ABSTRACT
Light is the most important environmental cue to entrain the circadian clock in most animals. In the fruit fly Drosophila melanogaster, the light entrainment mechanisms of the clock have been well-studied. The Drosophila brain contains approximately 150 neurons that rhythmically express circadian clock genes. These neurons are called “clock neurons” and control behavioral activity rhythms. Many clock neurons express the Cryptochrome (CRY) protein, which is sensitive to UV and blue light, and thus enables clock neurons deep in the brain to directly perceive light. In addition to the CRY protein, external photoreceptors in the Drosophila eyes play an important role in circadian light-input pathways. Recent studies have provided new insights into the mechanisms that integrate these light inputs into the circadian network of the brain. In this review, we will summarize the current knowledge on the light entrainment pathways in the Drosophila circadian clock.
Circadian pacemaker neurons in the Drosophila brain
Most animals possess circadian clocks that measure the time of day and allow the organism to adapt to daily environmental changes. The clock generates circadian rhythms of approximately 24 hours in many biological processes, such as behavior, metabolism, and physiology, enabling animals to anticipate and adapt to environmental changes. The oscillatory mechanism of the clock is self-sustained but must be synchronized to external time cues in order to allow adaption to changing environmental conditions. In most cases, light is the most critical entrainment cue and must be integrated into circadian clock circuits in the brain.
The brain of most animals contains a central clock. In the fruit fly Drosophila melanogaster, approximately 150 neurons in the brain have been identified as clock neurons based on the cyclic expression of the genes and proteins that play central roles in the circadian clock. These molecules are referred to as clock genes or proteins. Clock neurons are located in distinct clusters in the Drosophila central brain. Each cluster is named according to its location and the size of individual neurons, as shown in It remains unclear whether all clusters of clock neurons are important for controlling behavioral rhythms; however, several studies have suggested that they are not all of equal importance.Citation2
Figure 1. The circadian clock network in the Drosophila brain. The left hemisphere shows the distribution of the Cryptochrome (CRY)-positive (blue) and CRY-negative (white) clock neurons. The right hemisphere shows the compound eye and the Hofbauer-Buchner (HB) eyelet. The ocelli are located on top of the brain. The small and large lateral neuron (s-LNv and l-LNv) clusters express the Pigment-dispersing factor (PDF) protein and are regarded as the clock neurons controlling the morning (M) activity peak. The lateral dorsal neurons (LNd) and the 5th s-LNv are the evening (E) clock neurons. The dorsal neuron (DN) clusters are not well characterized. The HB eyelet is directly connected to the s-LNv neurons through projections to the surrounding area. In contrast, the connections between the compound eyes and the clock neurons have not yet been elucidated.
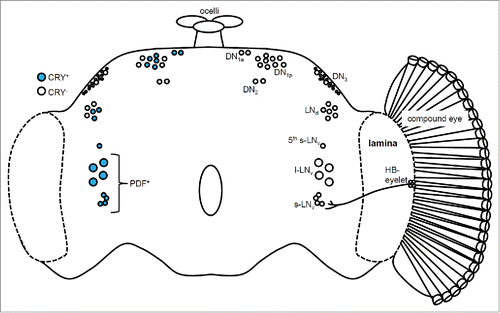
The best-studied clock neurons are the large and small lateral ventral neurons (l-LNvs and s-LNvs). These neurons express Pigment-dispersing factor (PDF), a neuropeptide that acts as a circadian neuromodulator.Citation3 PDF plays an important role in the circadian network as an intercellular messenger, synchronizing daily rhythms between PDF neurons and other clock neurons.Citation4-8 Pdf-null flies display weak circadian activity rhythms with a shortened period of approximately 22 hours when kept in constant darkness (DD) and a phase-advanced evening activity peak in the presence of a 12-hour:12-hour light-dark cycle (LD).Citation9 Interestingly, flies lacking the l-LNv and s-LNv neuron clusters show identical behavioral phenotypes, suggesting that PDF is the principal output of these neurons. Thus, PDF-expressing clock neurons as well as PDF itself strongly influence activity rhythms.
