ABSTRACT
The Dead Sea is unique compared to other extreme halophilic habitats. Its salinity exceeds 34%, and it is getting saltier. The Dead Sea environment is characterized by a dominance of divalent cations, with magnesium chloride (MgCl2) levels approaching the predicted 2.3 M upper limit for life, an acidic pH of 6.0, and high levels of absorbed ultraviolet radiation. Consequently, only organisms adapted to such a polyextreme environment can survive in the surface, sinkholes, sediments, muds, and underwater springs of the Dead Sea. Metagenomic sequence analysis and amino acid profiling indicated that the Dead Sea is predominantly composed of halophiles that have various adaptation mechanisms and produce metabolites that can be utilized for biotechnological purposes. A variety of products have been obtained from halophilic microorganisms isolated from the Dead Sea, such as antimicrobials, bioplastics, biofuels, extremozymes, retinal proteins, colored pigments, exopolysaccharides, and compatible solutes. These resources find applications in agriculture, food, biofuel production, industry, and bioremediation for the detoxification of wastewater and soil. Utilizing halophiles as a bioprocessing platform offers advantages such as reduced energy consumption, decreased freshwater demand, minimized capital investment, and continuous production.
1. Introduction
The Dead Sea, located 430 meters below sea level, is the lowest point on Earth [Citation1]. It is characterized by high salinity of 34.2% [Citation1]. The Dead Sea is named after the absence of any macroscopic living organisms [Citation2]. Its inhospitality toward higher organisms has been recognized since ancient times. A notable depiction is seen in the Madaba mosaic from the sixth century, which shows a map of the Dead Sea with two fish being washed into it and one dying, while the other struggles to return northward to the Jordan River [Citation3]. The Dead Sea consists of a deeper northern basin and a shallow southern basin (), which is dried and used for commercial mineral production [Citation4].
Figure 1. The Dead Sea as seen by satellite image in 2019 (https://earthobservatory.nasa.gov/images/145373/getting-saltier).
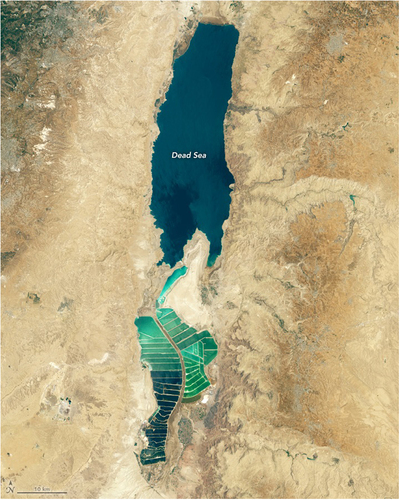
The water level of the Dead Sea relies on a balance between the influx of freshwater and the process of evaporation. The Jordan River serves as the primary source of freshwater inflow, complemented by numerous water springs and an intricate network of subterranean springs [Citation5]. The diversion of the Jordan River, the construction of numerous dams on side wadis, and the increasing aridification trend in the Eastern Mediterranean in recent years have further worsened the water balance of the Dead Sea [Citation4]. Consequently, water budget of the Dead Sea is negative, and its level has dropped by an average of 1 m per year during the past decade. The water is supersaturated with sodium, and massive amounts of halite (NaCl) precipitate to the bottom [Citation6]. Moreover, the water balance of the Dead Sea is significantly impacted by the commercial operations located at its southern end, where substantial amounts of brine are extracted for the production of potash and other byproducts. On average, approximately 550 million cubic meters of brine are withdrawn annually, with about 45% to 50% of this volume returning to the lake as highly concentrated end-brine [Citation4]. These extreme salinity conditions significantly impact biodiversity by imposing robust selective pressure, leading to the emergence of halophilic and halotolerant microbes [Citation7]. The Dead Sea is becoming a progressively more extreme environment for microorganisms owing to continual evaporation, exposure to UV radiation, elevated temperatures, and high salinity. The salinity of the Dead Sea is anticipated to rise in the coming years owing to reduced district precipitation and increased evaporation [Citation8].
