Abstract
Widely thought to be a housekeeping process, the regulation and synthesis of rRNA emerges as a potentially central mechanism for the maintenance of synaptic plasticity and memory. We have recently shown that an essential component of late-phase synaptic plasticity is rRNA biosynthesis — the rate-limiting step in the production of new ribosomes. We hypothesize that a particular population of ribosomes is generated upon learning-associated neural activity to alter the rate of synthesis of plasticity factors at tagged synapses that will support the maintenance of synaptic plasticity and memory.
In 1950, Katz and Halstead first proposed that memory formation required new protein synthesisCitation1 —a hypothesis that was not tested until decades later.Citation2-4 It is now well accepted that for memory to become consolidated, new transcription must accompany new, activity-dependent protein synthesis.Citation5,6
Persistent experience-evoked changes in synaptic efficacy are widely believed to form the basis of learning and memory (reviewed by).Citation7 Long-term potentiation (LTP) is a persistent form of synaptic plasticity used to investigate the physiological basis of long-term memory (LTM) at the synaptic and cellular level. Like memory, LTP can be divided into a transient translation-independent phase and an enduring late phase (L-LTP) that requires new transcription and protein synthesis.Citation7,8 Because of the crucial relevance of new transcription and protein synthesis for the transition between transient to consolidated memory, most efforts to understand experience-induced changes in neuronal gene expression have focused on the regulation and synthesis of RNA polymerase II transcripts, that is, precursor mRNA, snRNA and microRNA and their protein products.Citation6,9
In a recent article we reported findings that provide new insight into the molecular mechanism of long-term synaptic plasticity. We demonstrated for the first time that nucleolar integrity—and specifically, new ribosomal RNA (rRNA) synthesis is required for the maintenance of LTP.Citation10 rRNAs are the transcription products of RNA polymerase I (Pol I). Widely thought to be a housekeeping process, the regulation and synthesis of rRNA in learning and memory has remained largely unexplored until now when it emerges as a potentially central mechanism for the maintenance of synaptic plasticity.
Hypothesis
The rRNAs are essential components of ribosomes.Citation11 The requirement of Pol I-dependent transcription during LTP suggests that during long-term synaptic plasticity pre-existing rRNAs, in pre-existing ribosomes, are not sufficient to sustain LTP expression. Our overarching hypothesis is based on a speculative model where Pol I-dependent gene expression is selectively regulated to produce new rRNA; hence, new ribosomes, to carry out the protein synthesis required to support long-term synaptic plasticity at learning-activated (“tagged”) synapses (). To test our hypothesis we are addressing the following questions: 1) How does synaptic plasticity regulate the formation of new ribosomes? 2) Are these plasticity-induced new ribosomes functionally different from other ribosomes? 3) How do these new, and perhaps distinct, ribosomes support the maintenance of synaptic plasticity and memory? And 4) do all forms of plasticity and learning and memory require new ribosomes? The latter question becomes particularly relevant in light of a recent article in which Pol I transcription was disrupted in mouse hippocampal neurons by the conditional knockout of the nucleolar transcription factor TIF-IA.Citation12 TIF-1A is required for Pol I directed rRNA transcription. In characterizing the effect of Pol I disruption 1 month or more after tamoxifen induced TIF-1A ablation, the authors observed impairment in tetanic induced LTP (early and L-LTP), but no changes in LTM as measured by performance in the Morris Water Maze (a hippocampus dependent spatial learning task). However, at different times after ablation the animals exhibited variable changes in spatial learning and re-learning skills, an apparent upregulation of the mTOR pathway, and increased neurogenesis in the Dentate Gyrus suggesting a robust activation of neuroprotective compensatory mechanisms as a result of the hippocampal TIF-1A ablation.Citation12 An interesting question is whether the spatial learning tested in this study (Morris Water Maze) would be affected by acute disruption of Pol I activity.
