ABSTRACT
Metazoan body plans combine well-defined primary, secondary, and in many bilaterians, tertiary body axes with structural asymmetries at multiple scales. Despite decades of study, how axis-defining symmetries and system-defining asymmetries co-emerge during both evolution and development remain open questions. Regeneration studies in asexual planaria have demonstrated an array of viable forms with symmetrized and, in some cases, duplicated body axes. We suggest that such forms may point toward an ancestral eumetazoan form with characteristics of both cnidarians and placazoa.
Introduction
What is the connection between spatial symmetry breaking and multicellularity? To what extent can an ur-metazoan ancestor by envisaged as an initially adventitious, spherically symmetric aggregation of ancestral unicells, e.g. of choanoflagellates as suggested by the “choanoblastaea” model [Citation1], see also [Citation2,Citation3]? Spatial asymmetries clearly predate multicellularity: the Bacilli and the spiral bacteria are classified by their non-spherically symmetric shapes. Even E. coli exhibits substrate-dependent chirality at the colony scale [Citation4]. Unicellular eukaryotes exhibit a vast array of internal and external spatial asymmetries. How are such spatial asymmetries translated to the scale of a multicellular organism, particularly a metazoan with well-defined cell layers and multiple distinct organ systems arranged in a specific, population-invariant pattern? The ability to systematically manipulate body-axis asymmetries during whole-body regeneration (WBR) may provide a route toward answering these questions. Organisms capable of WBR are found in all five primary metazoan clades, including the placozoa [Citation5], sponges [Citation6], and ctenophores [Citation7] as well as bilaterians and cnidarians [Citation8]; hence WBR is widely regarded as an ancestral metazoan trait [Citation8–Citation10]. Here we will focus on WBR outcomes in asexual freshwater planaria (Platyhelminthes, Turbellaria, Tricladida), by far the most extensively manipulated WBR model system [Citation11,Citation12], mentioning supporting results from acoel worms (Xenacoelomorpha) and Hydra (Hydrazoa) where available.
Distinct body axes, along which differentiated structures can be asymmetrically arranged, provide the basis for Eumetazoan morphologies. With the advent of whole-genome sequencing and transcriptomics, it has become evident that the eumetazoan sister clades of cnidarians and bilaterians employ homologous “developmental toolkits” for body-axis specification [Citation13–Citation16]. Considerable molecular as well as embryological evidence supports homology between the primary cnidarian aboral – oral (A-O) and bilaterian anterior – posterior (A-P) axes [Citation3,Citation17–Citation19]. While a second, dorsal – ventral (D-V) axis breaking the otherwise cylindrical symmetry around the A-P is a defining bilaterian trait, both molecular and anatomical evidence support a secondary (“directive”) axis in at least some cnidarians [Citation20–Citation23]. A third, left – right (L-R) asymmetry appears in some arthropods (e.g. in lobsters) and is ubiquitous in vertebrates [Citation24,Citation25]. We focus here on the early-appearing A-P and D-V axes and their morphological correlates, particularly the gut and central nervous system (CNS) axes.
While many treatments are known that specifically disrupt axis specification in multicellular systems (e.g. Wnt, BMP, or bioelectrical pathways for the AP, DV, and LR axes; see below), these processes remain difficult to manipulate arbitrarily and with full control with molecular or embryological methods in either cnidarians and bilaterians. It is not, for example, completely clear at the molecular or cellular level how the morphological asymmetries of the CNS or the gut, or the behavioral asymmetry of forward locomotion, are aligned along the A-P axis in bilaterals. Nor is it known, outside of planaria and acoels (see below), whether such morphological or behavioral asymmetries can be selectively reversed, e.g. to produce A-P symmetric nervous systems or guts. Some putatively basal, acoel bilaterians have rudimentary, net-like nervous systems without evident ganglia or nerve cords, while others have more elaborated structures [Citation26–Citation28], suggesting that the correlation between CNS axis and A-P axis is not universal in bilaterians. The vermiform myxozoa, e.g. Buddenbrockia [Citation29,Citation30] exhibit forward locomotion driven by coordinated, A-P aligned muscle groups, but are radially symmetric cnidarians that altogether lack nervous systems. While such organisms are morphological outliers and may exhibit substantial derived loss of function, their existence renders reconstruction of ancestral axis-specification mechanisms and, in particular, the morphology and expected behavioral repertoire of the common eumetazoan ancestor less than straightforward even given extensive comparative genomics.
Here we suggest that WBR [Citation8–Citation10] provides a tractable alternative to embryonic development for asking fundamental questions about body-axis specification and deep ancestral morphology. We use the term “WBR” to indicate regeneration of the whole body from non-germ cells following either natural or laboratory-induced injuries. In the asexual freshwater planaria of primary interest here, specific experimental manipulations of WBR can symmetrize the A-P axis, including the nervous system and gut [Citation11], add ectopic A-P axes [Citation31], or remove the A-P axis altogether to produce outcomes radially symmetric around the remaining D-V axis [Citation32]; see also [Citation12] for review and specific details below. These manipulations support a suggested homology between the ventral nerve cord (VNC) of bilaterians and the circumoral nerve ring of cnidarians. We reconstruct a hypothesized ancestral eumetazoan characterized by a D-V axis, a blind gut, a nerve ring with a surrounding nerve net, and asexual reproduction. We suggest that the primary function of the nervous system is this animal was not locomotion or feeding but the regulation of body size and morphology.