The Dead Sea exhibits exceptionally high magnesium and calcium concentrations that continue to increase. The distinctive composition of Dead Sea water is marked by a unique equilibrium, where divalent cations (2.17 M Mg2+ and 0.525 M Ca2+) dominate over monovalent cations (1.53 M Na+ and 0.227 M K+). The anions consist mainly of Cl– (7.26 M) with a minor presence of 1% Br– (0.071 M) [Citation9]. The ongoing trend of declining water levels and halite precipitation persists. The diminishing of sodium concentrations leads to a rapid increase in the ratio between chaotropic ions that interfere with water structure and kosmotropic ions that promote water structuring. Calcium and magnesium ions are considered chaotropic ions because they reduce the order of water molecules and interfere with the hydrogen bonding network. In addition, they destabilize the structure of biomolecules such as proteins and nucleic acids [Citation10]. Dead Sea brine is enriched in MgCl2 due to the precipitation of NaCl, with levels approaching the predicted 2.3 M upper limit for life. This makes the Dead Sea inhospitable to microbial growth or metabolism [Citation11].
Over the last several decades, the surface water salinity of the Dead Sea has risen, leading it to become holomictic and start precipitating halite (NaCl) in 1980 [Citation12]. The observation of halite precipitation in the Dead Sea despite a seemingly sub-saturation concentration of Na+ ions can be explained by the influence of the common ion effect on solution equilibria. Natural brines like the Dead Sea contain a complex mixture of dissolved salts. Many of these salts, such as KCl, MgCl2, and CaCl2, dissociate in solution to release Cl− ions. The presence of these common Cl− ions from other sources effectively reduces the availability of free Cl− ions to solvate Na+ ions. With a decreased availability of solvating Cl− ions, Na+ ions experience a stronger electrostatic attraction to each other, promoting their aggregation and precipitation as halite crystals. This phenomenon occurs even if the total concentration of Na+ in the Dead Sea is lower than the saturation concentration for NaCl in a pure solution [Citation13].
Since 1980, the Dead Sea has experienced seasonal stratification, with a warmer epilimnion during the entire eight months of the summer and a vertically mixed water column during winter [Citation12]. This phenomenon resulted in the formation of two distinct water masses within the lake. In the upper layer, extending to approximately 40 meters, temperatures ranged from 19 to 37°C and the water contained high levels of sulfates and bicarbonates. The water below the transitional zone between 140 and 100 m was consistently 22°C in temperature and showed a greater salinity [Citation14]. This deeper layer surrounded by hydrogen sulfide, along with prominent concentrations of Mg2+, K+, Clˉ, and Brˉ. At the bottom of the water, salt builds up because of the saturation of deep water with NaCl. The challenges of population dynamics and limiting factors for microorganisms become apparent when the southern part of the Dead Sea, particularly the shallower section, undergoes evaporation. This process initiates vertical mixing, extending down to the bottom layer, and promoting aeration. The occurrence of hydrogen sulfide in contact with the bottom water of the Dead Sea serves as an environmental stressor for the ecosystem [Citation15].
Salinity, ionic composition, and pH play critical roles in determining the microbial population in hypersaline brines. The pH of the Dead Sea brine is around 6.0, making it acidic [Citation16]. The concentration of Ca2+ (and to a minor level Mg2+) is pivotal for verifying the ultimate pH of the brine. Equilibrium involving CO3 2-, HCO3-, and dissolved CO2 represents a principal buffer system in aquatic environments. The presence of Ca2+, which leads to the precipitation of insoluble calcite (CaCO3) and the removal of CO3 2-, significantly influences this equilibrium. In addition, Mg2+ levels affect the system by removing CO3 2- as dolomite (CaMg(CO3)2) [Citation17].
Metagenomic sequence analysis and amino acid profiling indicate that the modern, vanishing Dead Sea is predominantly composed of halophiles that have never been previously isolated or sequenced [Citation18]. Under these extreme conditions, microorganisms adapt to the unique ecosystem of the Dead Sea. This review presents an up-to-date analysis of microbial populations found across diverse environments within the Dead Sea, encompassing surface water, underwater springs, sinkholes, and mud. Furthermore, it examines, in detail, the potential biotechnological uses of these microbial communities, providing perspectives on how these applications might address prevailing challenges in the future.