The Gal4/UAS system is a genetic tool commonly used for tissue-specific manipulation of gene expression in Drosophila.Citation10 Several useful Gal4 lines have been available since the year 2000 that target subsets of clock neurons, allowing us to study their function. Pioneering studies using the Gal4/UAS system were performed by Grima et al. and Stoleru et al. in 2004.Citation11,12 These studies investigated different subsets of clock neurons by restoring clock function to specific neuron clusters in the clock-impaired period (per) mutant or by ablating clusters expressing the apoptosis-inducing head involution defective gene. The flies without functional PDF neurons lost anticipatory morning (M) activity, whereas the flies without functional lateral dorsal (LNd) and dorsal neuron (DN) clusters lost anticipatory evening (E) activity under LD conditions. Thus, both studies concluded that the PDF-positive clock neurons are important for morning activity, and LNd and DN clusters are important for evening activity. In 2006, the 5th s-LNv was identified as a non-PDF-expressing clock neuron that was located in close proximity to the l-LNv cluster and was classified as an E clock neuron.Citation13
CRY function in clock neurons
The structure of the CRY protein is very similar to that of the bacterial 6–4 photolyase. The CRY protein was first identified in Arabidopsis thaliana,Citation14 suggesting that it is highly conserved. Cryb mutant flies display failures in light entrainment.Citation15-17 Whereas wild-type flies are able to synchronize their circadian rhythms to a new LD condition within one day, cry mutant flies require approximately 7 d to adapt. The circadian clock responds to a light pulse during the night by advancing or delaying activity rhythms, depending on the time at which the light-pulse is given. These responses can be described by a phase-response curve. Compared to wild-type flies, cry0 mutants are significantly less sensitive to light pulses and display reduced phase responses.Citation18
CRY is expressed in many clock neurons, including M and E neurons.Citation19,20 Upon light exposure, CRY binds to the Timeless (TIM) protein, an essential clock component, and leads to its ubiquitination by the Jetlag protein and subsequent degradation.Citation21,22 The light-induced degradation of TIM destabilizes the Period (PER) protein, another core clock component and a binding partner of TIM, and thus pauses the circadian oscillation of PER and TIM levels.
These interactions between the PER, TIM, and CRY proteins provide a simple and clear-cut explanation of how the Drosophila clock is reset by light. However, not all clock neurons express CRY, and levels of CRY expression differ among clock neurons.Citation20 Furthermore, not all clock neurons show similar responses to light-pulses given during the night.Citation23 For example, Tang et al. (2010) demonstrated that a light pulse early in the night induces degradation of TIM in E neurons, but not in M neurons, suggesting that the phase resetting of the clock by a light pulse is not the same in all clock neurons and depends on the time of day.Citation23 More interestingly, CRY-dependent light input to M neurons can cause CRY-independent TIM degradation in E neurons.Citation24 Thus, M neurons can direct the light response of E neurons through neuronal communication.
A bright light pulse lasting for several minutes during the night is sufficient to induce TIM degradation in all clock neurons.Citation25,26 In response to a light pulse of reduced intensity and increased duration, the 5th s-LNv neuron shows the most efficient TIM degradation compared to other clock neurons,Citation27 suggesting that individual clock neurons differ in CRY-dependent light sensitivity. These studies used light pulses to examine light responses, revealing fine temporal properties of the clock in response to a short light-pulse. However, responses to light pulses differ from entrainment to LD conditions that consist of distinct periods of day and night.
When CRY is re-expressed in E neurons using the Gal4/UAS system in the cry mutant background, these flies show responses similar to those of wild-type flies to an 8-hour phase delay of the LD cycle, re-entraining nearly within one day.Citation26 In contrast, cry0 mutants require nearly a week to completely synchronize to the phase-delayed LD cycle. Interestingly, E neurons in cry0 mutants show rapid re-entrainment of molecular cycling of the clock protein Par Domain Protein (PDP1). However, re-entrainment of clock protein cycling in other clock neurons occurs slowly, similar to re-entrainment of behavioral rhythms.Citation26 Thus, E neurons are the first to reset in response to light input from the visual system, followed by other clock neurons.