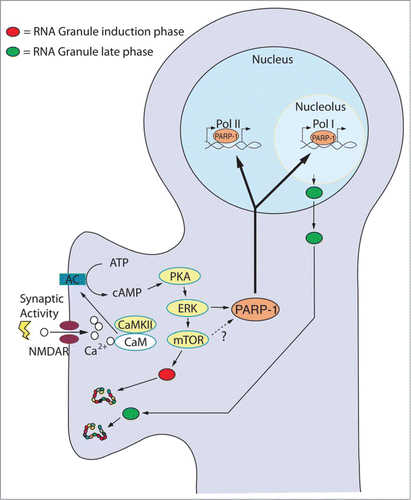
Figure 1. Hypothetical Model for the Transduction of Synaptic Stimuli to Long-Term Plasticity. Synaptic stimulation triggers adenylate cyclase (AC) resulting in the rapid release of cAMP and the activation of the cAMP-PKA-ERK pathway. Stimuli leading to long-term plasticity activate mTOR-dependent translation of preexisting RNA granules (red). Simultaneously, the PKA-ERK pathway induces the synthesis and activation of chromatin remodeling factors (e.g. PARP-1) that opens the chromatin allowing plasticity-dependent transcription to take place. Crucial among the new transcripts are precursor rRNAs required for the formation of new ribosomes. We hypothesize that new and qualitatively different ribosomes are assembled into new RNA granules (green) and shipped to activated synapses to maintain, through local protein synthesis, the long-lasting changes required for long-term synaptic plasticity and memory.
Ribosome Diversity
In 2002, Mauro and Edelman proposed the “ribosome filter” hypothesis introducing the idea that differential binding of mRNAs to the ribosomal subunits may affect the efficiency of translation.Citation12 Ribosomal subunits would act as regulatory elements that mediate interaction between particular mRNAs and components of the translational machinery.Citation13,14 This notion suggests that ribosomes may not simply be the homogeneous indiscriminant arbiters of translation as traditionally assumed, but might exhibit sufficient heterogeneity to play a regulatory role in translation. Sources of ribosome heterogeneity include: 1) ribosomal protein composition (paralogues), 2) post-translational modification of ribosomal proteins and ribosome-associated factors, 3) post-transcriptional modification of rRNA, and 4) rRNA gene (rDNA) sequence variants.Citation15-17
In eukaryotes, rDNA exist as multiple tandem repeats totaling, in some cases, hundreds of copies. Each transcription unit produces a 45S precursor rRNA that contains highly conserved coding regions as well as variable ones. Length and sequence heterogeneity in the non-coding and coding regions of rDNA allows for the possibility of functional rRNA variants (v-rRNAs) as have been described for miceCitation16 and humans.Citation17 Therefore, it seems possible that rDNA variants might provide the structural and/ or catalytic basis for specialized ribosomes and ribosomal diversity during plasticity and memory.
Recently, the existence of physiologically relevant v-rRNAs has been confirmed in organisms ranging from Arabidopsis thaliana to Homo sapiens.Citation17,18 For example, in Arabidopsis, 4 v-rRNAs were identified that differed in their expression according to tissue type and stage of development.Citation18 In mice, 7 v-rRNAs were cloned and characterized as being differentially expressed.Citation16 As in the Arabidopsis study, the 7 v-RNAs were found to be transcriptionally regulated in a manner corresponding to differences in DNA methylation sites. Interestingly, the epigenetic regulator poly(ADP-ribose) polymerase-1 (PARP-1) has been shown to regulate DNA methylation patterns (reviewed by),Citation19 chromatin availability and transcriptional activation in response to environmental cues (reviewed by),Citation20 and ribosome biogenesis.Citation21
Many studies have noted an increase in RNA synthesis, including rDNA gene expression, in correlation with neural plasticity and learning and memory models (See for example,).Citation14,22-25 In our recent article, we show for the first time that an essential component of late-phase, activity-dependent gene expression is rRNA biosynthesis — the rate-limiting step in the production of new ribosomes.Citation10 The requirement for de novo rRNA synthesis provides a new insight into the mechanism of long-term synaptic plasticity and suggests that ribosomal quantity and /or quality regulates the maintenance of long-term synaptic plasticity. Ribosomal biogenesis requires both Pol I driven transcription and the efficient processing of nascent rRNA transcripts — 2 processes that have been shown to be regulated by PARP-1.Citation21,24 Our finding that plasticity-induced Pol I activity depends upon PARP adds to the evidence for a key role of this epigenetic regulator in long-term synaptic plasticity and memory.Citation10,24,26–28
Klann and SweattCitation29 have proposed that a self-perpetuating positive feedback mechanism maintains an altered pattern of local translation that is required for the formation and maintenance of a memory engram. In agreement with this model, we propose that a particular population of ribosomes is recruited at or nearby selected (tagged) synapses to alter the rate of synthesis of plasticity factors that will support the maintenance of synaptic plasticity and memory. While there is good evidence supporting ribosomal diversity, our goal is to determine whether functional ribosome diversity is a cellular strategy important for the maintenance of synaptic plasticity and memory.
