ABSTRACT
The neurotensin receptor-3 also known as sortilin was the first member of the small family of vacuolar protein sorting 10 protein domain (Vps10p) discovered two decades ago in the human brain. The expression of sortilin is not confined to the nervous system but sortilin is ubiquitously expressed in many tissues. Sortilin has multiple roles in the cell as a receptor or a co-receptor, in protein transport of many interacting partners to the plasma membrane, to the endocytic pathway and to the lysosomes for protein degradation. Sortilin could be considered as the cells own shuttle system. In many human diseases including neurological diseases and cancer, sortilin expression has been shown to be deregulated. In addition, some studies have highlighted that the extracellular domain of sortilin is shedded into the culture media by an unknown mechanism. Sortilin can be released in exosomes and appears to control some mechanisms of exosome biogenesis. In lung cancer cells, sortilin can associate with two receptor tyrosine kinase receptors called the TES complex found in exosomes. Exosomes carrying the TES complex can convey a microenvironment control through the activation of ErbB signaling pathways and the release of angiogenic factors. Deregulation of sortilin function is now emerging to be implicated in four major human diseases- cardiovascular disease, Type 2 diabetes mellitus, Alzheimer disease and cancer.
KEYWORDS:
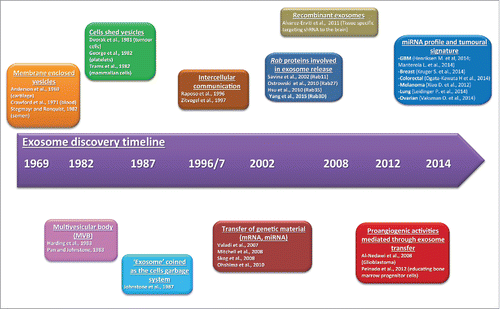
Exosomes discovery timeline
Exosomes are small extracellular vesicles (“cell bubbles”) secreted by most eukaryotic cells. They range from 30 to 100nm in size found in the cell culture media and many biological fluids such as blood, saliva and urine, and hence have a potential involvement in intercellular communication. They were originally described in 1983 such as small released vesicles from the multivesicular body (or also known as multivesicular late endosome) fusion with the plasma membrane during red blood cell maturationCitation1 (). For many years these vesicles were believed to be the cell's own garage disposal route. Some years later, these scarcely understood microvesicles have been called exosomes.Citation2 Since the last decade, exosomal research is growing exponentially, especially after the discovery of distinct subsets of RNAs into the exosomal cargo,Citation3 which has enriched the knowledge of the molecular cocktail that may be shuttled by exosomes. In doing so, many laboratories have investigated the close link between exosome secretion and disease, indicative but not exhaustive, such as cardiomyocyte hypertrophy,Citation4 diabetesCitation5 and cancer.Citation6-8 Remarkably, cancer cells may take advantage of exosome secretion in order to control the tumor microenvironment, and could endorse thereby the aggressiveness and the tumorigenic features of the tumor, such as angiogenesis,Citation6 invasionCitation8,9 and therapeutic escape.Citation7 Encouragingly, exosomes are shedding light on their utility as disease diagnostic markers,Citation10,11 as well as in the development of novel cancer treatment,Citation12 and could take the lion's share of this major challenge.