2. Microbial diversity in the Dead Sea
In the three domains of life -Archaea, Bacteria, and Eukarya- both halophilic and halotolerant microorganisms exist. The primary component of microbial biomass in the Dead Sea is aerobic halophilic archaea (). Under anaerobic conditions, methanogens and halophilic members of the methanogenic branch of Euryarchaeota can thrive at salt concentrations near saturation [Citation19]. Halophiles are rare in the Eukarya domain, with the green alga Dunaliella being the most notable example. Dunaliella is halotolerant rather than truly halophilic and can grow at relatively low salt concentrations. The Bacteria domain encompasses a wide variety of halophilic and halotolerant bacteria across many evolutionary groups, including Cyanobacteria and the Flavobacterium branch [Citation20]. Different branches of Proteobacteria contain halophilic and non-halophilic microorganisms. In general, it may be stated that most halophiles within the domain Bacteria are moderate rather than extreme halophiles. However, there are few types that resemble the archaeal halophiles of the family Halobacteriaceae regarding their tolerance and need for salt, notably several photosynthetic purple bacteria of the genus Halorhodospira and Actinomycetes [Citation21].
Among the various factors studied, salinity emerged as the primary environmental factor influencing microbial diversity. Other factors, including exposure to high and low temperatures, low oxygen conditions, and in some cases, low pH, also had important effects to some extent. Salinity and microbial diversity indices showed a strong negative correlation, highlighting the enormous restriction that salinity places on the microbial diversity. Under these conditions, the most widespread species are bacteria and archaea [Citation22].
Based on the optimal salt concentration at which they exhibit the best growth, Kushner and Kamekura’s (1988) classification divides halophilic microorganisms into three types: slight halophiles (marine bacteria), which grow best in media containing 1–3% NaCl; moderate halophiles, which grow best in media containing 3–15% NaCl; and extreme halophiles, which grow best in media containing 15–30% NaCl. Both halophilic and halotolerant bacteria are found in three domains: Archaea, Bacteria, and Eukarya [Citation23].
Despite the highly hypersaline conditions, the Dead Sea boasts a distinctive ecosystem that accommodates a diverse array of halophilic microorganisms that are well suited to its surroundings. Archaea emerged as the predominant group, comprising 52% of the sequences, while bacteria constituted 45%. Collectively, prokaryotic sequences, constituting 97% of the total, were found to be prevalent [Citation22]. Two major phyla, Proteobacteria (55.9%) and Firmicutes (41.7%), were the predominant bacterial groups, whereas other phyla such as Bacteroidetes, Actinobacteria, and Cyanobacteria were present, but in relatively minor abundance. The study revealed that Acinetobacter (45%) and Bacillus (35%) were the dominant bacterial genera. The remaining genera collectively represent only 10% of the total bacterial population. Among the Archaea, the predominant genera were Halorhabdus, constituting 52% of the archaeal community; Natronomonas (12%), Halobellus (4%), Haloplanus (4%), Halobacterium (3%), Halomicrobium (3%), Halogranum (3%), Halorubrum (3%), Halorientalis (2%), and Halomarina (2%). Bacteria constituted a substantial proportion, accounting for 45% of the microbial community. Firmicutes (42%) and Proteobacteria (56%) were the two most abundant bacterial phyla. Other phyla with lower proportions were also found, including Spirochaetes (0.01%), Cyanobacteria (0.08%), and Bacteroidetes (1%) [Citation22].
Historically, only a few phenotypic or documented morphological features have been used to support the taxonomy of halophilic bacteria found in the Dead Sea water at various depths and salinities. Molecular phylogeny, which includes 16S rRNA gene analysis, amplified ribosomal DNA restriction analysis (ARDRA), and random amplified polymorphic DNA (RAPD) to identify and analyze the biodiversity of bacterial strains, has been made available with great assistance for the analysis of microbial populations [Citation24]. The small subunit rRNA gene is extensively used in phylogenetic tree reconstruction among bacteria because of its ubiquitous presence, manageable size for easy sequencing, and accessibility to a significant database. Additionally, the highly variable length that varies between species flanks the highly conserved portions of rRNA that are essential for structure and function [Citation25].
