, John V, Budman DR. Trastuzumab-DM1: a clinical update of the novel antibody-drug conjugate for HER2-overexpressing breast cancer. Mol Med 2012; 18:1473-9; PMID:23196784; http://dx.doi.org/10.2119/molmed.2012.00302
- Corrigan PA, Cicci TA, Auten JJ, Lowe DK. Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate. Ann Pharmacother 2014; 48:1484-93; PMID:25082874; http://dx.doi.org/10.1177/1060028014545354
- Wang L, Amphlett G, Blattler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci 2005; 14:2436-46; PMID:16081651; http://dx.doi.org/10.1110/ps.051478705
- Goldmacher VS, Amphlett G, Wang L, Lazar AC. Statistics of the distribution of the abundance of molecules with various drug loads in maytansinoid antibody-drug conjugates. Mol Pharm 2015; 12:1738-44; PMID:25635630; http://dx.doi.org/10.1021/mp5007536
- Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 2004; 10:7063-70; PMID:15501986; http://dx.doi.org/10.1158/1078-0432.CCR-04-0789
- Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J. Heterogeneity of monoclonal antibodies. J Pharm Sci 2008; 97:2426-47; PMID:17828757; http://dx.doi.org/10.1002/jps.21180
- Zhang Z, Pan H, Chen X. Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom Rev 2009; 28:147-76; PMID:18720354; http://dx.doi.org/10.1002/mas.20190
- Banks DD, Gadgil HS, Pipes GD, Bondarenko PV, Hobbs V, Scavezze JL, Kim J, Jiang XR, Mukku V, Dillon TM. Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm Sci 2008; 97:775-90; PMID:17786988; http://dx.doi.org/10.1002/jps.21014
- Pristatsky P, Cohen SL, Krantz D, Acevedo J, Ionescu R, Vlasak J. Evidence for trisulfide bonds in a recombinant variant of a human IgG2 monoclonal antibody. Anal Chem 2009; 81:6148-55; PMID:19591437; http://dx.doi.org/10.1021/ac9006254
- Gu S, Wen D, Weinreb PH, Sun Y, Zhang L, Foley SF, Kshirsagar R, Evans D, Mi S, Meier W, et al. Characterization of trisulfide modification in antibodies. Anal Biochem 2010; 400:89-98; PMID:20085742; http://dx.doi.org/10.1016/j.ab.2010.01.019
- Nielsen RW, Tachibana C, Hansen NE, Winther JR. Trisulfides in proteins. Antioxid Redox Signal 2011; 15:67-75; PMID:20977350; http://dx.doi.org/10.1089/ars.2010.3677
- Cumnock K, Tully T, Cornell C, Hutchinson M, Gorrell J, Skidmore K, Chen Y, Jacobson F. Trisulfide modification impacts the reduction step in antibody-drug conjugation process. Bioconjug Chem 2013; 24:1154-60; PMID:23713462; http://dx.doi.org/10.1021/bc4000299
- Kita A, Ponniah G, Nowak C, Liu H. Characterization of Cysteinylation and Trisulfide Bonds in a Recombinant Monoclonal Antibody. Anal Chem 2016; 88:5430-7; PMID:27115984; http://dx.doi.org/10.1021/acs.analchem.6b00822
- Cornell C, Karanjit A, Chen Y, Jacobson F. A high-throughput hydrophilic interaction liquid chromatography coupled with a charged aerosol detector method to assess trisulfides in IgG1 monoclonal antibodies using tris(2-carboxyethyl)phosphine reaction products: Tris(2-carboxyethyl)phosphine-oxide and tris(2-carboxyethyl)phosphine-sulfide. J Chromatogr A 2016; 1457:107-15; PMID:27345209; http://dx.doi.org/10.1016/j.chroma.2016.06.037
- Kshirsagar R, McElearney K, Gilbert A, Sinacore M, Ryll T. Controlling trisulfide modification in recombinant monoclonal antibody produced in fed-batch cell culture. Biotechnol Bioeng 2012; 109:2523-32; PMID:22473825; http://dx.doi.org/10.1002/bit.24511
- Luo Q, Chung HH, Borths C, Janson M, Wen J, Joubert MK, Wypych J. Structural characterization of a monoclonal antibody-maytansinoid immunoconjugate. Anal Chem 2016; 88:695-702; PMID:26629796; http://dx.doi.org/10.1021/acs.analchem.5b03709
- Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol 2016; 17:e254-62; PMID:27299281; http://dx.doi.org/10.1016/S1470-2045(16)30030-4
- Lambert JM. Antibody-drug conjugates: targeted delivery and future prospects. Ther Deliv 2016; 7:279-82; PMID:27075948; http://dx.doi.org/10.4155/tde-2016-0010
- Huang RY, Chen G. Characterization of antibody-drug conjugates by mass spectrometry: advances and future trends. Drug Discov Today 2016; 21:850-5; PMID:27080148; http://dx.doi.org/10.1016/j.drudis.2016.04.004
- Beck A, Terral G, Debaene F, Wagner-Rousset E, Marcoux J, Janin-Bussat MC, Colas O, Van Dorsselaer A, Cianférani S. Cutting-edge mass spectrometry methods for the multi-level structural characterization of antibody-drug conjugates. Expert Rev Proteomics 2016; 13:157-83; PMID:26653789; http://dx.doi.org/10.1586/14789450.2016.1132167