ABSTRACT
The maintenance of the epithelial architecture during tissue proliferation is achieved by apical positioning of the midbody after cell division. Consequently, midbody mislocalization contributes to epithelial architecture disruption, a fundamental event during epithelial tumorigenesis. Studies in 3D polarized epithelial MDCK or Caco2 cell models, where midbody misplacement leads to multiple ectopic but fully polarized lumen-containing cysts, revealed that this phenotype can be caused by 2 different scenarios: the loss of mitotic spindle orientation or the loss of asymmetric abscission. In addition, we have recently proposed a third cellular mechanism where the midbody mislocalization is achieved through cytokinesis acceleration driven by the cancer-promoting phosphatase of regenerating liver (PRL)-3. Here we critically review these findings, and we furthermore present new data indicating that midbodies themselves might act as signal unit for polarization since they can infer apical characteristics to a basal membrane.
The epithelium is a fundamental tissue in metazoans that acts as a selective permeable membrane for most of the animal organs. It consists of a layer of tightly attached polarized cells that line from the entire outside of the organism until internal hollow small lumens. Its characteristic architecture must be strictly regulated since defects on the specific architecture are the source of almost 90% of all human cancers.Citation1-3 Thus, epithelial tissue polarization and organization have been extensively studied in 3-dimensional (3D) cellular models like single hollow lumen-containing 3D MDCK (Madin-Darby Canine Kidney) or Caco-2 (caucasian colon adenocarcinoma) cysts and in branched tubular MCF-7 (caucasian breast adenocarcinoma) structures.Citation4-10
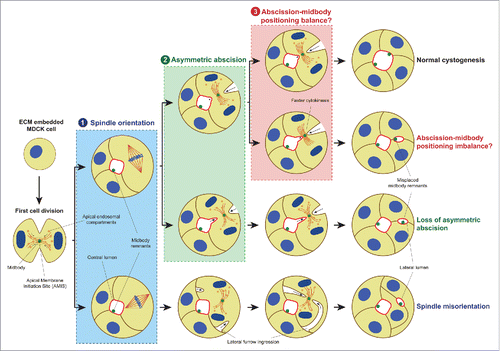
In MDCK cysts, a lumen is formed de novo by cord hollowing and starts during the first mitosis where vesicles containing apical markers move along the mitotic spindle microtubules to the midbody,Citation4,11-13 a structure created within the cleavage furrow near the end of cytokinesis.Citation14,15 Then, the Apical Membrane Initiation Site (AMIS) is formed, where the apical membrane will finally be positioned and the lumen will be opened.Citation8,12,13 This process is independent of a preexisting apical-basal axis since the AMIS is formed around the midbody.Citation8,12 Therefore, in subsequent cell divisions the midbody must be placed in the already specified apical membrane to ensure that a central single lumen is maintained.Citation8 The apical positioning of the midbody is driven through a 2-step process. First, the mitotic spindle is oriented perpendicular to the apical-basal axis generating a radial cleavage furrow. Then, this furrow ingresses from the basal and the apical membrane asymmetrically toward the lumen to finally locate the midbody at the apical membrane at the end of the abscission (, pathway on the top).Citation5,8,12,13,16 Disruption of any of these steps would result in mislocalized midbodies that would open ectopic lumens leading to the disturbance of the epithelial architecture ().Citation8,16 Two mechanisms had been previously proposed in the literature: 1) the loss of the perpendicular spindle orientation, which leads to lateral instead of apico-basal furrow ingression (, process labeled ‘1’); and 2) the loss of asymmetric abscission, where the midbody stays in the lateral membrane after cytokinesis (, process labeled ‘2’). The loss of spindle orientation has been extensively studied,Citation8,13 but the symmetric furrow ingression remains as a theoretical scenario empirically suggested by forced recruitment of E-Cadherin to the basal membrane.Citation8,13,17 Recently, we have suggested a third alternative cellular mechanism caused by the accelertion of the cytokinetis pace, leading to abscission taking place before the midbody reaches the apical membrane, and lateral retention of the midbody (, process labeled ‘3’).Citation18 Here, we provide a critical review of that work and cast light on the relevance of the midbody in cell polarity by proposing it as a polarity signal unit.