Planaria exemplify basal bilaterian morphology and WBR capability
While the early phylogeny of the Metazoa remains controversial, there is broad agreement across models that the Cnidarians and the Bilaterians are sister clades [Citation15,Citation33]. The early phylogeny of Bilaterians is similarly controversial, with numerous models now recognizing the Acoelamorpha as basal bilaterians [Citation34–Citation38]; see [Citation39] for a discussion of conflicts between molecular and developmental-morphological phylogenetic analyses. These animals are characterized by an unsegmented body plan, blind gut, and in some species, an L-R symmetric, multiple-VNC nervous system [Citation18,Citation40], although as noted above, nervous-system morphology is highly variable [Citation26,Citation28]. The development of robust acoel model systems including Isodiametra pulchra [Citation41,Citation42] and Hofstenia miamia [Citation43–Citation45] has allowed the biology of these organisms, including their regenerative capabilities, to be characterized. Srivastava et al. [Citation45] showed that Hofstenia miamia is capable of WBR mediated by the Wnt and BMP pathways as it is in both planaria and Hydra [see also Citation43]. Regeneration is enabled by somatic stem cells (neoblasts) expressing piwi homologs, as it is in WBR-capable planaria [Citation39]. In contrast to Hofstenia miamia, Isodiametra pulchra is capable of posterior regeneration, but not WBR [Citation42]. Such variability in WBR capability is also observed in planaria [Citation12].
Despite recent progress with acoels, the asexual planarian model systems Dugesia japonica and Schmidtea mediterranea remain the best-characterized and most extensively manipulated organisms with which to study WBR. While the rhabditophoran Platyhelminths, which include the planarians, are no longer regarded as a basal taxon, they share many of the morphological characteristics of the acoels, including unsegmented body plan, blind gut, and L-R symmetric, two-VNC nervous system [Citation18]. Whether these morphological commonalities are ancestral or derived in either extant acoels or extant planaria remains unknown. Both acoels and planaria exhibit atypical embryonic development [Citation46,Citation47]; whether these characteristics are ancestral or derived also remains unknown.
As with morphology, basal bilaterian reproductive strategy remains controversial [Citation48,Citation49]. While sexual reproduction far pre-dates multicellularity, obligate sexuality appears to be a multicellular innovation in both animal and plant lineages, consistent with Red Queen type arguments [Citation50]. Demosponges and cnidarians such as Hydra exhibit opportunistic sexuality with budding and WBR [Citation51,Citation52], suggesting that obligate sexuality is derived from this more flexible strategy [Citation9,Citation53]. Characterized acoels include male-female and cross-fertilizing hermaphroditic species as well as asexuals that reproduce by budding or fission [Citation40]. Characterized planaria include obligate sexual, opportunistic sexual, or asexual species, with some species alternating between sexual reproduction and parthenogenesis or between sexual and vegetative (fission followed by WBR) reproduction [Citation54]. Asexual planaria can be sexualized by feeding them closely related sexual planaria, suggesting that intercellular morphogen-based signaling promotes or enforces sexuality [Citation55,Citation56], inducing stem-cell lineages that would otherwise reproduce to replicate themselves instead to undergo a stem – germ – stem lineage cycle [Citation53,Citation57].
Manipulating WBR in planaria
Asexual planaria reproduce by fission transverse to the A-P axis followed by WBR of missing anterior or posterior structures [Citation58]; fission is a size and environmental conditions dependent biomechanical process [Citation59] regulated in part by Wnt and BMP pathways [Citation60]. Experimental transverse amputation of both head and tail produce trunk fragments that regenerate both anterior and posterior structures. While amputation of both head and tail does not occur during reproductive fission in the wild, both it and the other manipulations described below are possible outcomes of predation in the wild and engage the same molecular and bioelectric pathways active in reproductive transverse fission. A large number of molecular, pharmaceutical, and bioelectric manipulations have been shown to disrupt WBR in head, tail, trunk, and smaller fragments [Citation11,Citation61]. It is now well-established that the Wnt pathway implements A-P axis specification [Citation62–Citation65], with either bioelectric asymmetry [Citation66] or morphogen transport to the wound site [Citation65] as initiating events. Elements of the Hedgehog (Hh) pathway regulate Wnt pathway activity in both anterior and posterior compartments [Citation67]. Regeneration of specific anterior structures including brain and eyes also depends on the ERK and FGF pathways [Citation65,Citation68,Citation69]. Molecular manipulations implicate the BMP pathway as specifying the D-V axis [Citation11] as in other bilaterians [Citation14].
Here we are primarily interested in manipulations that symmetrize the A-P axis, i.e. replace the asymmetric A-P axial morphology with a symmetric A-P-A morphology, or introduce one or more ectopic A-P axes, with radially symmetric forms in which the A-P axis appears to have been altogether eliminated as the limiting case. If a morphologically normal worm is cut at 60% and 80% of its length to make a “pre-tail” (PT) fragment and the fragment is allowed to regenerate, a morphologically normal worm will result. If, however, the PT fragment is treated immediately post-amputation with β-catenin RNAi [Citation70,Citation71], octonol (8OH), a gap-junction blocker [Citation31], or a depolarizing ionophore [Citation66], a two-headed (2H) phenotype results with dose-dependent penetrance [complementary manipulations produce two-tailed phenotypes; see Citation31,Citation61]. Examination of these 2 H worms reveals that the pharynx has also been duplicated, and the ventral cilia are oriented toward the point of duplication, i.e. in the “posterior” direction from each head [Citation31] as shown in . The nervous system is also duplicated, with both copies functional in directing behavior [Citation72]. Crucially, the VNCs are not only duplicated but are continuous across the duplication point, yielding a nervous system with two complete brains connected by two uninterrupted and apparently fully functional VNCs [Citation31,Citation65,Citation72]. Hence, not only has a head grown from the posterior wound, but the entire anatomy anterior to the anterior-facing wound has been duplicated from the posterior wound. The A-P axis has, in other words, been symmetrized to an A-P-A axis around a point at roughly 70% of the worm’s length, as shown in .