Light-activated CRY also influences the neural activity of l-LNv neurons by increasing action potential firing.Citation28,29 This effect is independent of the CRY-TIM interaction and opsin-based photoreception. The role of these CRY-induced increases in neuronal activity is thus far unknown, but a possible involvement in light entrainment should be considered.
Light entrainment via the visual system
Drosophila have 3 different external photoreceptors: compound eyes, ocelli, and Hofbauer-Buchner eyelets (HB eyelets). The compound eyes are the largest photoreceptive structure and are thought to be the most important to light entrainment.Citation30 Studies using eye mutants have demonstrated that the compound eyes play roles in measuring day length and detecting moonlight.Citation31-33 Flies display 2 distinct activity peaks at approximately dawn and dusk in 12 hour:12 hour LD cycles, termed the M and E peaks, respectively.Citation34 These 2 activity peaks respond to changing photoperiod and to dim light during the night. Under long-day conditions or during nights with moonlight, the M and E peaks move away from one another, thus creating a larger phase angle between the peaks.Citation2 Drosophila mutants lacking compound eyes cannot adapt their M and E activity peaks to long-day conditions.Citation31 and do not respond to moonlight.Citation32,33 These findings suggest that the light input from the visual system affects the M and E neurons in opposite manners, namely phase-advances the M oscillator and phase-delays the E oscillator. Moonlight also has direct effects on activity, known as masking effects, in that it increases nighttime activity levels.Citation35 These masking effects are mediated by the compound eyes but are not influenced by CRY.Citation31,33 The compound eyes consist of approximately 800 ommatidia, each of which contains 8 photoreceptor cells (R1–8) expressing different rhodopsins (Rh1–6).Citation36 Mutant flies lacking Rh1 and Rh6, which are expressed in R1–6 and R8 cells, respectively, do not display masking effects on activity behavior in response to moonlight.Citation33
Twilight, the gradual change in light intensity at dawn and dusk, also affects activity rhythms, though it is often neglected in laboratory experiments. Simulation of twilight conditions causes shifts of the M and E activity peaks into the dim light zones.Citation37 The effects of twilight on behavior exceed those of moonlight, and activity rhythms during twilight simulation more strongly resemble activity rhythms under natural conditions.Citation38-41 The effects of twilight on behavior are also mediated by the compound eyes, particularly by the 2 inner photoreceptor cells R7 and R8.Citation40 Thus, different light-sensing mechanisms have different roles in modulating and entraining activity rhythms.
The role of the ocelli in circadian light entrainment has not been well studied. Comparisons between mutants that lack all external photoreceptors or lack only the compound eyes showed significant contribution of the compound eyes to entrainment, compared to the minor contribution of the ocelli.Citation31 The same is true for the HB eyelets, which are remnants of the larval photoreceptors, the Bolwig´s organs, and express the rhodopsin 5 and 6 (rh5 and rh6) genes.Citation42,43 In both larval and adult brains, the projections of Bolwig´s organs and HB eyelets directly contact the PDF-positive LNvs.Citation30,44,45 The larval Bolwig´s organs use acetylcholine as a neurotransmitter, whereas the adult HB eyelets express both acetylcholine and histamine.Citation43,46 Application of cholinergic agonists increases Ca2+ and cyclic AMP (cAMP) levels in both dissociated larval LNvs and in adult LNv clusters in intact brains.Citation47,48 Bolwig´s organs, together with CRY, are essential for light entrainment of the larval clock neurons.Citation49 However, it remains unclear how significantly HB eyelets contribute to light entrainment of the adult clock. Two studies have investigated the roles of the Rh5 and Rh6 proteins that are expressed in the HB eyelets and in photoreceptor cell R8 of the compound eyes.Citation50,51 Flies with triple-mutation of rh5, rh6, and cry display slower light re-entrainment than cry single mutants in following a 6-hour phase shift of the LD cycle, suggesting that the HB eyelets contribute to light entrainment.
