Abstract
Exocytic post-fusion events play an important role determining the composition and quantity of cellular secretion. In particular, Ca2+-dependent regulation of fusion pore dilation/closure is a key regulator for fine-tuning vesicle content secretion. This requires a tight temporal and spatial integration of vesicle fusion with the PM, Ca2+ signals and translation of the Ca2+ signal into fusion pore dilation via auxiliary factors. Yet, it is still mostly elusive how this is achieved in slow and non-excitable secretory cells, where initial Ca2+ signals triggering fusions will abate before onset of the post-fusion phase. New results suggest, that the vesicles themselves provide the necessary itinerary to sense and link vesicle fusion to generation of local Ca2+ signals and fusion pore expansion.
Regulated secretion is a fundamental process in many types of eukaryotic cells. In general, vesicle contents are released via exocytosis of secretory vesicles. During exocytosis a sequence of highly regulated steps leads to fusion of exocytic vesicles with the plasma membrane (PM), opening of a fusion pore and finally content release.Citation1-4 Secretory output can thereby be adjusted either by regulating the number of secretory vesicles fusing with the PM during the so-called exocytic pre-fusion phase or by facilitating content release from fused secretory granules during the exocytic post fusion phase. In particular, for exocytosis of large secretory granules and secretion of bulky vesicle contents increasing evidence suggests that regulatory mechanisms during the post-fusion phase determine the composition and quantity of cellular secretion. Several mechanisms have been found that promote and facilitate post-fusion vesicle content release. In particular, regulation of fusion pore dilation/closure has been identified as key regulator for fine-tuning vesicle content secretion. It is well established that fusion pore expansion is regulated by Ca2+.Citation5 In addition, a range of factors including myosin II,Citation6 synaptotagmins,Citation7,8 dynaminCitation9 and F-actinCitation10 and others,Citation11 have been suggested as molecular mediators for Ca2+-dependent fusion pore transitions in exocytosis of large vesicles in non-neuronal cells. These models suggest a tight temporal and spatial integration of vesicle fusion with the PM, Ca2+ signals and translation of the Ca2+ signal into fusion pore dilation via auxiliary factors. This is easily conceivable in neurons and neuro-endocrine cells where exocytosis of vesicles occurs within milliseconds from stimulation and Ca2+ signals initiated during the pre-fusion phase last sufficiently long into the post-fusion phase to provide sufficient Ca2+ for fusion and fusion pore dilation.Citation12 However, the conundrum remains what are the molecular entities to achieve such spatial and temporal integration in slow and non-excitable secretory cells? One possibility is that the vesicles themselves provide the necessary itinerary to sense and link vesicle fusion to generation of local Ca2+ signals and fusion pore expansion. This would be of particular advantage in secretory cells that lack defined “active zones” endowed with protein machinery necessary for exocytosis. Yet, so far a definite example for such vesicular control of fusion pore expansion was still missing.
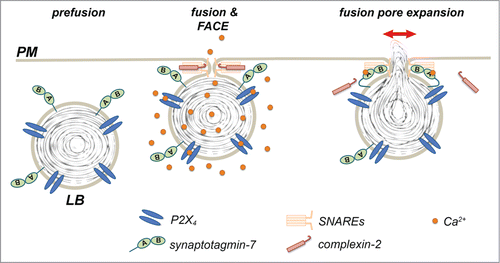
We have recently reported that lamellar bodies (LBs), large, lysosome related storage organelles for lung surfactant in alveolar type II cells, constitute vesicles in control of post-fusion regulation of secretion. Previous results from our laboratory already reported that P2X4 receptors are localized on the limiting membrane of LBs and that selective activation of these receptors upon fusion of the vesicle with the PM results in a localized “fusion-activated“ Ca2+-entry (FACE) that facilitates fusion pore expansion.Citation13 Yet, specific mechanisms linking this locally restricted Ca2+ signal and fusion pore expansion were still elusive. Now Neuland et al. demonstrated that synaptotagmin-7 (syt-7) is also expressed on LBs and provides a molecular link between FACE and regulation of fusion pore dilation. Specifically, they propose that Ca2+ provided by FACE binds to the C2A domain of syt-7. Syt-7 then antagonises the recruitment of complexin-2 to the fused vesicle inhibiting complexin-2 mediated restriction of fusion pore expansion.Citation14 In summary these studies suggest that lamellar bodies themselves harbour all necessary molecules to provide a spatially and temporally restricted rise in Ca2+-signaling linked to vesicle fusion (P2X4 receptors, FACE) and control fusion pore expansion (syt-7). Therefore, LB exocytosis constitutes a model for “vesicular control of secretion."