Figure 1. Mechanisms for ectopic lumen formation through post-mitotic midbody mispositioning. Pathway on the TOP: At early cystogenesis stage, apical components-containing vesicles are recruited around the midbody during the first cell division to form the AMIS, which will turn into a lumen after abscission. In subsequent cell divisions, to ensure the single lumen maintenance, the midbody is delivered to the apical membrane by a planar orientation of the mitotic spindle to the apical-basal axis (1) followed by asymmetric furrow ingression (2), during which the midbody is positioned at the apical membrane (3). Loss of mitotic spindle orientation (1, lower pathway) or loss of asymmetric furrow ingression (2, lower pathway) may affect the apical positioning of the midbody leading to ectopic lumen development. Furthermore, we suggested a novel mechanism in which abscission takes place before the midbody reaches its luminal position through faster cytokinesis (3, lower pathway), and which might also lead to lateral retention of the midbody leading to the same multiple lumen phenotype. Moreover, midbody remnants from previous cell divisions are kept delineating the apical surface. Apical membrane, light red; γ-tubulin, dark red; nuclei and chromosomes, blue; midbody and midbody remnants, green; basolateral membrane, brown; apical endosomal compartment, orange.
Cytokinesis acceleration as a new mechanism to alter the apical midbody position in the epithelium
We discovered the new mechanism by studying the cancer promoting phosphatase of regenerating liver -3 (PRL-3).Citation18,19 It is a dual specificity phosphatase (DSP) and belongs to the family of the PRLs (PRL-1, -2 and -3). All of them are implicated in promoting cancer progression.Citation19 Few protein and non-protein PRL-3 substrates have been suggested, but none is thoroughly established yet.Citation19 PRL-3 is known to promote epithelial tumor progression and metastasis but the molecular mechanism behind this clinical outcome had not been investigated before in the context of epithelial architecture and cell polarity.Citation19 Therefore, we recently studied if PRL-3 activity would induce a cancer-relevant phenotype in polarized epithelial cells using MDCK, Caco-2 and MCF-7 epithelial cell 3D models.Citation18 Indeed, we showed that MDCK and Caco-2 cysts overexpressing the wild type version of PRL-3 were frequently abnormal with fully polarized multiple lumens compared with single central lumen in parental MDCK and Caco-2 cysts while the cell polarity was not disrupted. Moreover, we corroborated this finding in MCF-7 3D structures. The branch ends of these complex structures contained multiple lumens when PRL-3 was present in parental MCF-7 cells, but showed single-lumen containing branch ends upon PRL-3 knockdown.Citation18 PRL-3 substrate candidates related to epithelial polarity like ezrin or phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) were analyzed to uncover the molecular mechanism behind this observed phenotype, but no change in the phosphorylation status or localization was observed. These conclusions were drawn upon observation of static fixed confocal pictures of the 3D cysts, and therefore phosphorylation variation during dynamic processes were not possible to monitor due to physical and technical challenges impossible to solve with the state-of-art technology. Therefore, improvements in 3D cyst image acquisition would be required to further investigate the PRL-3 phenotype in this context.
Further investigation on the cellular process that underlies the ectopic lumen formation upon PRL-3 aberrant expression showed that they arise from mispositioned midbodies. However, PRL-3 did not disrupt the spindle orientation and asymmetric furrow ingression, the two already postulated mechanisms,Citation8 suggesting that a third alternative mechanism might exist.Citation18 Since cytokinesis is fundamental for midbody positioning and PRL-3 was enriched in the cytokinetic bridge, we studied the role of PRL-3 in this cellular process.Citation18 Indeed, we showed that PRL-3 expression accelerates abscission timing.Citation18 Therefore, since the midbody is a structure derived from cell division, and spatial disturbance of cytokinesis leads to midbody mispositioning in the epithelial context, a temporal alteration of the abscission would likely infer also an alteration in the final position of the midbody. For that reason, we proposed a third scenario where the acceleration of cytokinesis timing by PRL-3 might be the cause of the ectopic lumen phenotype observed in 3D epithelial cysts (, process labeled ‘3’). Since it is highly challenging with the currently available technologies to follow the apical or lateral positioning of the midbody by life-cell imaging in the 3D cysts, an alternative mechanism where the midbody reaches the apical membrane after cytokinesis and then it is located back in the lateral membrane by the action of PRL-3 cannot be excluded. However, this is an unlikely scenario given that PRL-3 does not influence cell polarity itself,Citation18 that it is enriched in the cytokinetic bridge,Citation18 and that cytokinesis timing and midbody positioning are intertwined.Citation13,18
Future work on PRL-3's role in cytokinesis promises to reveal the molecular mechanism behind this phenotype. Proposed PRL-3 substrates like PI(4,5)P2 or ezrin, which where initially not studied in the context of cytokinesis,Citation18 or PRL-3 interacting proteins like CNNM magnesium transporters,Citation20,21 of which CNNM4 does not co-localize with PRL-3 in MDCK cysts during interphase (own unpublished data), can still play a role in PRL-3's effect on cytokinesis and with that in the disruption of epithelial architecture. Nevertheless, studying cytokinesis in 3D polarized cells remains a challenge as these systems cannot be synchronized, and the event of cytokinesis is rather rare and difficult to detect in statistically relevant numbers using microscopy techniques.