Figure 1. (a) Cutting a PT fragment from a WT worm and treating with 8OH, a depolarizing ionophore, or β-catenin RNAi yields a dose-dependent 2 H phenotype in which all structures anterior to the anterior-facing wound are duplicated. (b) This transformation resymmetrizes the A-P axis around a point at roughly 70% of the worm’s length, equivalent to acting with abstract operators “Copy70(π)(•)” and “Rotate70(•)” in sequence
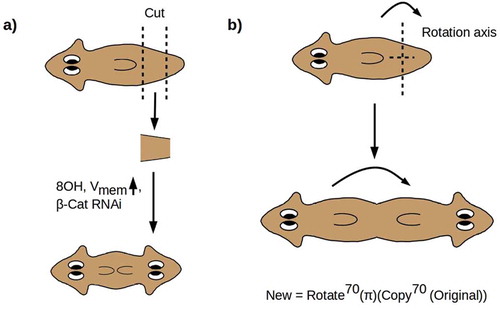
The symmetrization of the A-P axis can be represented geometrically as an abstract rotation by π radians (180°) of a copy of the anterior 70% of the anatomy of the animal (). The axis of rotation is the preserved D-V axis. This “rotation” of the A-P axis is implemented by regenerative growth from the posterior-facing blastema, while regenerative growth from the anterior-facing blastema reproduces the original A-P axis [Citation31,Citation65,Citation72]. Symmetrization of the A-P axis does not erase the distinction between anterior and posterior; it rather duplicates it to produce a bidirectional A-P-A axis with its midpoint at 70% of the original length. The symmetrized animal has duplicated anterior and no posterior anatomy.
Symmetrized 2 H planaria regenerate to produce 2 H progeny for as many generations as have been observed, indicating a stable alteration of morphology. Intriguingly, the morphologically normal outcomes of 8OH treatment under the above conditions are not wild-type, but are rather “cryptic” worms that continue to regenerate 2 H progeny, at the same percentage as in the original experiment, for multiple rounds of regeneration in plain water with no further perturbations [Citation73]. The production of 2 H progeny can be reversed by ionophore treatment, indicating that the “memory” for the 2 H morphology is bioelectric.
Prima facie similar axis duplication results have been obtained in acoels [Citation45,Citation74] and Hydra [Citation75]; however, neither multi-generation inheritance of the axis-duplicated phenotype across multiple rounds of regeneration or any analog of the “cryptic” phenotype has been demonstrated in these systems.
Experiments in which the two VNCs are nicked midway through a PT fragment produce symmetric 4 H worms as shown in [Citation31]. Here again, the entire anterior anatomy is regenerated from the two side nicks, producing two symmetrized A-P axes at right angles. As in 2 H animals, the continuity of the VNCs is preserved, with each of the four brains connected by VNCs to the two neighboring brains [Citation31]. This outcome can be represented geometrically as a repeated copy-and-rotate operation as shown in .
Figure 2. (a) Nicking the two VNCs produces symmetric outcomes with two A-P axes. (b) The outcome can be represented by a repeated copy-and-rotate operation
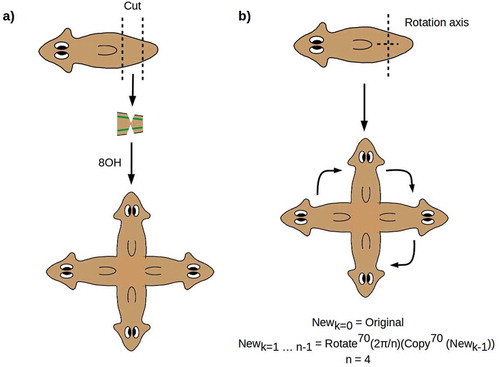
The effective duplication of a symmetrized A-P axis in the cruciform 4 H animals produced by Oviedo et al. [Citation31] suggests that radially symmetric, hypercephalized outcomes such as sketched in could be produced by making multiple “copies” of the A-P axis and “rotating” them around a central D-V axis. From a geometric point of view, making a large number of copies of the anterior morphology and rotating them in such a way that the heads are evenly spaced is equivalent to simply deleting the A-P axis to produce a radially symmetric, completely anterior morphology. Every radial direction from the central D-V axis is, in this case, “anterior”; hence, any regenerative mechanism that “anteriorized” the animal in a radially symmetric way could be expected to yield this outcome. Such radially symmetric, hypercephalized outcomes were observed by Iglesias et al. [Citation32] up to 4 weeks following β-catenin RNAi treatment of amputation fragments. Consistently with the 2 H and cruciform 4 H regenerates discussed above, these outcomes have a continuous, circumferential “VNC” nerve cord [Citation32]. As predicted, the D-V axis remains unaffected, indicating a lack of significant cross-talk between the A-P (Wnt) and D-V (BMP) axis specification systems. Pharyngeal anatomy is, however, disorganized or lost altogether in these radially symmetric animals, in contrast to its preservation and apparent function in 2 H and 4 H animals, suggesting that radially symmetric anteriorization disorganizes tissue specification near the “origin” of the radial axis. Eyes with optic nerves are present in association with some, but not all, of the apparently proto-cephalic clusters of neurons distributed roughly uniformly along the circumferential “VNC” [Citation32], suggesting some loss of tissue specification distally along the radial axis.