In the Dead Sea ecosystem, bacteria and archaea are the most extensively spread creatures. Though there are relatively few species that have been identified, the overall biomass of approximately 105 bacteria and 104 algae cells per milliliter is extremely high. Phylogenetic analysis confirmed that halophilic archaeobacteria and eubacteria belong to several evolutionary branches. While most of these bacteria are eubacteria, archaeobacteria, which are also composed of mild and moderately halophilic bacteria, are commonly used to represent halophilic bacteria [Citation24]. In addition to bacteria and archaea, six distinct fungal species were recovered from the Dead Sea: Aspergillus, versicolor, Chaetomium globosum, Hortaea werneckii, Aureobasidium Pullulans, Eurotium spp., and Gymnascella spp. However, Chaetomium globosum and Aspergillus versicolor are the most prevalent genera [Citation26]. Buchalo et al., (1998) reported the occurrence of filamentous fungi in Dead Sea water belonging to the phylum Ascomycota and Deuteromycota. These include Gymnascella marismortui, Ulocladium chlamydosporum and Penicillium westlingii [Citation27].
In saltwater, microbial activity is predominantly governed by salinity, which is driven by energy constraints. Crenarchaeota (Thermoproteota) was consistently detected in the sediment layer and was mostly ubiquitous in zones with low to moderate salinity levels. Most of these microorganisms are anaerobic heterotrophs that use proteins and sugars for a variety of biochemical and physiological functions, and some even participate in the sulfur geochemical cycle (oxidation and reduction) [Citation28]. The anaerobic methanotrophic group within Halobacterota, specifically the family Methanophagaceae, is an anaerobic microorganisms that metabolize methane as their source of carbon and chemical energy. These taxa exhibit adaptability to a broad temperature range and thrive in high-salt environments [Citation29].
Salinization and desalination of lakes are growing environmental concerns caused by water alterations, global climate change, undesired freshening, and drying. These changes can significantly shape the community composition of microorganisms [Citation30,Citation31]. Measuring the presence and activity of microorganisms becomes challenging when halite crystals form on the water column of the Dead Sea during a drop in its level. However, it is reasonable to think that microorganisms in different hypersaline lakes might exhibit distinct responses to fluctuations in salinity levels. Microbes have to cope with increased or decreased osmotic pressure by adjusting their physiology and morphology [Citation32]. Salinity significantly influences the diversity of microorganisms present, the composition of communities, and the roles they play. A reduction in microbial diversity due to salinity fluctuations could result in the development of a transitional microbial group with alterations in both taxonomic and functional components [Citation33]. Due to increasing salinity, ultimately numerous halophilic microorganisms will eventually die owing to osmotic stress [Citation34], resulting in a decrease in microbial diversity [Citation35]. Nonetheless, halophilic organisms typically do not display fast, unpredictable, and extreme changes when exposed to a salinity stressor. Diatoms have been shown to respond better to short-term rapid salinity fluctuations, while Cyanobacteria better tolerate long-term exposure to undiluted Dead Sea water [Citation36]. Microorganisms with adaptable strategies are chosen through repeated, abrupt, and fluctuating alterations in salinity [Citation37].
As demonstrated in , extremely halophilic bacteria flourish in the mud layer of the Dead Sea. Archaeal genera such as Halobaculum and Halomicroarcula were discovered in the deepest parts and within the mud, respectively. Additionally, Dimastigamoeba was isolated from the Dead Sea mud, along with the green alga Dunaliella viridis, which was cultivated from a mud sample [Citation46]. The majority of them require natural brines for in vitro culture on agar plates, coupled with a range of other nutrients such as milk or fish extract for growth. A few of them also require complex nutrients such as yeast extract for survival. In contrast, moderate halophiles that display a surface water layer build large amounts of certain organic osmolytes in the cytoplasm, which serve as osmoprotectants and maintain osmotic balance without interfering with the cell’s regular metabolism [Citation47,Citation48]. Laboratory analysis of 24 mud samples collected from three locations on the eastern shore of the Dead Sea was performed to identify the physical and chemical factors that affect bacterial growth [Citation49]. Chemical analysis of the mud samples revealed high CaO (20.61–27.86 wt. %), CO2 (15.47–25.01 wt.%), and SiO2 (23.74–33.66 wt.%), while the total soluble salts, chlorides, and sulfates were 10.19, 4.48, and 0.056 wt.%, respectively. Under such conditions, extremely halophilic bacteria proliferate remarkably slowly [Citation49].
Table 1. Microbial diversity of the Dead Sea surface water, mud, sediments, underwater springs and sinkholes.