The compound eyes use histamine as a neurotransmitter. Flies with double-mutation of cry and the hdc gene encoding histidine decarboxylase are unable to synchronize to an LD cycle.Citation31 Drosophila express 2 histamine receptor genes: ort and hisCl1. Both receptors are expressed in interneurons located between the lamina and the medulla and may also be present in other brain regions.Citation52,53 Serotonin and dopamine may be involved in intermediate pathways.Citation54,55 However, it is not yet known how histamine receptor-positive cells transmit signals to clock neurons.
CRY is also expressed in the compound eyes. A recent study demonstrated that CRY interacts with the phototransduction complex through the Inactivation No Afterpotential D protein in the compound eyes.Citation56 Cry mutant flies show reduced nocturnal light sensitivity in electroretinograms and a weaker optomotor response compared to wild-type flies. However, CRY expressed in the eyes may have a minor contribution to light entrainment, since flies expressing cry only in the eyes do not show significant improvement in entrainment compared to cry mutants.Citation26,57
PDF neurons and light entrainment
PDF is a circadian neuropeptide specifically expressed in the l-LNv and s-LNv clusters and serves as an intercellular communication signal between clock neurons.Citation3,6 The PDF receptor (PDFR) is expressed in many clock neurons including the s-LNvs.Citation58 Pdf mutants or flies lacking PDF-expressing neurons show a phase-advanced E activity peak under LD conditions.Citation9 They are also incapable of shifting M and E peaks under long-day conditions.Citation7 These phenotypes are similar to those observed in eye mutants.Citation31 Furthermore, ablation of the l-LNv cluster attenuates the response to a light-pulse late in the night.Citation59 These results suggest that PDF and the PDF neurons are important for light entrainment.
Flies with double-mutation of Pdf and cry show an intriguing phenotype, completely lacking the E peak under LD conditions.Citation60,61 This phenotype suggests a model in which CRY and the visual system entrain the M and E neurons by either direct or indirect mechanisms; furthermore, PDF-expressing neurons transduce light input from the visual system to E neurons, such that E neurons in Pdf-cry double mutants are unable to receive light input. Im and Taghert (2011) additionally demonstrates that the M peak in the double mutants is a masking effect but is not driven by the circadian clock, indicating that PDF and CRY are essential for light entrainment.Citation62
Thermogenetics is a suitable genetic tool for the manipulation of clock neuron activity because the effect of temperature on the clock is relatively moderate compared to that of light, which is used for optogenetics. TrpA1 is a temperature-sensitive cation channel that induces neuronal depolarization in response to increasing temperature.Citation63 Interestingly, it appears that phase-response curves in response to temperature pulses applied to flies overexpressing trpA1 in PDF neurons mimic phase-response curves in response to light pulses.Citation64 Thus, light inputs, likely from the visual system, may normally excite PDF neurons, consequently resetting downstream neurons such as the E neurons and leading to behavioral phase shifts. This pathway may be mediated by specific adenylate cyclases, cAMP, and the protein kinase A signaling cascade that promotes TIM degradation in E neurons.Citation64-67 The neuronal circuits linking the visual system to the clock neurons have not yet been described, with the exception of the direct connection between HB eyelets and PDF neurons described above. It has not yet been demonstrated that light inputs from all visual organs converge on the PDF neurons, not on the E neurons, which would support the hypothesis that PDF neurons provide the only pathway for light inputs to the clock that are not mediated by CRY. This has already been shown for the larval brain, which has simpler circadian circuits involving only 18 clock neurons and only 2 light-input pathways: Bolwig´s organs and the CRY protein.Citation49
Conclusions
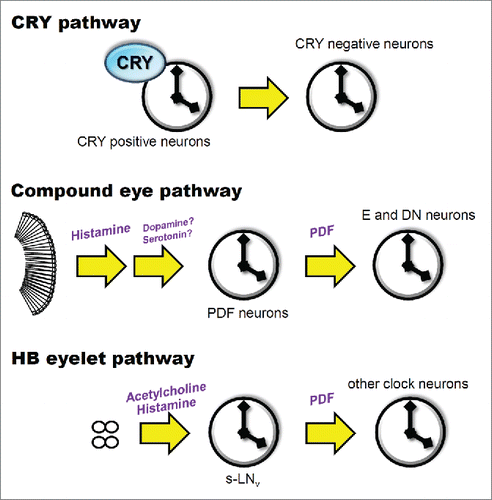
Previous studies have revealed the astonishing complexity of light entrainment in the circadian system of the fly, which has otherwise been considered a rather simple organism. The compound eyes convey light signals to the PDF neurons via histaminergic signaling through interneurons, leading to the resetting of the molecular clock in the PDF neurons. Bolwig´s organs and HB eyelets use both acetylcholine and histamine to signal to PDF neurons; this pathway seems to consist of a direct connection between the visual organs and clock neurons. Both pathways first reset PDF-expressing neurons, which in turn reset the PDF-negative clock neurons. CRY is expressed in the clock neurons and directly interacts with TIM to reset the molecular clock. Interestingly, light entrainment of the E neurons is especially important for behavioral rhythms. Although more detailed studies are required for an exhaustive understanding of the complete mechanism, including unknown light-input pathways,Citation68,69 the relentless efforts of many researchers in our field have been steadily revealing new details on how the Drosophila light-input systems entrain the neuronal clock network ().