Figure 3. (a) Radially symmetric, hypercephalized outcome of multiple A-P axis duplication and symmetrization as the number n of duplicates becomes large. Such outcomes have been observed following β-catenin RNAi [Citation32]. (b, c) Radially symmetric, hypercephalized outcomes, visualized with synapsin staining, obtained by allowing PT fragments from cryptic worms [Citation73] to regenerate in plain water. d) detail of apparently duplicated circumferential VNC in (c), showing nearly continuous clustering of neurons into apparently proto-cephalic structures [cf. Citation32, Figure 3]
![Figure 3. (a) Radially symmetric, hypercephalized outcome of multiple A-P axis duplication and symmetrization as the number n of duplicates becomes large. Such outcomes have been observed following β-catenin RNAi [Citation32]. (b, c) Radially symmetric, hypercephalized outcomes, visualized with synapsin staining, obtained by allowing PT fragments from cryptic worms [Citation73] to regenerate in plain water. d) detail of apparently duplicated circumferential VNC in (c), showing nearly continuous clustering of neurons into apparently proto-cephalic structures [cf. Citation32, Figure 3]](/cms/asset/e5765a0e-63d1-4997-938e-0b3e353dc97c/kcib_a_1729601_f0003_oc.jpg)
Radially symmetric, hypercephalized outcomes with continuous, circumferential nerve cords () have also been observed following regeneration, in plain water without further perturbation, of PT fragments of cryptic worms [Citation73]. The VNCs appear to be duplicated in some of these regenerates (,). Neither pharyngeal structures nor eyes are observed in these preparations. These observations suggest that the bioelectric changes that define the cryptic phenotype can have far-reaching and variable consequences for regenerative morphology that await further, detailed investigation.
In summary, the planarian A-P axis appears to be not just symmetrizable, but highly manipulable, in regeneration-based assays. Both molecular (e.g. β-catenin RNAi) and bioelectric (8OH, ionophore) manipulations can lead to axis symmetrization and duplication. The cryptic phenotype identified with 8OH treatment is the first known example of reversible, bioelectric, epigenetic inheritance [Citation73]; a similar reversible bioelectric manipulation has now also been demonstrated in Hydra [Citation76]. In the absence of rescue manipulations, the altered phenotypes are stable across multiple generations in viable individual planaria, and may be permanent. These results suggest that while the A-P axis is “primary” in planaria as in other bilaterians, it is in a highly plastic state that may reflect loss of evolved constraints on the standard bilaterian body plan.
The planarian A-P axis as a transitional state
In cnidarians, Wnt pathway components including the Disheveled (Dsh) receptor and β-catenin effector are expressed in a decreasing gradient from the Oral to the Aboral pole [Citation18,Citation19]. Let us call this the “Wnt – anti-Wnt” axis, where here “anti-Wnt” refers to either a Wnt inhibitor or opposite-pole determinant. The Wnt – anti-Wnt axis is aligned with the gut in cnidarians, orthogonal to the circumoral nerve ring, and aligned with the long axis of the nerve net driving whole-body contractions and motility [Citation77].
In bilaterians, Wnt pathway components are expressed in a decreasing gradient from posterior to anterior. In bilaterians possessing a through-gut, the Wnt – anti-Wnt axis is aligned with the gut and the nerve cord(s) extending from the anterior brain. The anterior, anti-Wnt direction is the direction of both motion and the mouth.
The structure of the pharynx and hence the location and orientation of the combined mouth/anus is variable in both acoels and flatworms. In the planaria of interest here, the mouth opens ventrally, aligned with the D-V axis [Citation78], along which the pharynx can also be extended as sketched in . The planarian blind gut has the orientation with respect to the D-V axis that the cnidarian blind gut has with respect to the O-A axis. Symmetrizing the A-P axis in planaria duplicates the mouth and pharynx while maintaining their D-V orientation (). In the radially symmetric forms shown in , the D-V axis has become the “primary” body axis about which anatomical structures are radially symmetric; the radial symmetry in this case is analogous to the radial symmetry of cnidarians around the O-A axis.
Figure 4. (a) The planarian mouth opening is aligned along the D-V axis, with respect to which the blind gut has the radial symmetry of the blind gut in cnidarians. (b) Symmetrizing the A-P axis duplicates the mouth-opening axis while preserving its D-V orientation
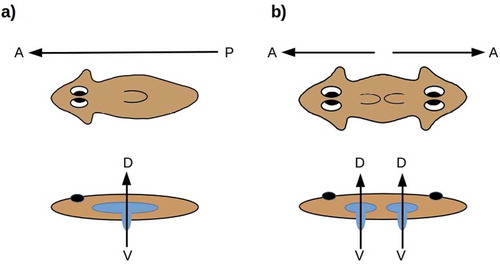
From this perspective, the idea of a “primary axis” appears somewhat ambiguous in planaria. Known manipulations of the D-V axis, moreover, are neither as thorough-going or as extensive as the A-P manipulations reviewed here [Citation11]; no D-V analogs of the multiple A-P duplications shown in or are known. Topologically, the planarian A-P axis is analogous to the cnidarian directive (secondary) axis; each specifies two opposing “sides” of a central, invaginated body cavity. Could the plasticity of the planarian A-P axis reflect an ancestral state in which this axis was secondary, as the directive axis is in cnidarians?
Reconstructing an ancestral eumetazoan
Deep metazoan phylogeny remains highly controversial, with active disagreement about whether porifera or ctenophores are more basal and considerable uncertainty about the placement of placazoa [Citation79–Citation81]. All empirical phylogeny, however, equally suffers from the problem that only extant (or well-preserved fossil) species are accessible for analysis. An empirically informed theoretical phylogeny may therefore have value in considering questions of eumetazoan ancestry.