Sortilin
Sortilin is a newly identified member of a small family of proteins characterized to contain a Vps10p domain. Sortilin can function alone or as part of a co-receptor as well as a transporter of proteins from the trans-Golgi network (TGN).Citation13 As a co-receptor of p75 neurotrophin receptor (p75NTR), sortilin binds the immature, pro-forms of nerve growth factor (NGF) or brain derived neurotrophic growth factor (BDNF) and induces apoptosis in neuronal cells.Citation14 Sortilin's cytoplasmic tail shares similarity to the mannose-6-phosphate receptor with colocalisation to the endosomes and endosome to TGN cargo vesicles.Citation15 In addition, sphingolipid activator proteins, acid sphinomyelinase, and cathepsin D and H have been shown to be trafficked by sortilin to the lysosomes.Citation16-18 These studies demonstrate that sortilin has a dual role both in endocytosis and in receptor trafficking allowing the correct sorting of ligands from the cell surface to lysosomes and the traffic of pro-neurotrophins (proNTs) such as the neuropeptide neurotensin (NT), proNGF and proBDNF.Citation14,19-23
Role of sortilin in cancer
Given the important function of pro-neurotrophin receptors such as sortilin play in cellular development, cell survival and death.Citation24 An imbalance in cellular homeostasis can be affected by neurotrophin signaling which could lead to the progression of cancer.Citation24,25 Not surprising, sortilin expression is elevated in several human cancer cells including brain, prostate, colon, pancreas, skin, pituitary.Citation25-29 Some of the initial studies demonstrated that a furin-cleaved form of sortilin could bind NT at the cell surfaceCitation19,20 and traffic NT to the endocytic pathway while maintaining a constant level of sortilin expression at the cell surface.Citation21 In a later study, sortilin was shown to be released from cells requiring cleavage of sortilin luminal domain by a protein kinase C-dependent protease.Citation30 However, the mechanism used for sortilin release from these cells and the consequence to the microenvironment was uncertain. In colon cancer, sortilin forms a dimeric complex with NTSR1 which is internalised upon NT stimulation.Citation31 The binding of NT to sortilin-NTSR1 and trafficking of this complex induces signaling pathways by modification of mitogen-activated protein (MAP) kinases and the turnover of phosphoinositide (PI) facilitated by NTSR1.Citation31 It is not known why sortilin is released from cancer cells but evidence is now emerging to implicate that sortilin may modify the neighboring cells/environment. Massa and colleagues studied the human adenocarcinoma epithelial cell line (HT29) to assess the ability of soluble sortilin to be released and internalised using radioreceptor assays and microscopy.Citation32 The binding of soluble sortilin is independent from the transactivation of the epidermal growth factor receptor (EGFR) resulting in raised intracellular calcium concentration and significant activation of PI3 kinase pathway through Akt phosphorylation dependent upon of FAK/Src phosphorylation.Citation32 The PI3 kinase pathway is implicated in the survival mechanisms of cancer cells.Citation33 The action of soluble sortilin could be explained to have an autocrine/paracrine function.
A number of reports have hinted that NT mediated by sortilin stimulated by an autocrine/paracrine function could be a mechanism associated with the tumorigenesis.Citation26-28 The cell responds to two types of neurotrophin signal, one elicited by the p75NTR and the other by Trk tyrosine kinase receptors.Citation34 Sortilin can interact with either of these receptors but the consequential outcome affects cell survival. Sortilin traffics from the TGN to the cell surface through the secretory pathway where it interacts with p75NTR that can signal a pro-neurotrophin-induced cell death. The signals induce cell death by the pathway of c-Jun N-terminal kinase 3 and caspases 3, 6 and 9.Citation35-38 Trk interaction with sortilin promotes cell survival and in the case of neuronal cells stimulates cell survival, differentiation, innervation and plasticity /effect cell survival. Sortilin can associate with all the Trk receptors (A, B and C)Citation24,25 implicating an important role in cell survival that is disrupted in human disease.
Sortilin is a key component of exosome biogenesis
Unprecedented reports have found that sortilin expression level is associated to different types of cancer.Citation24-26 Some of these studies have implicated that sortilin could play a role in the tumorigenesis process.Citation24,26 Our team has been interested in these links between sortilin and cancer and at the same time the cross-talk between the epidermal growth factor receptor (EGFR) and tyrosine kinase receptor (Trk) signaling pathways.Citation13 We have discovered that sortilin can form a novel complex with TrkB and EGFR found in exosomes that are released from lung cancer cells conveying a microenvironmental control upon endothelial cells.Citation39 In this study, we examined closely the secretion mechanism utilized for the extracellular domain of sortilin from human lung cancer cells (A549) and the effect on the microenvironment. We show for the first time that sortilin uses a ‘canonical pathway' and can be found in exosomes. We demonstrate that sortilin is a key component of exosomes mediating communication between A549 and endothelial cells (). Sortilin is already known to play a prime function in cancer cells; however we have reported herein that it plays a new role in both the assembly of a tyrosine kinase complex and its exosome release. This novel complex called ‘TES' complex expressed by exosomes results in the linkage of two tyrosine kinase receptors, TrkB and EGFR with sortilin. We demonstrate in this study that the TES complex coveys a control on the microenvironment i.e. endothelial cells and initiates the activation of angiogenesis via exosome transfer. Therefore, our data suggested that sortilin and its partners have a paracrine through exosome transfer and control of the microenvironment. This novel complex containing sortilin could play the role as a molecular switch in cancer progression by promoting angiogenesis.