The post-mitotic midbody might act as a signal unit in for apical specificity
Midbody remnants can participate in non-cytokinetic functions.Citation17,22,23 Accordingly, our results confirm that midbodies have essential functions in de novo lumenogenesis and lumen growth during cytokinesis and right after abscission,Citation8,12,16,18 but their fate beyond that time line remained previously unexplored. In the process of our published research, we realized that midbody remnants persist long after lumen opening where they delineate the edge of the central and also ectopic lumens instead of being degraded,Citation18 suggesting a role long after cell division in polarity maintenance or specification. One hypothesis, based on the midbody being the center for the delivery of apical components,Citation11-13 is that the midbody might support the stability or maintenance of apical polarity by inferring apical polarity to membranes in polarized systems.
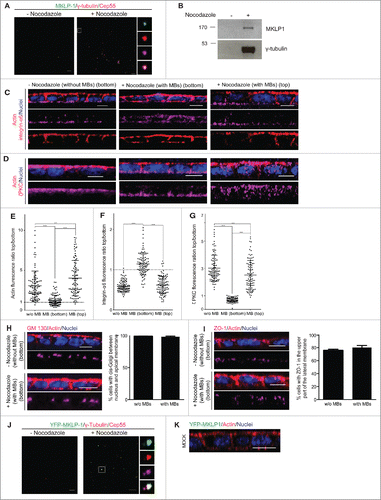
Here, we wanted to further investigate this hypothesis. To this end, we delivered midbody remnants purified from non-polarized 2-dimensional (2D) culture (,),Citation24 to the top (apical membrane) or bottom (basal membrane) side of a 2D polarized monolayer of MDCK cells. Ectopic midbodies delivered to the bottom induced a partial shift of the localization of apical (actin and ζPKC) and basal (integrin-α6) markers 24 hours after being delivered (). However, other polarity markers like ZO-118or the cis-Golgi matrix protein GM130Citation25 did not show any changes in localization (,) suggesting a selective shift of polarity.Citation26 As control we treated the cells in the same manner with samples obtained through the same purification procedure using non-synchronized cells, which did not contain midbodies (,). Cells treated in this manner did not show a shift of polarity markers (), showing the dependence on the presence of the midbodies for the polarity shift. To discard the possibility that the actin shift could be due to an engulfment of the midbody in a phagocytotic manner,Citation27 ectopic midbodies purified from YFP-MKLP-1 overexpressing MDCK cells () were added on top or to the bottom of MDCK 2D polarized cell cultures. Since we observed no engulfment of the YFP-tagged midbodies (), we confirmed that the observed actin enrichment is not due to phagocytosis.