The extreme halophiles proliferate incredibly slowly in the lab or even fail to grow [Citation50]. Different strategies were employed to increase the growth rate of halophilic archaea for future applications such as optimization nutrient supply (e.g., carbon and nitrogen sources) and adjusting environmental conditions (e.g., salt concentration, temperature, pH). Using bile acid-free peptone or alternative media components is essential, as some commercial peptone preparations may contain bile acids that can lyse haloarchaeal cells. Certain archaea are highly sensitive to contaminants so that high-quality agar specifically designed for culturing halophiles or washing regular agar to remove inhibitors is crucial. Also, many detergents commonly used in lab cleaning can harm haloarchaea. To avoid this, a thorough rinse with distilled water after washing all glassware is essential [Citation51]. Recent advances in molecular biology have helped us understand the metabolic capabilities encoded in archaeal genomes. This knowledge is proving invaluable in efforts to cultivate more archaea [Citation52]. Multiple innovative cultivation techniques for archaea were developed such as co-culturing archaea with bacteria or other archaea which may have mutual benefits to the interacting partners [Citation53]. Another strategy was developed based on recent understanding of direct interspecies electron transfer (DIET) phenomenon. This technique utilized soluble artificial oxidants to sustain the growth of anaerobic methanotrophic archaea by decoupling archaeal methane oxidation from sulfate reduction [Citation54]. Other methods for culturing archaea utilized single-cell isolation [Citation55], high throughput culturing (HTC) [Citation56], and simulation of the natural habitat [Citation57].
Several microorganisms found in the Dead Sea have distinct characteristics. The green microalga Dunaliella, which possesses a large cup-shaped chloroplast and no stiff cell wall, has an extremely high intracellular concentration of glycerol (up to 2.1 M) [Citation58]. Halobacterium spp. exhibited remarkable selectivity for K+ and an exceptionally high intercellular K+ concentration (up to 4.8 M). Obligate halophilic bacteria, including the pleomorphic Halobacterium spp., and the green alga Dunaliella, make up the majority of the indigenous flora of the Dead Sea. In contrast, archaea are widely distributed throughout the water column and occupy various slots including mud [Citation2], sediment [Citation43,Citation59], and surface water [Citation22] of the Dead Sea. The archaeal communities were ubiquitous in the low, moderately, and high saline niches [Citation2,Citation43]. In the sediments, aerobic (Haloferax volcanii, Haloplanus natans, Halorubrum sodomense, Haoarcula marismortui, Halobaculum gomorrense) and anaerobic (Halobacteroides halobius, Sporohalobacter lortetii, Orenia marismortui, Selenihalanaerobacter shriftii) heterotrophic archaea primarily utilize carbohydrates and proteins for a range of physiological and metabolic processes [Citation43].
Microorganism blooms have sometimes been seen in the magnesium- and calcium-rich waters of the Dead Sea. Dense populations of extremely halophilic Archaea (from the family Halobacteriaceae) and the alga Dunaliella salina frequently give salt-saturated brines a red hue [Citation60]. In the past, dense colonies of halobacteria and Dunaliella were formed when the water column becomes stratified due to the entry of freshwater during winter floods and the availability of phosphate [Citation33]. As long as the water column stays stratified, dense bacterial colonies can persist for long periods of time.
The biomass in the top water layers is significantly reduced by an overturn of the water column until the environment is favorable for the growth of an algal and bacterial bloom. The microbial population observed during the algal bloom and the resident community that occurs during the inter-bloom interval differ significantly, according to previous studies. There has never been a period when all haloarchaea live on the Dead Sea’s surface. As a result, it is difficult to distinguish between the environmental and interbloom population components of haloarchaeal blooms. Microbial blooms, which are primarily composed of the unicellular green alga Dunaliella and the extremely halophilic archaea, only occur when the upper layers of the water column are significantly diluted after rainfall. Dead Sea water turned red in 1992 as a result of dilution levels as high as 70% in the top 5 m of the water column, which supported archaea concentrations as high as 3.5 × 107 mL−1. Dunaliella was no longer observed in the Dead Sea after 1996 [Citation40]. Owing to its extraordinary resilience to a variety of environmental stressors, especially those associated with hypersaline environments, Dunaliella has garnered a great deal of interest. Glycerol buildup, which acts as an osmoregulator, is the main mechanism for halotolerance [Citation61].