Figure 2. Three light-input pathways to the Drosophila clock. CRY is expressed in many clock neurons, which transmit light information to CRY-negative clock neurons. Histamine, dopamine, and serotonin have been suggested as neurotransmitters that convey light inputs from the compound eyes. The HB eyelets use acetylcholine and histamine as neurotransmitters and directly target the PDF neurons. PDF plays a role in intercellular communication between the PDF neurons and other clock neurons that express the PDF receptor.
Abbreviations
| CRY | = | Cryptochrome |
| cyclic AMP | = | cAMP |
| DD | = | constant darkness |
| DN | = | dorsal neuron |
| E | = | evening |
| HB eyelets | = | Hofbauer-Buchner eyelets |
| LD | = | light-dark |
| LN | = | lateral neuron |
| M | = | morning |
| = | Pigment-dispersing factor | |
| PDP1 | = | Par Domain Protein 1 |
| PER | = | Period |
| TIM | = | Timeless |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the JSPS (KAKENHI 23870021, 25840121, and 15H05600) and the German Research Foundation (DFG; Fo207/10–3 and SFB1047, INST 93/784–1).
References
- Hermann-Luibl C, Helfrich-Förster C. Clock network in Drosophila. Curr Opin Insect Sci 2015; 7:65-70; http://dx.doi.org/10.1016/j.cois.2014.11.003
- Yoshii T, Rieger D, Helfrich-Förster C. Two clocks in the brain: an update of the morning and evening oscillator model in Drosophila. Prog Brain Res 2012; 199:59-82; PMID:22877659; http://dx.doi.org/10.1016/B978-0-444-59427-3.00027-7
- Helfrich-Förster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 1995; 92:612-6; PMID:7831339; http://dx.doi.org/10.1073/pnas.92.2.612
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol 2003; 1:32-40; http://dx.doi.org/10.1371/journal.pbio.0000013
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci 2004; 24:7951-7; PMID:15356209; http://dx.doi.org/10.1523/JNEUROSCI.2370-04.2004
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 2008; 58:223-37; PMID:18439407; http://dx.doi.org/10.1016/j.neuron.2008.02.018
- Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J Neurosci 2009; 29:2597-610; PMID:19244536; http://dx.doi.org/10.1523/JNEUROSCI.5439-08.2009
- Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science 2014; 343:1516-20; PMID:24675961; http://dx.doi.org/10.1126/science.1251285
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 1999; 99:791-802; PMID:10619432; http://dx.doi.org/10.1016/S0092-8674(00)81676-1
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993; 118:401-15; PMID:8223268
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 2004; 431:869-73; PMID:15483616; http://dx.doi.org/10.1038/nature02935
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 2004; 431:862-8; PMID:15483615; http://dx.doi.org/10.1038/nature02926
- Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci 2006; 26:2531-43; PMID:16510731; http://dx.doi.org/10.1523/JNEUROSCI.1234-05.2006
- Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen LO, van der Horst GT, Batschauer A, Ahmad M. The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol 2011; 62:335-64; PMID:21526969; http://dx.doi.org/10.1146/annurev-arplant-042110-103759
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 1998; 95:681-92; PMID:9845370; http://dx.doi.org/10.1016/S0092-8674(00)81638-4
- Emery P, Stanewsky R, Hall JC, Rosbash M. Drosophila cryptochromes: a unique circadian-rhythm photoreceptor. Nature 2000; 404:456-7; PMID:10761904; http://dx.doi.org/10.1038/35006558
- Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 2001; 30:249-61; PMID:11343659; http://dx.doi.org/10.1016/S0896-6273(01)00277-X
- Kistenpfennig C, Hirsh J, Yoshii T, Helfrich-Förster C. Phase-shifting the fruit fly clock without cryptochrome. J Biol Rhythms 2012; 27:117-25; PMID:22476772; http://dx.doi.org/10.1177/0748730411434390
- Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor cryptochrome is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms 2008; 23:296-307; PMID:18663237; http://dx.doi.org/10.1177/0748730408318588
- Yoshii T, Todo T, Wülbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol 2008; 508:952-66; PMID:18399544; http://dx.doi.org/10.1002/cne.21702
- Koh K, Zheng X, Sehgal A. Jetlag resets the Drosophila circadian clock by promoting light-induced degradation of timeless. Science 2006; 312:1809-12; PMID:16794082; http://dx.doi.org/10.1126/science.1124951
- Peschel N, Chen KF, Szabo G, Stanewsky R. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr Biol 2009; 19:241-7; PMID:19185492; http://dx.doi.org/10.1016/j.cub.2008.12.042
- Tang CH, Hinteregger E, Shang Y, Rosbash M. Light-mediated TIM degradation within Drosophila pacemaker neurons (s-LNvs) is neither necessary nor sufficient for delay zone phase shifts. Neuron 2010; 66:378-85; PMID:20471351; http://dx.doi.org/10.1016/j.neuron.2010.04.015
- Lamba P, Bilodeau-Wentworth D, Emery P, Zhang Y. Morning and evening oscillators cooperate to reset circadian behavior in response to light input. Cell Rep 2014; 7:601-8; PMID:24746814; http://dx.doi.org/10.1016/j.celrep.2014.03.044
- Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila Cryptochrome structural domains in circadian photoreception. Science 2004; 304:1503-6; PMID:15178801; http://dx.doi.org/10.1126/science.1096973
- Yoshii T, Hermann-Luibl C, Kistenpfennig C, Schmid B, Tomioka K, Helfrich-Förster C. Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila. J Neurosci 2015; 35:6131-41; PMID:25878285; http://dx.doi.org/10.1523/JNEUROSCI.0070-15.2015
- Vinayak P, Coupar J, Hughes SE, Fozdar P, Kilby J, Garren E, Yoshii T, Hirsh J. Exquisite light sensitivity of Drosophila melanogaster cryptochrome. PLoS Genet 2013; 9:e1003615; PMID:23874218; http://dx.doi.org/10.1371/journal.pgen.1003615
- Fogle KJ, Parson KG, Dahm NA, Holmes TC. Cryptochrome is a blue-light sensor that regulates neuronal firing rate. Science 2011; 331:1409-13; PMID:21385718; http://dx.doi.org/10.1126/science.1199702
- Fogle KJ, Baik LS, Houl JH, Tran TT, Roberts L, Dahm NA, Cao Y, Zhou M, Holmes TC. Cryptochrome-mediated phototransduction by modulation of the potassium ion channel β-subunit redox sensor. Proc Natl Acad Sci USA 2015; 112:2245-50; PMID:25646452; http://dx.doi.org/10.1073/pnas.1416586112
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci 2002; 22:9255-66; PMID:12417651
- Rieger D, Stanewsky R, Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms 2003; 18:377-91; PMID:14582854; http://dx.doi.org/10.1177/0748730403256997
- Bachleitner W, Kempinger L, Wülbeck C, Rieger D, Helfrich-Förster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci USA 2007; 104:3538-43; PMID:17307880; http://dx.doi.org/10.1073/pnas.0606870104
- Schlichting M, Grebler R, Peschel N, Yoshii T, Helfrich-Förster C. Moonlight detection by Drosophila's endogenous clock depends on multiple photopigments in the compound eyes. J Biol Rhythms 2014; 29:75-86; PMID:24682202; http://dx.doi.org/10.1177/0748730413520428