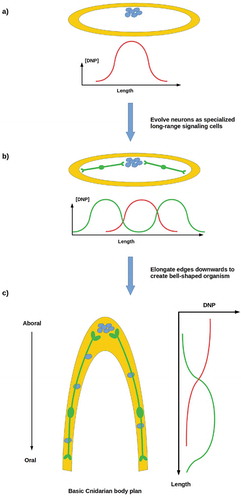
Standard models of the emergence of animal multicellularity are based on the aggregation of closely related cells [Citation82], typically choanoflagellates [Citation1–Citation3]. We have recently proposed an alternative, non-aggregative model in which ancestral, free-living stem cells produce a protective “body” comprising their own reproductively disabled progeny as a means of self-defense in a challenging environment [Citation83]. The principal regulator in this scenario is a “do not proliferate” (DNP) signal that the parent stem cell employs to shut down proliferative capability in its progeny, rendering them fully “somatic” cells with no independent genetic interests or fitness. As Wnt-pathway components are already used for proliferation suppression of prestalk cells in Dictyostelium [Citation84,Citation85], it is plausible on phylogenetic grounds that this DNP signal may be a Wnt or Wnt analog. The DNP signal is assumed to be secreted only by proliferative stem cells and to be short-range; hence its distribution within a multicellular system will depend on whether the system’s proliferative cells are dispersed or clustered, as sketched in . A primitive organism comprising a cellular envelope enclosing a uniform distribution of dispersed proliferative cells may be expected, assuming cell-cycle synchronization or some other mechanism to coordinate stem-cell proliferation, to have relatively uniform internal [DNP] and to be stable. However, such an organism with clustered proliferative cells is expected to have non-uniform internal [DNP] and to be unstable due to uncontrolled reproduction by “somatic” cells in which independent proliferation has not been fully suppressed ().
Figure 5. (a) An ancestral proliferative cell produces progeny for protection, employing a short-range “do not proliferate” (DNP) signal to suppress their proliferation. (b) A somatic cell layer enclosing dispersed proliferate cells has uniform [DNP]; if proliferative cells cluster, [DNP] is non-uniform. (c) A primitive organism comprising a cell layer enclosing dispersed proliferative cells is stable; one enclosing clustered proliferative cell has insufficient [DNP] to prevent rogue proliferation at its margins, so is not stable
![Figure 5. (a) An ancestral proliferative cell produces progeny for protection, employing a short-range “do not proliferate” (DNP) signal to suppress their proliferation. (b) A somatic cell layer enclosing dispersed proliferate cells has uniform [DNP]; if proliferative cells cluster, [DNP] is non-uniform. (c) A primitive organism comprising a cell layer enclosing dispersed proliferative cells is stable; one enclosing clustered proliferative cell has insufficient [DNP] to prevent rogue proliferation at its margins, so is not stable](/cms/asset/4644c070-2cec-4808-b21f-e7bdf8636af1/kcib_a_1729601_f0005_oc.jpg)
Reproductive stability is possible with clustered proliferative cells if longer-range communication of a DNP-like signal that suppresses rogue cell division by somatic cells is possible. Neurons provide an ideal solution to this problem, as they allow long-range, error-correcting communication between source cells and specific target cells [Citation86]. Neurons likewise provide a means of coordinating the proliferation of dispersed populations of stem cells that are too far apart or too distant within a cell lineage to be reproductively coordinated by other mechanisms. An organism with neurons can adopt a more complex body plan, e.g. by elongating its periphery into an invagination as sketched in . The three extant animal lineages with complex body plans – the ctenophores, cnidarians, and bilaterians – all have neurons. Structural, biochemical, and molecular differences between ctenophore neurons and those of cnidarians and bilaterians suggest convergent evolution to a common function [Citation87]. We have suggested that the primary ancestral function of neurons in all three lineages is the long-distance control of cell proliferation that enables a stable multicellular morphology even with clustered stem cells [Citation86]. While this hypothesis remains to be tested, manipulations in Xenopus embryos provide initial evidence for CNS regulation of distal morphogenesis [Citation88,Citation89].
Figure 6. (a, b) A reproductively unstable system can achieve stability by employing neurons to transmit a DNP-like signal (green curves) to distant somatic cells in order to suppress rogue cell division. (c) Neurons enable the development of complex anatomies, e.g. invaginated body cavities
These theoretical considerations suggest an interpretation of the radially symmetric, hypercephalized regeneration outcomes shown in as regressions toward an ancestral state, one that may pre-date not only planaria but eumetazoa in general. Only one extant animal lineage comprises flat, approximately radially symmetric organisms: the placozoa [Citation5,Citation90]. Placozoa have no differentiated neurons but have neurotransmitters and behavior. The dispersed, interior “fiber” cells of placozoa are extended and may serve a communicative function, e.g. by paracrine signaling as in sponges [Citation91]. Characterized placozoa do not have differentiated mouths or guts, but appear to digest food externally and absorb nutrients through distributed ventral-surface cells [Citation5]. Placozoa are predominately asexual, reproducing by budding or fission with WBR, but also exhibit opportunistic sexuality. They are often regarded as amorphous but have a primary D-V axis, the axis normal to the substrate, around which they are approximately radially symmetric. Their anatomy resembles the left side of .
Do the radially symmetric, gutless, hypercephalized regeneration outcomes in , resemble placozoa with neurons added? While gross morphology suggests that placozoa may be their closest affinity, further investigation of both parties is clearly required to answer this question. If these planarian regenerates indeed resemble placozoa at more than a superficial level, they may be pointing toward an ancestral eumetazoan with radial symmetry around a primary D-V axis, a blind or undifferentiated gut, a rudimentary circumferential nerve cord with radial branching, and asexual reproduction with WBR.
Conclusion
We have suggested here that WBR provides an alternative to embryology for studying the mechanisms of body-axis specification and their contributions to the evolution of complex morphologies. Asexual planaria appear to be particularly attractive model systems in this regard. The A-P axis of planaria, in particular, is highly malleable using molecular, pharmacological, and bioelectric manipulations. This axis can not only be symmetrized but also duplicated to such an extent that it effectively disappears, leaving a radially symmetric, fully anteriorized form. Whether the A-P axis of acoels, or of other bilaterians, is similarly manipulable remains to be seen; commonalities in axis-specification mechanisms across the bilaterians as well as specific results in acoels [Citation45,Citation74] suggest that they may be.