Knowledge holds that the Dead Sea itself contains freshwater drawn from freshwater springs that can be found at the bottom of the Sea. Green sulfur bacteria, cyanobacteria, and single-celled algae are among the additional microorganisms found close to these springs. Nearly 80 species of fungi are among the additional microorganisms discovered at the bottom of the Dead Sea’s bottom [Citation45]. The water flow in these undersea springs is characterized by sudden changes in both amplitude and frequency [Citation62]. The freshwater flow from the springs determines the salinity of the surrounding water by determining the distance between the water mixes and sediment. The severe and rapid variations in the flow cause the ambient biofilm salinity and other physicochemical parameters, such as pH and O2 concentration, to fluctuate greatly. A more thorough investigation found that the microbial communities surrounding these springs were significantly more diverse and had higher cell densities. Dense biofilms are also present, coating the rocks and sediments surrounding springs. Sulfate reducers, nitrifiers, iron oxidizers, and iron reducers bacteria were discovered together with their sequences based on comparative investigations of the community structure and geochemical reconstruction of spring water sources. Sulfide oxidation via chemolithotrophy and phototrophy is particularly significant. The water chemistry analysis revealed signs of microbial activity along the route, indicating that the springs provide the microbial communities of the Dead Sea with organic matter, phosphate, and nitrogen. Phosphates and carbonates are the most common accessory minerals from which elements are typically liberated in groundwater systems [Citation63].
3. Seasonal changes in microbial community of the Dead Sea
The Dead Sea, among other extreme environments, provides a special chance to study how microorganisms adapt to harsh conditions through their diversity. The physicochemical properties of the Dead Sea are greatly affected by seasonal changes, which in turn impact the diversity and composition of microbial communities. These variations are influenced by different environmental conditions that exist all year long. Between summer and winter, the Dead Sea experiences considerable temperature fluctuations. Extremely halophilic archaea, which can withstand and even flourish in hypersaline conditions, predominate the microbial community during summertime periods of peak salinity [Citation64]. During the winter months, a mild decrease in salinity leads to a slight increase in bacterial diversity, including those that are somewhat halophilic and less resistant to harsh conditions. Increased rainfall and river flow bring more freshwater into the Dead Sea, causing a temporary drop in salinity. This reduction in salinity facilitates the proliferation of various microbial species. The influx of freshwater and lower temperatures allows bacteria that are less resistant to extreme salinity to thrive. Notably, genera such as Salinibacter and certain halophilic Cyanobacteria have been observed to flourish during these periods [Citation65]. Advanced molecular techniques, including metagenomics and 16S rRNA sequencing, have been employed recently to gain a comprehensive understanding of the seasonal changes in the microbial community of the Dead Sea. Through this studies, it has been discovered that although the core community of halophiles remains relatively consistent, there are notable variations in the proportional representation of specific taxa [Citation37]. In the period of heavy rainfall, which usually spans from November to March, the amount of freshwater flowing into the Dead Sea basin can notably rise.
Biological monitoring started in 1980 has shown that only after heavy rainfall in the winter, blooms of the single-celled green alga Dunaliella and salt-loving Archaea from the family Halobacteriaceae can exist in the lake. The summer heat is unbearable, especially in August, the lake’s waters frequently produce a dense mist above the lake due to evaporation. Evaporation tends to rise during the Spring and early summer months, reaching its highest point in May, whereas the impact of winter on the change is minimal [Citation66]. Surveys conducted in winter and summer revealed varying abundant populations of heterotrophic bacterial community members in each season. The most notable difference between the two seasons was the prevalence of Actinobacteria (specifically Modestobacter spp.) in summer samples, compared to the dominance of Flavobacteria in winter months. During the analysis of the complete population, only a small number of organisms identified as Nitriliruptor spp., Modestobacter spp., and Ottowia spp. were regularly detected throughout all seasons [Citation67].
4. Biotechnological applications
4.1. Industrial uses of products isolated from Dead Sea microbes
4.1.1. Hydrolytic enzymes
Halophilic hydrolytic enzymes from Dead Sea microbes are valuable in industrial processes that require high salt concentrations. These enzymes include amylases, cellulases, lipases, elastase, malate dehydrogenase and proteases () and can be used in the food industry, leather processing, and the production of biofuels.
Table 2. Products of microbes isolated from the Dead Sea having potential for industrial applications.
Amylases have significant biotechnological applications in the food industry for producing high-fructose syrups and as anti-staling agents in baking. They enhance detergent formulations by breaking down starchy stains and are used in textile and paper industries for fabric desizing and paper coating [Citation83]. Additionally, amylases are utilized in pharmaceuticals for drug formulation and in biofuel production by hydrolyzing starch into fermentable sugars. Their efficiency and specificity make them invaluable across various sectors [Citation84].