Figure 2. Role of sortilin in EV biogenesis. Sortilin is initially synthesized in the constitutive secretory pathway as a precursor encoding a short propeptide sequence. The propeptide is cleaved by pro-protein convertases at the TGN allowing sortilin to enter the secretory pathway (stage 1). There are a number of likely routes that sortilin can be trafficked. Sortilin can be trafficked along a number of possible routes such as trafficking to the plasma membrane through constitutive secretory vesicles (stage 2). Alternatively, sortilin could be anterograde transported from the TGN by itself or with its binding partners to the early endosomes (stage 3). Sortilin present at the cell surface or in the endocytic pathway could be cleaved by disintegrin and metalloproteinase domain-converting protein (ADAM) 10 or ADAM17, and followed by g-secretase (stage 5). Following endoproteolytic cleavage, sortilin could form a heterotrimeric complex with TrkB and EGFR (TES complex) which is internalized through a clathrin-dependent endocytosis process into early endosomes (stage 6). At the plasma membrane, the purple spots represent clathrin associated with vesicles (clathrin-coated vesicles [CCV]) or the bilayered clathrin coats at the endosome. The intraluminal vesicles (ILV) are formed by an invagination event at the membrane of the late endosomes/multivesicular body (MVB). Sortilin may play a role in the recruitment of certain cargo such as its binding partners- TrkB and EGFR, which could be an ESCRT-dependent mechanism. The MVB and its content could be degraded via the lysosome-mediated pathway for degradation or alternatively the MVB are transported to the cell surface were they dock at the plasma membrane requiring Rab27A to release the vesicles into the extracellular space (stage 7). The exosomes carrying the TES complex could be released and taken up in the target cell. The uptake of TES-containing exosomes initiates cellular communication through upregulation of cell signaling events by the induction of cell survival through the EGFR cascade and the angiogenesis process (stage 8).
![Figure 2. Role of sortilin in EV biogenesis. Sortilin is initially synthesized in the constitutive secretory pathway as a precursor encoding a short propeptide sequence. The propeptide is cleaved by pro-protein convertases at the TGN allowing sortilin to enter the secretory pathway (stage 1). There are a number of likely routes that sortilin can be trafficked. Sortilin can be trafficked along a number of possible routes such as trafficking to the plasma membrane through constitutive secretory vesicles (stage 2). Alternatively, sortilin could be anterograde transported from the TGN by itself or with its binding partners to the early endosomes (stage 3). Sortilin present at the cell surface or in the endocytic pathway could be cleaved by disintegrin and metalloproteinase domain-converting protein (ADAM) 10 or ADAM17, and followed by g-secretase (stage 5). Following endoproteolytic cleavage, sortilin could form a heterotrimeric complex with TrkB and EGFR (TES complex) which is internalized through a clathrin-dependent endocytosis process into early endosomes (stage 6). At the plasma membrane, the purple spots represent clathrin associated with vesicles (clathrin-coated vesicles [CCV]) or the bilayered clathrin coats at the endosome. The intraluminal vesicles (ILV) are formed by an invagination event at the membrane of the late endosomes/multivesicular body (MVB). Sortilin may play a role in the recruitment of certain cargo such as its binding partners- TrkB and EGFR, which could be an ESCRT-dependent mechanism. The MVB and its content could be degraded via the lysosome-mediated pathway for