- Helfrich-Förster C. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster–sex-specific differences suggest a different quality of activity. J Biol Rhythms 2000; 15:135-54; PMID:10762032; http://dx.doi.org/10.1177/074873040001500208
- Kempinger L, Dittmann R, Rieger D, Helfrich-Förster C. The nocturnal activity of fruit flies exposed to artificial moonlight is partly caused by direct light effects on the activity level that bypass the endogenous clock. Chronobiol Int 2009; 26:151-66; http://dx.doi.org/10.1080/07420520902747124
- Behnia R, Desplan C. Visual circuits in flies: beginning to see the whole picture. Curr Opin Neurobiol 2015; 34:125-32; PMID:25881091; http://dx.doi.org/10.1016/j.conb.2015.03.010
- Rieger D, Fraunholz C, Popp J, Bichler D, Dittmann R, Helfrich-Förster C. The fruit fly Drosophila melanogaster favors dim light and times its activity peaks to early dawn and late dusk. J Biol Rhythms 2007; 22:387-99; PMID:17876060; http://dx.doi.org/10.1177/0748730407306198
- Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 2012; 484:371-5; PMID:22495312; http://dx.doi.org/10.1038/nature10991
- Menegazzi P, Vanin S, Yoshii T, Rieger D, Hermann C, Dusik V, Kyriacou CP, Helfrich-Förster C, Costa R. Drosophila clock neurons under natural conditions. J Biol Rhythm 2013; 28:3-14; http://dx.doi.org/10.1177/0748730412471303
- Schlichting M, Grebler R, Menegazzi P, Helfrich-Förster C. Twilight dominates over moonlight in adjusting Drosophila's activity pattern. J Biol Rhythms 2015; 30:117-28; PMID:25838418; http://dx.doi.org/10.1177/0748730415575245
- Schlichting M, Menegazzi P, Helfrich-Förster C. Normal vision can compensate for the loss of the circadian clock. Proc Biol Sci 2015; 282(1815). pii:20151846 PMID:26378222
- Hofbauer A, Buchner E. Dose Drosophila have seven eyes? Naturwissenschaften 1989; 76:335-6; http://dx.doi.org/10.1007/BF00368438
- Yasuyama K, Meinertzhagen IA. Extraretinal photoreceptors at the compound eye's posterior margin in Drosophila melanogaster. J Comp Neurol 1999; 412:193-202; PMID:10441750; http://dx.doi.org/10.1002/(SICI)1096-9861(19990920)412:2<193::AID-CNE1>3.0.CO;2-0
- Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 2002; 129:1443-53; PMID:11880353
- Yuan Q, Xiang Y, Yan Z, Han C, Jan LY, Jan YN. Light-induced structural and functional plasticity in Drosophila larval visual system. Science 2011; 333:1458-62; PMID:21903815; http://dx.doi.org/10.1126/science.1207121
- Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res 1991; 266:391-8; PMID:1684918; http://dx.doi.org/10.1007/BF00318195
- Wegener C, Hamasaka Y, Nässel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol 2004; 91:912-23; PMID:14534288; http://dx.doi.org/10.1152/jn.00678.2003
- Lelito KR, Shafer OT. Reciprocal cholinergic and GABAergic modulation of the small ventrolateral pacemaker neurons of Drosophila's circadian clock neuron network. J Neurophysiol 2012; 107:2096-108; PMID:22279191; http://dx.doi.org/10.1152/jn.00931.2011
- Klarsfeld A, Picot M, Vias C, Chelot E, Rouyer F. Identifying specific light inputs for each subgroup of brain clock neurons in Drosophila larvae. J Neurosci 2011; 31:17406-15; PMID:22131402; http://dx.doi.org/10.1523/JNEUROSCI.5159-10.2011
- Veleri S, Rieger D, Helfrich-Förster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J Biol Rhythms 2007; 22:29-42; PMID:17229923; http://dx.doi.org/10.1177/0748730406295754