Bacillus spp. isolates obtained from Dead Sea mud possessed amylase activity [Citation68]. Thermo-stable amylase activity was reported from Bacillus strain HUTBS62 isolated from hot springs near the Dead Sea. The optimum pH and temperature for its catalytic activity was pH 4.4 and 90°C, respectively. The purified amylase demonstrated stability in acidic and high-temperature conditions, possessing unique properties that make it ideal for various industrial food applications [Citation69]. In another study, thermo-stable amyloglucosidase was extracted from Halobacterium sodomense with a temperature optimum of around 65°C in the presence of 1.4 M NaCl, and around 75°C in the presence of 3.9 M NaCl. The enzyme required salt concentrations higher than 1 M for optimal activity [Citation70].
Cellulases, enzymes that break down cellulose, are pivotal in numerous biotechnological applications. They are extensively used in biofuel production by converting lignocellulosic biomass into fermentable sugars for bioethanol, addressing the growing demand for sustainable energy sources. Additionally, cellulases have applications in the textile industry for fabric care, in the paper and pulp industry for improving paper quality, and in agriculture for enhancing soil quality [Citation85]. Their role in the food industry includes clarifying fruit juices and extracting essential oils. Recent advances focus on optimizing microbial cellulase production to enhance efficiency and cost-effectiveness in these industries [Citation86]. Cellulase was extracted from the archeon Halomicroarcula pellucida strain GUMF5, inhabiting Dead Sea sediments. Maximum cellulase activity of 17.7 U/ml was seen at 20% (w/v) NaCl, at pH 7 and at 40°C [Citation71].
Malate dehydrogenase finds biotechnological uses in diagnostic assays, biocatalysis for compound synthesis, metabolic engineering for increased metabolite production, and biofuel enhancement. Also, it aids bioremediation by metabolizing organic pollutants, contributes to drug discovery as a target for inhibitors, and plays a role in environmental and pharmaceutical advancements [Citation87]. When halophilic and nonhalophilic malate dehydrogenases were compared they were having similar molecular weights and composed of the same number (two) of subunits. However, halophilic malate dehydrogenase have excess of negatively charged groups [Citation88]. From the extreme halophilic bacterium Salinibacter ruber a malate dehydrogenase was extracted. Unlike most other halophilic enzymes, which unfold when exposed to low salt concentrations, S. ruber malate dehydrogenase remains completely stable in the absence of salt. Additionally, its amino acid composition lacks the pronounced acidic characteristics typical of halophilic proteins [Citation73]. In a second study, the gene coding for the enzyme malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui was successfully cloned in Escherichia coli [Citation89].
Lipases are utilized in biotechnology for biodiesel production, leather, textile, pharmaceuticals, medicals and food processing to modify lipid content. They also find applications in detergent formulation for stain removal and wastewater treatment for fat degradation [Citation90]. Lipase activity was detected in Bacillus spp. isolates [Citation68]. Further investigation and characterization of their stability and activity is needed.
Microbial proteases are particularly favored over plant and animal sources because microorganisms offer faster growth, higher production efficiency, greater diversity, longer shelf life, and ease of genetic manipulation. These advantages make microbial proteases highly suitable for industrial use [Citation91]. Extracellular serine protease was isolated from Halobacterium halobium with extraction yield ranging from 35–49%. The enzyme activity was completely lost when NaCl concentration fell below 2 M [Citation74]. Halophilic extracellular proteases, like those optimized for 4.3 M NaCl, find applications in detergent formulation, leather processing, food fermentation, bioremediation, and pharmaceutical production due to their stability in saline conditions [Citation92].
Enzymes derived from halophiles exhibit exceptional stability at saturated salt concentrations, which opens up the possibility of novel applications [Citation43]. This stability lowers production costs and improves efficiency. In the Dead Sea, Bacillus spp., which constitutes 35% of the bacterial genera, generate substantial quantities of enzymes that have applications in various industries [Citation22]. Furthermore, halophilic bacteria and archaea provide extremozymes and stable genetic elements for genetic engineering. Their enzymes, such as DNA polymerases, function efficiently in high-salt conditions, facilitating polymerase chain reaction (PCR) and other molecular biology techniques. Additionally, unique promoters and regulatory elements from these organisms enable gene expression in extreme environments, expanding the toolkit for synthetic biology. These properties are crucial for developing robust biotechnological applications in fields requiring high-salt tolerance [Citation93,Citation94].