Nucleolar Integrity and Neurodegenerative Disorders
An important hallmark of neurodegenerative diseases is the occurrence of aberrations in the epigenetic code of acetylation, methylation and PARylationCitation30 (reviewed by).Citation31,32 Nucleolar impairment may be a common denominator in several neurodegenerative disorders such as Huntington's, Parkinson's and Alzheimer's disease (reviewed by).Citation33 Our data demonstrate that nucleolar integrity is necessary for long-term synaptic plasticity and strengthens the connection between the structure and function of the nucleolar complex. We suggest that the impairment of memory and cognition occurring in the above-mentioned neurodegenerative disorders manifest through nucleolar function deficits and aberrant nucleolar DNA methylation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Katz J, Halstead WC. Protein organization and mental function. Comp Psychol Monographs 1950; 20:1-38
- Flexner JB, Flexner LB, Stellar E. Memory in mice as affected by intracerebral puromycin. ScienSce 1963; 141:57-9; PMID:13945541; http://dx.doi.org/10.1126/science.141.3575.57
- Castellucci VF, Frost WN, Goelet P, Montarolo PG, Schacher S, Morgan JA, Blumenfeld H, Kandel ER. Cell and molecular analysis of long-term sensitization in Aplysia. J Physiol 1986; 81:349-57; PMID:3572827
- Schacher S, Castellucci VF, Kandel ER. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science 1988; 240:1667-9; PMID:2454509; http://dx.doi.org/10.1126/science.2454509
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 1996; 273:1402-6; PMID:8703078; http://dx.doi.org/10.1126/science.273.5280.1402
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 2009; 89:121-45; PMID:19126756; http://dx.doi.org/10.1152/physrev.00017.2008
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science 2001; 294:1030-8; PMID:11691980; http://dx.doi.org/10.1126/science.1067020
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 2004; 44:5-21; PMID:15450156; http://dx.doi.org/10.1016/j.neuron.2004.09.012
- West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol 2011; 3; PMID:21555405; http://dx.doi.org/10.1101/cshperspect.a005744
- Allen KD, Gourov AV, Harte C, Gao P, Lee C, Sylvain D, Splett JM, Oxberry WC, van de Nes PS, Troy-Regier MJ, et al. Nucleolar integrity is required for the maintenance of long-term synaptic plasticity. PLoS One 2014; 9:e104364; PMID:25089620; http://dx.doi.org/10.1371/journal.pone.0104364
- Welch M, Majerfeld I, Yarus M. 23S rRNA similarity from selection for peptidyl transferase mimicry. Biochemistry 1997; 36:6614-23; PMID:9184141; http://dx.doi.org/10.1021/bi963135j
- Kiryk A, Sowodniok K, Kreiner G, Rodriguez-Parkitna J, Sonmez A, Gorkiewicz T, Bierhoff H, Wawrzyniak M, Janusz AK, Liss B, et al. Impaired rRNA synthesis triggers homeostatic responses in hippocampal neurons. Front Cell Neurosci 2013; 7:207; PMID:24273493; http://dx.doi.org/10.3389/fncel.2013.00207
- Mauro VP, Edelman GM. The ribosome filter hypothesis. Proc Natl Acad Sci U S A 2002; 99:12031-6; PMID:12221294; http://dx.doi.org/10.1073/pnas.192442499
- Zemp JW, Wilson JE, Schlesinger K, Boggan WO, Glassman E. Brain function and macromolecules. I. Incorporation of uridine into RNA of mouse brain during short-term training experience. Proc Natl Acad Sci U S A 1966; 55:1423-31; PMID:5227661; http://dx.doi.org/10.1073/pnas.55.6.1423
- Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol 2012; 13:355-69; PMID:22617470; http://dx.doi.org/10.1038/nrm3359
- Tseng H, Chou W, Wang J, Zhang X, Zhang S, Schultz RM. Mouse ribosomal RNA genes contain multiple differentially regulated variants. PLoS One 2008; 3:e1843; PMID:18365001; http://dx.doi.org/10.1371/journal.pone.0001843