The evolutionary emergence of complex body plans appears intimately connected to the emergence of neurons as specialized long-range signaling systems [Citation86]. We suggest that the emergence of neurons in a radially symmetric, placozoan-like animal may have set the stage for the differentiation of the eumetazoan lineages.
Further work is clearly required to elaborate and test these hypotheses. Life-history studies of the symmetrized forms shown in , have already been initiated; if such forms can be reliably produced and maintained, functional investigation of their nervous and digestive systems will be possible. The results of Müller [Citation75] and Braun and Ori [Citation76] suggest that Hydra may be an attractive Cnidarian model system with which to pursue similar axis-symmetrization studies. Thorough investigation of cell-cell signaling mechanisms in Placozoa would complement these investigations. More broadly, the study of the regulation of morphogenesis outside of the nervous system per se by neural activity remains in its infancy. Recent as well as classical evidence of regulation of regeneration [Citation92] and of transformation and tumor growth by the nervous system [Citation93–Citation95] suggests that such studies may also have significant clinical relevance.
Author Contributions
CF and Ml jointly conceived of the ideas and wrote the paper.
Acknowledgments
We gratefully acknowledge support by an Allen Discovery Center award from the Paul G. Allen Frontiers Group (No. 12171), and the Templeton World Charity Foundation (No. TWCF0089/AB55).
Disclosure statement
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Nielsen C. Six major steps in animal evolution: are we derived sponge larvae?. Evol Devel. 2008;10:241–257.
- Funayama N. The stem cell system in demosponges: insights into the origin of somatic stem cells. Devel Growth Diff. 2010;52:1–14.
- Arendt D , Benito-Gutierrez E , Brunet T , et al. Gastric pouches and the mucociliary sole: setting the stage for nervous system evolution. Philos Trans Royal Soc B. 2015;370:20150286.
- Jauffred L , Vejborg RM , Korolev KS , et al. Chirality in microbial biofilms is mediated by close interactions between the cell surface and the substratum. Isme J. 2017;11:1688–1701.
- Miller DJ , Ball EE . Animal evolution: the enigmatic phylum Placozoa revisited. Curr Biol. 2005;15:R26–R28.
- Ereskovsky AV , Renard E , Borchiellini C . Cellular and molecular processes leading to embryo formation in sponges: evidences for high conservation of processes throughout animal evolution. Devel Genes Evol. 2013;223:5–22.
- Martindale MQ . The onset of regenerative properties in ctenophores. Curr Opin Genet Devel. 2016;40:113–119.
- Slack JMW . Animal regeneration: ancestral character or evolutionary novelty? EMBO Rep. 2017;18:1497–1508.
- Lai AG , Aboobaker AA . EvoRegen in animals: time to uncover deep conservation or convergence of adult stem cell evolution and regenerative processes. Dev Biol. 2018;433:118–131.
- Tiozzo S , Copley RR . Reconsidering regeneration in metazoans: an evo-devo approach. Front Ecol Evol. 2015;3:67.
- Elliott SA , Sánchez Alvarado A . The history and enduring contributions of planarians to the study of animal regeneration. WIRES Devel Biol. 2012;2:301–326.
- Rink JC . Stem cells, patterning and regeneration in planarians: self-organization at the organismal scale. In: Rink JC , editor. Planarian regeneration: methods and protocols (Methods in Molecular Biology). Vol. 1774. New York (NY): Springer; 2018. p. 57–172.
- Adamska M . Developmental signalling and emergence of animal multicellularity. In: Ruiz-Trillo I , Nedelcu AM , editors. Evolutionary transitions to multicellular life. Dordrecht: Springer; 2015. p. 425–450. DOI:10.1007/978-94-017-9642-2_20
- Niehrs C . On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857.
- Sebé-Pedrós A , Degnan BM , Ruiz-Trillo I . The origin of Metazoa: a unicellular perspective. Nat Rev Genet. 2017;18:498–512.
- Tweedt SM , Erwin DH . Origin of metazoan developmental toolkits and their expression in the fossil record. In: Ruiz-Trillo I , Nedelcu AM , editors. Evolutionary transitions to multicellular life. Dordrecht: Springer; 2015. p. 47–78. DOI:10.1007/978-94-017-9642-2_3
- Loh KM , van Amerongen R , Nusse R . Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Devel Cell. 2016;38:643–655.
- Martindale MQ , Hejnol A . A developmental perspective: changes in the position of the blastopore during bilaterian evolution. Devel Cell. 2009;17:162–174.
- Petersen CP , Reddien PW . Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068.
- Genikhovich G , Fried P , Prünster MM , et al. Axis patterning by BMPs: cnidarian network reveals evolutionary constraints. Cell Rep. 2015;10:1646–1654.
- He S , Del Viso F , Chen C-Y , et al. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science. 2018;361:1377–1380.
- Layden MJ , Rentzsch F , Röttinger E . The rise of the starlet sea anemone Nematostella vectensis as a model system to investigate development and regeneration. WIRES Devel Biol. 2016;5:408–428.
- Wijesena N , Simmons DK , Martindale MQ . Antagonistic BMP–cWNT signaling in the cnidarian Nematostella vectensis reveals insight into the evolution of mesoderm. Proc Natl Acad Sci USA. 2017;114:E5608–E5615.
- Neville AC . Animal asymmetry. London: Edward Arnold; 1976.
- Palmer AR . Animal asymmetry. Curr Biol. 2009;19:R473–R477.
- Gavilán B , Perea-Atienza E , Martínez P . Xenacoelomorpha: a case of independent nervous system centralization? Philos Trans Royal Soc B. 2016;371:20150039.
- Hejnol A , Rentzsch F . Neural nets. Curr Biol. 2015;25:R775–R792.