- Szular J, Sehadova H, Gentile C, Szabo G, Chou WH, Britt SG, Stanewsky R. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-β signaling. J Biol Rhyth 2012; 27:25-36; http://dx.doi.org/10.1177/0748730411431673
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, et al. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci 2006; 26:7245-56; PMID:16822982; http://dx.doi.org/10.1523/JNEUROSCI.5426-05.2006
- Pantazis A, Segaran A, Liu CH, Nikolaev A, Rister J, Thum AS, Chun B, Hyun S, Lee Y, Kim J. Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J Neurosci 2008; 28:7250-9; PMID:18632929; http://dx.doi.org/10.1523/JNEUROSCI.1654-08.2008
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron 2005; 47:115-27; PMID:15996552; http://dx.doi.org/10.1016/j.neuron.2005.05.027
- Hirsh J, Riemensperger T, Coulom H, Iche M, Coupar J, Birman S. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr Biol 2010; 20:209-14; PMID:20096587; http://dx.doi.org/10.1016/j.cub.2009.11.037
- Mazzotta G, Rossi A, Leonardi E, Mason M, Bertolucci C, Caccin L, Spolaore B, Martin AJ, Schlichting M, Grebler R, et al. Fly cryptochrome and the visual system. Proc Natl Acad Sci USA 2013; 110:6163-8; PMID:23536301; http://dx.doi.org/10.1073/pnas.1212317110
- Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron 2000; 26:493-504; PMID:10839367; http://dx.doi.org/10.1016/S0896-6273(00)81181-2
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol 2010; 518:1925-45; PMID:20394051; http://dx.doi.org/10.1002/cne.22311
- Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci USA 2008; 105:19587-94; PMID:19060186; http://dx.doi.org/10.1073/pnas.0809577105
- Cusumano P, Klarsfeld A, Chelot E, Picot M, Richier B, Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci 2009; 12:1431-7; PMID:19820704; http://dx.doi.org/10.1038/nn.2429
- Zhang L, Lear BC, Seluzicki A, Allada R. The Cryptochrome photoreceptor gates PDF neuropeptide signaling to set circadian network hierarchy in Drosophila. Curr Biol 2009; 19:2050-5; PMID:19913424; http://dx.doi.org/10.1016/j.cub.2009.10.058
- Im SH, Li W, Taghert PH. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PLoS One 2011; 6:e18974; PMID:21559487; http://dx.doi.org/10.1371/journal.pone.0018974
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008; 454:217-20; PMID:18548007; http://dx.doi.org/10.1038/nature07001
- Guo F, Cerullo I, Chen X, Rosbash M. PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. ELife 2014; 17:3
- Duvall LB, Taghert PH. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PLoS Biol 2012; 10:e1001337; PMID:22679392; http://dx.doi.org/10.1371/journal.pbio.1001337
- Li Y, Guo F, Shen J, Rosbash M. PDF and cAMP enhance PER stability in Drosophila clock neurons. Proc Natl Acad Sci U S A 2014; 111:E1284-90; PMID:24707054; http://dx.doi.org/10.1073/pnas.1402562111
- Seluzicki A, Flourakis M, Kula-Eversole E, Zhang L, Kilman V, Allada R. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS Biol 2014; 12:e1001810; PMID:24643294; http://dx.doi.org/10.1371/journal.pbio.1001810
- Veleri S, Brandes C, Helfrich-Förster C, Hall JC, Stanewsky R. A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr Biol 2003; 13:1758-67; PMID:14561400; http://dx.doi.org/10.1016/j.cub.2003.09.030
- Chen KF, Peschel N, Zavodska R, Sehadova H, Stanewsky R. Quasimodo, a Novel GPI-anchored zona pellucida protein involved in light input to the Drosophila circadian clock. Curr Biol 2011; 21:719-29; PMID:21530261; http://dx.doi.org/10.1016/j.cub.2011.03.049