- Kuo BA, Gonzalez IL, Gillespie DA, Sylvester JE. Human ribosomal RNA variants from a single individual and their expression in different tissues. Nucleic Acids Res 1996; 24:4817-24; PMID:8972871; http://dx.doi.org/10.1093/nar/24.23.4817
- Pontvianne F, Abou-Ellail M, Douet J, Comella P, Matia I, Chandrasekhara C, Debures A, Blevins T, Cooke R, Medina FJ, et al. Nucleolin is required for DNA methylation state and the expression of rRNA gene variants in Arabidopsis thaliana. PLoS Genet 2010; 6:e1001225; PMID:21124873; http://dx.doi.org/10.1371/journal.pgen.1001225
- Caiafa P, Guastafierro T, Zampieri M. Epigenetics: poly(ADP-ribosyl)ation of PARP-1 regulates genomic methylation patterns. FASEB J 2009; 23:672-8; PMID:19001527; http://dx.doi.org/10.1096/fj.08–123265
- Ji Y, Tulin AV. The roles of PARP1 in gene control and cell differentiation. Curr Opin Genet Devel 2010; 20:512-8; PMID:20591646; http://dx.doi.org/10.1016/j.gde.2010.06.001
- Boamah EK, Kotova E, Garabedian M, Jarnik M, Tulin AV. Poly(ADP-Ribose) polymerase 1 (PARP-1) regulates ribosomal biogenesis in Drosophila nucleoli. PLoS Genet 2012; 8:e1002442; PMID:22242017; http://dx.doi.org/10.1371/journal.pgen.1002442
- Rother S, Schmidt R, Brysch W, Schlingensiepen KH. Learning-induced expression of meningeal ependymin mRNA and demonstration of ependymin in neurons and glial cells. J Neurochem 1995; 65:1456-64; PMID:7561838; http://dx.doi.org/10.1046/j.1471–4159.1995.65041456.x
- Vargas JP, Rodr inverted question markiguez F, Liqm JC, Arias JL, Salas C. Spatial learning-induced increase in the argyrophilic nucleolar organizer region of dorsolateral telencephalic neurons in goldfish. Brain Res 2000; 865:77-84; PMID:10814734; http://dx.doi.org/10.1016/S0006–8993(00)02220–4
- Hernandez AI, Wolk J, Hu JY, Liu J, Kurosu T, Schwartz JH, Schacher S. Poly-(ADP-ribose) polymerase-1 is necessary for long-term facilitation in Aplysia. J Neurosci 2009; 29:9553-62; PMID:19641118; http://dx.doi.org/10.1523/JNEUROSCI.1512–09.2009
- Jordan BA, Fernholz BD, Khatri L, Ziff EB. Activity-dependent AIDA-1 nuclear signaling regulates nucleolar numbers and protein synthesis in neurons. Nat Neurosci 2007; 10:427-35; PMID:17334360
- Cohen-Armon M, Visochek L, Katzoff A, Levitan D, Susswein AJ, Klein R, Valbrun M, Schwartz JH. Long-term memory requires polyADP-ribosylation. Science 2004; 304:1820-2; PMID:15205535; http://dx.doi.org/10.1126/science.1096775
- Goldberg S, Visochek L, Giladi E, Gozes I, Cohen-Armon M. PolyADP-ribosylation is required for long-term memory formation in mammals. J Neurochem 2009; 111:72-9; PMID:19645746; http://dx.doi.org/10.1111/j.1471–4159.2009.06296.x
- Fontan-Lozano A, Suarez-Pereira I, Horrillo A, del-Pozo-Martin Y, Hmadcha A, Carrion AM. Histone H1 poly[ADP]-ribosylation regulates the chromatin alterations required for learning consolidation. J Neurosci 2010; 30:13305-13; PMID:20926656; http://dx.doi.org/10.1523/JNEUROSCI.3010–10.2010
- Klann E, Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem 2008; 89:247-59; PMID:17919940; http://dx.doi.org/10.1016/j.nlm.2007.08.009
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 1997; 88:615-26; PMID:9054501; http://dx.doi.org/10.1016/S0092–8674(00)81904–2
- Lee J, Ryu H. Epigenetic modification is linked to Alzheimer's disease: is it a maker or a marker? BMB Rep 2010; 43:649-55; PMID:21034526; http://dx.doi.org/10.5483/BMBRep.2010.43.10.649
- Day JJ, Sweatt JD. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology 2012; 37:247-60; PMID:21593731; http://dx.doi.org/10.1038/npp.2011.85
- Parlato R, Kreiner G. Nucleolar activity in neurodegenerative diseases: a missing piece of the puzzle? J Mol Med (Berl) 2013; 91:541-7; PMID:23179684; http://dx.doi.org/10.1007/s00109–012-0981–1