- Martínez P , Perea-Atienza E , Gavilán B , et al. The study of xenacoelomorph nervous systems: molecular and morphological perspectives. Invert Zool. 2017;14:32–44.
- Gruhl A , Okamura B . Development and myogenesis of the vermiform Buddenbrockia (Myxozoa) and implications for cnidarian body plan evolution. EvoDevo. 2012;3:10.
- Jiménez-Guri E , Philippe H , Okamura B , et al. Buddenbrockia is a cnidarian worm. Science. 2007;317:116–118.
- Oviedo NJ , Morokuma J , Walentek P , et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339:188–199.
- Iglesias M , Gomez-Skarmeta JL , Saló E , et al. Silencing of Smed-βcatenin1 generates radial-like hypercephalized planarians. Development. 2008;135:1215–1221.
- Erwin DH . Early metazoan life: divergence, environment and ecology. Philos Trans Royal Soc B. 2015;370:20150036.
- Jondelius U , Ruiz-Trillo I , Baguñà J , et al. The Nemertodermatida are basal bilaterians and not members of the Platyhelminthes. Zool Scripta. 2002;31:201–215.
- Giribet G . Assembling the lophotrochozoan (=spiralian) tree of life. Philos Trans Royal Soc B. 2008;363:1513–1522.
- Hejnol A , Obst M , Stamatakis A , et al. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc R Soc B. 2009;276:4261–4270.
- Dunn CW , Giribet G , Edgecombe GD , et al. Animal phylogeny and its evolutionary implications. Annu Rev Ecol Evol Syst. 2014;45:371–395.
- Cannon JT , Vellutini BC , Smith J III , et al. Xenacoelomorpha is the sister group to Nephrozoa. Nature. 2016;530:89–93.
- Egger B , Steinke D , Tarui H , et al. To be or not to be a flatworm: the Acoel controversy. PLoS ONE. 2009;4(5):e5502.
- Bouriat SJ , Hejnol A . Acoels. Curr Biol. 2009;19:R279–R280.
- De Mulder K , Kuales G , Willems M , et al. Characterization of the stem cell system of the acoel Isodiametra pulchra. BMC Devel Biol. 2009;9:69.
- Perea-Atienza E , Botta M , Salvenmoser W , et al. Posterior regeneration in Isodiametra pulchra (Acoela, Acoelomorpha). Front Zool. 2013;10:64.
- Gehrke AR , Neverett E , Luo Y-J , et al. Acoel genome reveals the regulatory landscape of whole-body regeneration. Science. 2019;363:eaau6173.
- Raz AA , Srivastava M , Salvamoser R , et al. Acoel regeneration mechanisms indicate an ancient role for muscle in regenerative patterning. Nat Comms. 2017;8:1260.
- Srivastava M , Mazza-Curll KL , van Wolfswinkel JC , et al. Whole-body acoel regeneration is controlled by Wnt and Bmp-Admp signaling. Curr Biol. 2014;24:1107–1113.
- Adell T , Martín-Durán JM , Saló E , et al. Platyhelminthes. In: Wanninger A , editor. Evolutionary developmental biology of invertebrates 2: lophotrochozoa (Spiralia). Wein: Springer; 2015. p. 21–40. DOI:10.1007/978-3-7091-1871-9_3
- Hejnol A . Acoelomorpha and Xenoturbellida. In: Wanninger A , editor. Evolutionary developmental biology of invertebrates 1: introduction, non-bilateria, acoelomorpha, xenoturbellida, chaetognatha. Wein: Springer; 2015. p. 203–214. DOI:10.1007/978-3-7091-1862-7_9
- Petralia RS , Mattson MP , Yao PJ . Aging and longevity in the simplest animals and the quest for immortality. Ageing Res Rev. 2014;16:66–82.
- Sköld HN , Obst M , Sköld M , et al. Stem cells in asexual reproduction of marine invertebrates. In: Rinkevich B , Matranga V , editors. Stem cells in marine organisms. Berlin: Springer; 2009. p. 105–137. DOI:10.1007/978-90-481-2767-2_5
- Lively CM . A review of Red Queen models for the persistence of obligate sexual reproduction. J Hered. 2010;101:S13–S20.
- Maldonado M , Riesgo A . Reproduction in the phylum Porifera: a synoptic overview. Treb Soc Catalana Biol. 2008;59:29–49.
- Watanabe H , Hoang VT , Mättner R , et al. Immortality and the base of multicellular life: lessons from cnidarian stem cells. Sem Cell Devel Biol. 2009;20:1114–1125.
- Solana J . Closing the circle of germline and stem cells: the primordial stem cell hypothesis. EvoDevo. 2013;4:2.
- Vila-Farré M , Rink JC . The ecology of freshwater planarians. In: Rink JC , editor. Planarian regeneration: methods and protocols. New York (NY): Humana; 2018. p. 173–205. DOI:10.1007/978-1-4939-7802-1_3
- Hoshi M , Kobayashi K , Arioka S , et al. Switch from asexual to sexual reproduction in the planarian Dugesia ryukyuensis. Integr Comp Biol. 2003;43:242–246.
- Kobayashi K , Koyanagi R , Matsumoto M , et al. Switching from asexual to sexual reproduction in the planarian Dugesia ryukyuensis: bioassay system and basic description of sexualizing process. Zool Sci. 1999;16:291–298.
- Fields C , Levin M . Are planaria individuals? What regenerative biology is telling us about the nature of multicellularity. Evol Biol. 2018;45:237–247.
- Newmark PA , Sánchez Alvarado A . Not your father’s planarian: a classic model enters the era of functional genomics. Nat Rev Genet. 2002;3:210–219.