Figure 2. The post-mitotic midbody infers apical specificity to basal membranes. (A) Representative confocal images of midbodies purified from synchronized cells (+ nocodazole) spinned down over poly-l-lysine coverslips and stained with Cep-55 (magenta), MKLP-1 (green) and γ-tubulin (red) antibodies. The same procedure from non-synchronized cells (- nocodazole) led to samples that did not contain midbodies. (B) Purified midbodies (+ nocodazole) and the control sample (- nocodazole) were immunoblotted with antibodies against MKLP-1 (upper picture) and γ-tubulin (lower picture) as midbody markers to confirm the purification. (C, D) Representative images of integrin α6 (C) or ζPKC (D) (magenta) in 2D polarized MDCK cell cultures treated with the control sample without (- nocodazole) midbodies or with the sample containing ectopic midbodies (+ nocodazole) (MBs), which both were added on top or to the bottom incubated 24 h. Actin was stained with phalloidin (red) and nuclei with Hoechst (blue). Scale bars 10 μm. E-G Fluorescence ratio between the top (apical) and the bottom (basal) membrane is represented for actin (E), integrin α6 (F) and ζPKC (G). n = 100. (H, I) Representative images of cis-Golgi marker GM130 (H) and thigh junction marker ZO-1 (I) in magenta in 2D polarized MDCK cell cultures treated with the control sample without (- nocodazole) midbodies or with the sample containing ectopic midbodies (+ nocodazole), which both were added to the bottom and incubated for 24 h. Nuclei were stained with Hoechst (blue) and actin with phalloidin (red). The percentage of cells with GM130 stained cis-Golgi present between the nucleus and the apical membrane and ZO-1 stained in the upper part of the lateral membrane was calculated. n = 90. J Purified midbodies from YFP-MKLP-1 (green) overexpressing MDCK cells and a non-synchronized control preparation were spinned down over poly-l-lysine coverslips and stained against Cep-55 (magenta) and γ-tubulin (red) to confirm the purification. Right: separated channels showing YFP-MKLP-1 (up), γ-tubulin (middle up), Cep55 (middle down) and merge (down). Scale bars 10 μm. K Representative images of 2D polarized monolayer of MDCK cells where ectopic midbodies purified from YFP-MKLP1 were delivered to the bottom or top (not shown) of the layer (n = 1950). Actin was stained with phalloidin (red) and nuclei with Hoechst (blue). Scale bars 10 μm. Values represent mean plus standard deviation of 3 experiments.
Taken together, these results indicate that midbodies might act as a signal unit containing apical specifiers needed for apical polarity, suggesting an active role of the post-mitotic midbody in cell polarity. This signal unit seems to be beneficial or required for apical stability, as the midbodies are not degraded over time.Citation18 However, other factors are important as well since the apical-basal axis orientation is not completely shifted by the midbodies in a pre-established polarized structure.
Conclusion
In our previous study, which we have reviewed and discussed here, we suggested a novel mechanism for the loss of epithelial organization in cancer where cytokinesis timing might be involved in causing the misplacement of midbody remnants without altering the mitotic spindle orientation or asymmetric abscission. We identified PRL-3 as an enzyme involved in this process. Moreover, in the process of our research, we also observed that midbody remnants stay on the edge of the lumen, indicating a role in polarity maintenance. Here, we cast light on the fate of midbody remnants after cytokinesis suggesting that they might act as polarity signal unit for apical membrane specificity. This short communication is a step toward proving this hypothesis, and further work is required to address how PRL-3 affects cytokinesis timing, if this temporal disruption is undoubtedly the direct cause of midbody misplacement, and what the components within the midbody are that allow for apical specification. A large part of these studies will however require advancements in the suitable technology.
Methods
2D MDCK polarized cultures
4 × 104 MDCK cells were plated on Transwell filters (12 mm, 0.4 μm pore size; Corning CoStar) and grown in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS), (both from Gibco, Life Technologies), 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma) and 2 mM L-glutamine (Sigma) for 3 d before the incubation with purified midbodies over 24 h.