It is tempting to speculate whether similar mechanisms can also be found in other secretory cells, in particular, cells harbouring lysosomes or lysosome-related organelles.Citation15-17 Many of the lysosome-related organelles contain bulky cargoes and release thereof is often regulated during the post-fusion phaseCitation18 including regulation of fusion pore expansion.Citation8 Although they might not necessarily rely on the same molecular entities, it is well established that P2X4 receptors are predominantly located within lysosomal compartments and inserted into the cell surface upon exocytosis.Citation19,20 Moreover, syt-7 is present on lysosomes and lysosome-related organelles and has been found to be implicated in exocytosis and secretion.Citation8,21,22 Further research is warranted to link these findings and determine whether vesicular control will be established as a more general scheme in secretion, particularly in non-neuronal cells.
Figure 1. Vesicular control of fusion pore expansion. P2X4 receptors and synaptotagmin-7 are expressed on LBs. Activation of P2X4 receptors upon fusion of secretory vesicles with the plasma membrane results in localized Ca2+-entry and a rise in the Ca2+ concentration around the fused vesicle (FACE). Ca2+ then binds to sytnaptotagmin-7, which in turn antagonises complexin-2 mediated restriction of fusion pore expansion resulting in fusion pore expansion and efficient secretion.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Bean AJ, Zhang X, Hokfelt T. Peptide secretion: what do we know? FASEB J 1994; 8:630-8; PMID:8005390
- Lindau M, Gomperts BD. Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta 1991; 1071:429-71; PMID:1751542; http://dx.doi.org/10.1016/0304-4157(91)90006-I
- Südhof TC. The synaptic vesicle cycle. Ann Rev Neurosci 2004; 27:509-47; PMID:15217342; http://dx.doi.org/10.1146/annurev.neuro.26.041002.131412
- Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca2+-triggered exocytosis. Science 2002; 298:781-5; PMID:12399579; http://dx.doi.org/10.1126/science.1075375
- Haller T, Dietl P, Pfaller K, Frick M, Mair N, Paulmichl M, Hess MW, Furst J, Maly K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J Cell Biol 2001; 155:279-89; PMID:11604423; http://dx.doi.org/10.1083/jcb.200102106
- Bhat P, Thorn P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol Biol Cell 2009; 20:1795-803; PMID:19158378; http://dx.doi.org/10.1091/mbc.E08-10-1048
- Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet beta -cells. J Biol Chem 2000; 275:36079-85; PMID:10938083; http://dx.doi.org/10.1074/jbc.M004284200
- Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol 2004; 2:E233; PMID:15226824; http://dx.doi.org/10.1371/journal.pbio.0020233
- Anantharam A, et al. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol Biol Cell 2011; 22:1907-18; PMID:21460182; http://dx.doi.org/10.1091/mbc.E11-02-0101
- Larina O, et al. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol Biol Cell 2007; 18:3502-11; PMID:17596517; http://dx.doi.org/10.1091/mbc.E07-01-0024
- Jackson MB, Chapman ER. The fusion pores of Ca2+ -triggered exocytosis. Nat Struct Mol Biol 2008; 15:684-9; PMID:18596819; http://dx.doi.org/10.1038/nsmb.1449
- Fernandez-Chacon R, Alvarez de Toledo G. Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS Letts 1995; 363:221-5; http://dx.doi.org/10.1016/0014-5793(95)00319-5
- Miklavc P, Mair N, Wittekindt OH, Haller T, Dietl P, Felder E, Timmler M, Frick M. Fusion-activated Ca2+ entry via vesicular P2´4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc Natl Acad Sci USA 2011; 108:14503-8; PMID:21844344; http://dx.doi.org/10.1073/pnas.1101039108
- Neuland K, Sharma N, Frick M. Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J Cell Sci 2014 Dec 15; 127(24):5218-27; PMID:25344253
- Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol 2002; 3:122-31; PMID:11836514; http://dx.doi.org/10.1038/nrm732
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol 2007; 8:622-32; PMID:17637737; http://dx.doi.org/10.1038/nrm2217
- Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J 2000; 14:1265-78; PMID:10877819
- Thorn P. New insights into the control of secretion. Commun Integr Biol 2009; 2:315-7; PMID:19721876; http://dx.doi.org/10.4161/cib.2.4.8262
- Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2´4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci 2007; 120:3838-49; PMID:17940064; http://dx.doi.org/10.1242/jcs.010348
- Toyomitsu E, Tsuda M, Yamashita T, Tozaki-Saitoh H, Tanaka Y, Inoue K. CCL2 promotes P2´4 receptor trafficking to the cell surface of microglia. Pur Signal 2012; 8:301-10; http://dx.doi.org/10.1007/s11302-011-9288-x
- Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol 2000; 148:1141-9; PMID:10725327; http://dx.doi.org/10.1083/jcb.148.6.1141
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 2001; 106:157-69; PMID:11511344; http://dx.doi.org/10.1016/S0092-8674(01)00421-4