- Malinowski PT , Cochet-Escartin O , Kaj KJ , et al. Mechanics dictate where and how freshwater planarians fission. Proc Natl Acad Sci USA. 2017;114:10888–10893.
- Arnold CP , Bentham-Pyle BW , Lange JJ , et al. Wnt and TGFβ coordinate growth and patterning to regulate size-dependent behaviour. Nature. 2019;572:655–659.
- Lobo D , Beane WS , Levin M . Modeling planarian regeneration: a primer for reverse-engineering the worm. PLoS Comp Biol. 2012;8:ee1002481.
- Lobo D , Levin M . Inferring regulatory networks from experimental morphological phenotypes: a computational method reverse-engineers planarian regeneration. PLoS Comp Biol. 2015;11:e1004295.
- Owlarn S , Bartscherer K . Go ahead, grow a head! A planarian’s guide to anterior regeneration. Regeneration. 2016;3:139–155.
- Petersen CP , Reddien PW . Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science. 2011;332:852–855.
- Pietak A , Bischof J , LaPalme J , et al. Neural control of body-plan axis in regenerating planaria. PLoS Comp Biol. 2019;15:e1006904.
- Durant F , Bischof J , Fields C , et al. The role of early bioelectric signals in the regeneration of planarian anterior/posterior polarity. Biophys J. 2019;116:948–961.
- Yazawa S , Umesono Y , Hayashi T , et al. Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci USA. 2009;106:22329–22334.
- Fraguas S , Barberán S , Iglesias M , et al. egr-4, a target of EGFR signaling, is required for the formation of the brain primordia and head regeneration in planarians. Development. 2014;141:1835–1847.
- Iglesias M , Almuedo-Castillo M , Aboobaker AA , et al. Early planarian brain regeneration is independent of blastema polarity mediated by the Wnt/β-catenin pathway. Devel Biol. 2011;358:68–78.
- Gurley KA , Rink JC , Sánchez Alvarado A . Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327.
- Petersen CP , Reddien PW . Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330.
- Bischof J , Day ME , Miller KA , et al. Nervous system and tissue polarity dynamically adapt to new morphologies in planaria. Preprint bioRXiv:815688. 2019. DOI:10.1101/815688
- Durant F , Morokuma J , Fields C , et al. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys J. 2017;112:2231–2243.
- Sikes JM , Bely AE . Making heads from tails: development of a reversed anterior-posterior axis during budding in an acoel. Devel Biol. 2010;338:86–97.
- Müller WA . Head formation at the basal end and mirror-image pattern duplication in Hydra vulgaris . Int J Devel Biol. 1996;40: 1119–1141. PMID: 9032017.
- Braun E , Ori H . Electric-induced reversal of morphogenesis in Hydra. Biophys J. 2019;117:1514–1523.
- Watanable H . Back through time: how cnidarians and basal metazoans shed light on ancient nervous systems. In: Shigeno S , et al., editor. brain evolution by design, diversity and commonality in animals. Tokyo: Springer; 2017. p. 45–75. DOI:10.1007/978-4-431-56469-0_3
- Sluys R , Riutort M . Planarian diversity and phylogeny. In: Rink JC , editor. Planarian regeneration: methods and protocols (Methods in Molecular Biology). Vol. 1774. New York (NY): Springer; 2018. p. 1–56.
- Feuda R , Dohrmann M , Pett W , et al. Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. Curr Biol. 2017;37:3864–3870.
- Simion P , Philippe H , Baurain D , et al. A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr Biol. 2017;27:958–967.
- Whelan NV , Kocot KM , Moroz TP , et al. Ctenophore relationships and their placement as the sister group to all other animals. Nat Evol Ecol. 2017;1:1737–1746.
- Hanschen ER , Shelton DE , Michod RE . Evolutionary transitions in individuality and recent models of multicellularity. In: Ruiz-Trillo I , Nedelcu AM , editors. Evolutionary transitions to multicellular life. Dordrecht: Springer; 2015. p. 165–188. DOI:10.1007/978-94-017-9642-2_9
- Fields C , Levin M . Somatic multicellularity as a satisficing solution to the prediction-error minimization problem. Commun Integr Biol 2019;12:119–132.
- Morris HR , Masento MS , Taylor GW , et al. Structure elucidation of two differentiation inducing factors (DIF-2 and DIF-3) from the cellular slime mould Dictyostelium discoideum. Biochem J. 1988;249:903–906.
- Morris HR , Taylor GW , Masento MS , et al. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature. 1987;328:811–814.
- Fields C , Bischof J , Levin M . Morphological coordination: a common ancestral function unifying neural and non-neural signaling. Physiology. 2020;35:16–30.
- Moroz LL , Kohn AB . Independent origins of neurons and synapses: insights from ctenophores. Philos Trans R Soc Lond B. 2016;371:20150041.
- Herrera-Rincon C , Levin M . Booting up the organism during development: prebehavioral functions of the vertebrate brain in guiding body morphogenesis. Commun Integr Biol. 2018;11:e1433440.
- Herrera-Rincon C , Pai VP , Moran KM , et al. The brain is required for normal muscle and nerve patterning during early Xenopus development. Nat Commun. 2017;8:587.
- Schierwater B , DeSalle R . Placozoa. Curr Biol. 2017;28:R97–R98.
- Nickel M . Evolutionary emergence of synaptic nervous systems: what can we learn from the non-synaptic, nerveless Porifera? Invert Biol. 2010;129:1–16.
- Kumar A , Brockes JP . Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012;35:691–699.
- Boilly B , Faulkner S , Jobling P , et al. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–354.
- Kuol N , Stojanovska L , Apostolopoulos V , et al. Role of the nervous system in cancer metastasis. J Exp Clin Cancer Res. 2018;37:5.
- Pawlowski A , Weddell G . Induction of tumors in denervated skin. Nature. 1967;213:1234–1236.