Antibodies and immunolabelling
Primary mouse antibodies used were anti-γ-Tubulin (1:100; Sigma, clone GTU-88) and anti-GM130 (1:100; BD Bioscience). Primary rabbit antibodies used were anti-MKLP1 (1:1000, Santa Cruz Biotechnology, clone N-19), anti-ZO-1 (1:200; Invitrogen) and anti-PKCζ (1:200;Santa Cruz Biotechnology). Primary rat antibody used was anti-integrin α6 (1:100; Millipore). Primary goat antibody used was anti-Cep55 (1:100; Santa Cruz Biotechnology). Alexa fluorophore-conjugated secondary antibodies (1:1000 for all secondary antibodies; Invitrogen) or rhodamine-phalloidin (1:1000; Invitrogen) and Hoechst to label nuclei (10 μg/mL) were used. The procedure for the immunofluorescence staining and image data acquisition and analysis was described previously in detail.Citation18
Isolation of midbodiesCitation24
Monolayer cultures of MDCK cells or MDCK stable expressing YFP-MKLP1 were synchronized in metaphase by adding nocodazole (Sigma) to a final concentration of 100 ng/mL for 16 h. Mitotically-arrested cells were harvested by shaking the culture flask. Cells were washed and resuspended with fresh medium and incubate at 37°C with gentle shaking for 25 min. Cells were subsequently pelleted at 200 g for 3 min and gently resuspended in 25 volumes of a hypotonic solution consisting of 1M hexylene glycol (2-methyl-2,4-pentanediol), 20 μM MgCl2, and 2 mM piperazine-N-N'-bis(2-ethane sulfonic acid) (PIPES), pH 7.2, at room temperature. Cells were immediately pelleted at 200 × g for 3 min and vigorously resuspended in 50 volumes of a lysing solution consisting of 1 M hexylene glycol, 1 mM EGTA, 1% Nonidet P-40, 2 mM PIPES, pH 7.2, at 37°C. Disruption of the cells and release of midbodies were completed by vigorous vortexing for 1 min in this solution. Midbodies were then stabilized by chilling on ice and by adding to the lysate 0.3 volume of cold 1M hexylene glycol, 50 mM 2-(N-morpholino) ethane sulfonic acid (MES), pH 6.3, to lower the pH. After centrifugation at 250 × g for 10 min to remove large debris, the supernatant was layered over a cushion of 40% glycerol (w/v) in 50 mM MES, pH 6.3, and centrifuged for 40 min at 2800 × g. This pellet was resuspended in 50 mM MES, pH 6.3, and centrifuged again through 40% glycerol and subsequently resuspended in MES buffer. Finally, the pellet obtained from a 10 cm culture dish untreated (no midbodies) or treated with nocodazole (with midbodies) was washed twice with fresh MDCK growth medium, and added to the 2D polarized live cell culture resuspended in cell medium. The controls were treated exactly the same, except the cells were not synchronized. Midbody purification was confirmed by SDS-PAGE and immunobloting against MKLP1 and γ–tubulin. The whole pellet obtained from a 10 cm culture dish untreated (no midbodies) or treated with nocodazole (with midbodies) was loaded in the acrylamide gel. Moreover, 250 μL of purified midbodies were spinned down at 4°C at 2000 g during 20 min in a 24-well for image acquisition.
Data analysis
Ordinary 2-tailed one-way ANOVA and Tukey's multiple comparison test were performed to study statistical significance: (***) p ≤ 0.001 and (****) p ≤ 0.0001. Statistical analysis and graphs were performed with Prism (GraphPad).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Martin Jechlinger for discussions for providing apico-basal polarity markers. We thank Pierre Neveu for discussions. We thank the Advanced Light Microscopy Facility at EMBL Heidelberg for support.
Funding
T.R. thanks EMBL and Marie Curie Actions for the EMBL Interdisciplinary Postdoc (EIPOD) fellowship. P.L. and G.V. thank the EMBL International PhD Program for support.