degradation or alternatively the MVB are transported to the cell surface were they dock at the plasma membrane requiring Rab27A to release the vesicles into the extracellular space (stage 7). The exosomes carrying the TES complex could be released and taken up in the target cell. The uptake of TES-containing exosomes initiates cellular communication through upregulation of cell signaling events by the induction of cell survival through the EGFR cascade and the angiogenesis process (stage 8).](/cms/asset/736573c4-1bbd-48ce-b63e-92c465bab136/kcib_a_1130192_f0002_c.gif)
The unanswered questions of sortilin's role in exosome/EV biogenesis
It is well appreciated that MVBs have two fates in the cell; they act as a platform to deliver cargo destined for lysosome-mediated degradation or as a portal to release ILVs/exosomes from the cell. The endosomal sorting of cargo is mediated by a sequence events involving four multiprotein complexes (ESCRT0, -I, -II, and –III). The clathrin coats condense and cluster cargo at the cytosolic face of the MVB membrane ready to be captured and recruited to ILVs. These early events of cargo recruitment are assisted by the ESCRT machinery, ESCRT‐0 and ESCRT‐I. In a previous study, the HRS gene found in the ESCRT-0 complex could be involved in the formation and secretion of exosomes.Citation40 Knocking down some of the genes that encode for components of the ESCRT-0 complex (HRS, STAM1 or TSG101) perturb exosome release and affect the size and/or protein content of the ILVs demonstrating an important role played by the ESCRT complex.Citation41 Our data suggested a possible unreported new role for sortilin as a possible cargo recruiter to ILVs through cargo recognition and sorting at the MVB. The challenge remains to determine several questions: (1) what is the intracellular route of sortilin trafficking through the secretory pathway; (2) at what stage is sortilin important for ILV formation at the MVB; (3) what is sortilin's mechanism to recruit cargo or the regulation of ILV formation; (4) and at the same time whether sortilin released as exosomes from cells plays a role in the angiogenesis process. Furthermore, an imbalance in sortilin expression in cancer could alter the content of exosomes regulating the delivery of both a genomic and proteomic content to the target cells. To this end, the challenge remains to define the exact role of sortilin in cancer thus providing clues to sortilin's global role in other types of human diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 1983; 97:329-39; PMID:6309857; http://dx.doi.org/10.1083/jcb.97.2.329
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987; 262:9412-20.
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 2014; 124:2136-46; PMID:24743145; http://dx.doi.org/10.1172/JCI70577
- Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 α impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem 2007; 282:9364-71; PMID:17202141; http://dx.doi.org/10.1074/jbc.M608985200
- Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2014; 6:715-31.
- Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 2012; 227:658-67; PMID:21465472; http://dx.doi.org/10.1002/jcp.22773
- Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer 2014; 13:256; PMID:25428807; http://dx.doi.org/10.1186/1476-4598-13-256
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al., VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438:820-7; PMID:16341007; http://dx.doi.org/10.1038/nature04186
- Hogan MC, Bakeberg JL, Gainullin VG, Irazabal MV, Harmon AJ, Lieske JC, Charlesworth MC, Johnson KL, Madden BJ, Zenka RM, et al. Identification of Biomarkers for PKD1 Using Urinary Exosomes. J Am Soc Nephrol 2014; 26:1661-70.