References
- McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol 2011; 21:727-35; PMID:21782440; https://doi.org/10.1016/j.tcb.2011.06.005
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell 2010; 141:757-74; PMID:20510924; https://doi.org/10.1016/j.cell.2010.05.011
- Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol 2011; 21:R126-36; PMID:21300279; https://doi.org/10.1016/j.cub.2010.12.003
- Schlüter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol 2009; 20:1444-52; PMID:19497970; https://doi.org/10.1681/ASN.2008090949
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010; 12:1035-45; PMID:20890297; https://doi.org/10.1038/ncb2106
- Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, et al. A molecular switch for the orientation of Epithelial cell polarization. Dev Cell 2014; 31:171-87; PMID:25307480; https://doi.org/10.1016/j.devcel.2014.08.027
- Rodríguez-Fraticelli AE, Bagwell J, Bosch-Fortea M, Boncompain G, Reglero-Real N, García-León MJ, Andrés G, Toribio ML, Alonso MA, Millán J, et al. Developmental regulation of apical endocytosis controls epithelial patterning in vertebrate tubular organs. Nat Cell Biol 2015; 17:241-50; PMID:25706235; https://doi.org/10.1038/ncb3106
- Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 2008; 183:625-33; PMID:19001128; https://doi.org/10.1083/jcb.200807121
- Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007; 4:359-65; PMID:17396127; https://doi.org/10.1038/nmeth1015
- do Amaral JB, Rezende-Teixeira P, Freitas VM, Machado-Santelli GM. MCF-7 cells as a three-dimensional model for the study of human breast cancer. Tissue Eng Part C Methods 2011; 17:1097-107; PMID:21882900; https://doi.org/10.1089/ten.tec.2011.0260
- Wang T, Yanger K, Stanger BZ, Cassio D, Bi E. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J Cell Sci 2014; 127:2483-92; PMID:24706948; https://doi.org/10.1242/jcs.139923
- Li D, Mangan A, Cicchini L, Margolis B, Prekeris R. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep 2014; 15:428-37; PMID:24591568; https://doi.org/10.1002/embr.201338128
- Overeem AW, Bryant DM, van IJzendoorn SCD. Mechanisms of apical-basal axis orientation and epithelial lumen positioning. Trends Cell Biol 2015; 25:476-85; PMID:25941134; https://doi.org/10.1016/j.tcb.2015.04.002
- Steigemann P, Gerlich DW. Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol 2009; 19:606-16; PMID:19733077; https://doi.org/10.1016/j.tcb.2009.07.008
- D'Avino PP, Capalbo L. Regulation of midbody formation and function by mitotic kinases. Semin Cell Dev Biol 2016; 53:57-63; PMID:26802517; https://doi.org/10.1016/j.semcdb.2016.01.018
- Schlüter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu C-J, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 2009; 20:4652-63; PMID:19776356; https://doi.org/10.1091/mbc.E09-02-0137
- Morais-de-Sá E, Sunkel C. Adherens junctions determine the apical position of the midbody during follicular epithelial cell division. EMBO Rep 2013; 14:696-703; PMID:23774295; https://doi.org/10.1038/embor.2013.85
- Luján P, Varsano G, Rubio T, Hennrich ML, Sachsenheimer T, Gálvez-Santisteban M, Martín-Belmonte F, Gavin A-C, Brügger B, Köhn M. PRL-3 disrupts epithelial architecture by altering the post-mitotic midbody position. J Cell Sci 2016; 129:4130-42; PMID:27656108
- Rios P, Li X, Köhn M. Molecular mechanisms of the PRL phosphatases. FEBS J 2013; 280:505-24; PMID:22413991; https://doi.org/10.1111/j.1742-4658.2012.08565.x
- Zhang H, Kozlov G, Li X, Wu H, Gulerez I, Gehring K. PRL3 phosphatase active site is required for binding the putative magnesium transporter CNNM3. Sci Rep 2017; 7:1-9; PMID:28127051
- Funato Y, Yamazaki D, Mizukami S, Du L, Kikuchi K, Miki H. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J Clin Invest 2014; 124:5398-410; PMID:25347473; https://doi.org/10.1172/JCI76614
- Chen C-T, Ettinger AW, Huttner WB, Doxsey SJ. Resurrecting remnants: the lives of post-mitotic midbodies. Trends Cell Biol 2013; 23:118-28; PMID:23245592; https://doi.org/10.1016/j.tcb.2012.10.012
- Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB, et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol 2011; 13:1214-23; PMID:21909099; https://doi.org/10.1038/ncb2332
- Mullins JM, Mclntosh JR. Isolation and Initial Characterization of the Mammalian Midbody. J Cell Biol 1982; 94(3):654-61; PMID:7130277; https://doi.org/10.1083/jcb.94.3.654
- Baschieri F, Confalonieri S, Bertalot G, Di Fiore PP, Dietmaier W, Leist M, Crespo P, Macara IG, Farhan H. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat Commun 2014; 5:4839; PMID:25208761; https://doi.org/10.1038/ncomms5839
- Kroschewski R, Hall a, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol 1999; 1:8-13; PMID:10559857
- Park H, Cox D. Cdc42 regulates Fc gamma receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol Biol Cell 2009; 20:4500-8; PMID:19741094; https://doi.org/10.1091/mbc.E09-03-0230