- Musante L, Tataruch DE, Holthofer H, Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne) 2014; 5:149; PMID:25309511
- Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm Res 2015; 32:2003-14
- Wilson CM, Naves T, Saada S, Pinet S, Vincent F, Lalloué F, Jauberteau MO. The implications of sortilin/vps10p domain receptors in neurological and human diseases. CNS Neurol Disord Drug Targets 2014; 13:1354-65; PMID:25345507; http://dx.doi.org/10.2174/1871527313666141023151642
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, et al., Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004; 427:843-8; PMID:14985763; http://dx.doi.org/10.1038/nature02319
- Mari M, Bujny MV, Zeuschner D, Geerts WJ, Griffith J, Petersen CM, Cullen PJ, Klumperman J, Geuze HJ. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 2008; 9:380-93; PMID:18088323; http://dx.doi.org/10.1111/j.1600-0854.2007.00686.x
- Canuel M, Lefrancois S, Zeng J, Morales CR. AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem Biophys Res Commun 2008; 366:724-30; PMID:18078806; http://dx.doi.org/10.1016/j.bbrc.2007.12.015
- Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J 2003; 22:6430-7; http://dx.doi.org/10.1093/emboj/cdg629
- Ni X, Morales CR, The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic 2006; 7:889-902; PMID:16787399; http://dx.doi.org/10.1111/j.1600-0854.2006.00429.x
- Mazella J, Zsürger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem 1998; 273:26273-6; PMID:9756851; http://dx.doi.org/10.1074/jbc.273.41.26273
- Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J 1999; 18:595-604; PMID:9927419; http://dx.doi.org/10.1093/emboj/18.3.595
- Navarro V, Martin S, Sarret P, Nielsen MS, Petersen CM, Vincent J, Mazella J. Pharmacological properties of the mouse neurotensin receptor 3. Maintenance of cell surface receptor during internalization of neurotensin. FEBS Lett, 2001. 495:100-5; PMID:11322955; http://dx.doi.org/10.1016/S0014-5793(01)02367-5
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 2005; 25:5455-63; PMID:15930396; http://dx.doi.org/10.1523/JNEUROSCI.5123-04.2005
- Nilsson SK, Christensen S, Raarup MK, Ryan RO, Nielsen MS, Olivecrona G. Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the VPS10p domain receptor families. J Biol Chem, 2008. 283:25920-7; PMID:18603531; http://dx.doi.org/10.1074/jbc.M802721200
- Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, Richner M, Erdmann B, Nyengaard JR, Tessarollo L, et al. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci 2011; 14:54-61.
- Akil H, Perraud A, Melin C, Jauberteau MO, Mathonnet M. Fine-tuning roles of endogenous brain-derived neurotrophic factor, TrkB and sortilin in colorectal cancer cell survival. PloS One 2011; 6:e25097.
- Dal Farra C, Sarret P, Navarro V, Botto JM, Mazella J, Vincent JP. Involvement of the neurotensin receptor subtype NTR3 in the growth effect of neurotensin on cancer cell lines. Int J Cancer 2001; 92:503-9.
- Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol 2008; 128:2031-40.
- Giorgi RR, Chile T, Bello AR, Reyes R, Fortes MA, Machado MC, Cescato VA, Musolino NR, Bronstein MD, Giannella-Neto D, et al. Expression of neurotensin and its receptors in pituitary adenomas. J Neuroendocrinol 2008; 20:1052-7.
- Xiong J, Zhou L, Yang M, Lim Y, Zhu YH, Fu DL, Li ZW, Zhong JH, Xiao ZC, Zhou XF. ProBDNF and its receptors are upregulated in glioma and inhibit the growth of glioma cells in vitro. Neuro Oncol 2013; 15:990-1007.
- Navarro V, Vincent JP, Mazella J. Shedding of the luminal domain of the neurotensin receptor-3/sortilin in the HT29 cell line. Biochem Biophys Res Commun 2002; 298:760-4.
- Martin S, Navarro V, Vincent JP, Mazella J. Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line. Gastroenterology 2002; 123:1135-43.
- Massa F, Devader C, Beraud-Dufour S, Brau F, Coppola T, Mazella J. Focal adhesion kinase dependent activation of the PI3 kinase pathway by the functional soluble form of neurotensin receptor-3 in HT29 cells. Int J Biochem Cell Biol 2013; 45:952-9.
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009; 8:627-44.
- Schecterson LC, Bothwell M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol 2010; 70:332-8.
- Skeldal S, Matusica D, Nykjaer A, Coulson EJ. Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR). BioEssays 2011; 33:614-25.
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 2006; 361:1545-64.
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci 2006; 26:7756-66.
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A 2004; 101:6226-30.
- Wilson CM, Naves T, Vincent F, Melloni B, Bonnaud F, Lalloue F, Jauberteau MO. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci 2014; 127:3983-97.
- Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun 2010; 399:384-90.
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126:5553-65.