4.1.2. Biodegradable plastics: Polyhydroxyalkanoates (PHAs)
PHA(s) constitute a category of biodegradable polyesters derived from (R)-hydroxyalkanoates such as polyhydroxy butyrate (PHB). These biopolymers, which are insoluble in water, are synthesized and stored by a diverse range of bacteria and haloarchaea in situations where there is an excess of carbon sources, but limitations in other essential nutrients. The growing interest in PHA(s) stems from their biodegradability, biocompatibility, and thermoplastic characteristics, positioning them as promising alternatives to plastics derived from petrochemicals. They find potential applications in various fields, such as packaging and biomedical materials, nonwoven fabrics, and as agents for flavor delivery [Citation75]. PHA constitute a group of intracellular polymers stored as reserves of energy and carbon by various archaea and bacteria. Extreme halophilic microorganisms, which flourish in environments with high salt concentrations, are regarded as potential candidates for the economically viable large-scale production of PHA [Citation95,Citation96]. Production of bioplastic PHA from natural sources is one of the many steps required to respond to planet threats and challenges, including the accumulation of non-biodegradable plastics [Citation76].
Haloarchaea are employed as cell factories for synthesizing PHAs directly from inexpensive raw materials, owing to their metabolic capabilities [Citation97]. The class Halobacteria within the phylum Euryarchaeota is known for thriving in halophilic environments. Among these, only 15 genera, including Haloferax, Natrinema, Haloarcula, Halococcus, Haloterrigena, Halobiforma, Halopiger, Haloquadratum, Halogeometricum, Natronococcus, Halogranum, Halorhabdus, Natronobacterium, Halobacterium, and Halorubrum, have been identified as PHA producers [Citation97].
The halophilic archaeon Haloferax mediterranei isolated from multi-pond solar salterns from the Dead Sea, was shown to produce PHA bioplastics from date palm fruit waste biomass as feedstock [Citation76]. Large efforts were paid to improve archaeal PHA production efficiency and scalability using different agro-industrial wastes like vinasse, a liquid residue from sugarcane-based ethanol industry to produce PHA by H. marismortui in shake flasks [Citation98]. Importantly, high salinity in the cultivation media of halophilic microorganisms reduces the likelihood of microbial contamination. This enables the implementation of an open and unsterile fermentation process for PHA production [Citation96].
4.1.3. Biofuels
Various types of biofuels are found in nature, including bioethanol, biobutanol, biogas, hydrogen, and biodiesel. Among these options, bioethanol is the most viable alternative. In hypersaline environments, halophiles play a direct role in fermenting sugars to produce ethanol and butanol [Citation99].
Archaea have been shown to naturally produce or can be engineered to produce a range of products such as biofuels [Citation100]. Researchers have documented the production of PHB from H. marismortui, a halophilic archaeon that lives in the Dead Sea [Citation75]. The energy contained within the PHB presents opportunities for utilization through different methods. Ongoing studies are focused on enhancing PHB production and developing techniques to extract its stored energy for applications in biofuel production.
Hydrogen has garnered significant attention from scientists owing to its ease of conversion into electricity and potential as a clean-burning fuel [Citation77]. A community of halophilic microorganisms strongly produce hydrogen from raw starch, among which halophiles are algae belonging to Dunaliella spp. living in the Dead Sea [Citation77].
4.1.4. Biocompatible osmolytes
To deal with the osmotic pressure caused by the high concentration of NaCl in Dead Sea habitats, halophiles utilize one of two distinct adaptation methods. They build up inorganic ions (K+, Na+, and Cl−) in the cytoplasm and produce some proteins that remain stable and functional in the presence of salts. In contrast, algae adapt to high salinity by implementing a mechanism that excludes salts from their intracellular fluid and utilizes glycerol or other compatible solutes for osmotic regulation [Citation101].
Compatible solutes, like glycerol, ectoine and betaine, are useful as stabilizers of biomolecules and whole cells [Citation102]. While Dunaliella is known to produce glycerol, the cost-effectiveness of producing glycerol through other methods or as a by-product of oil manufacturing makes the use of Dunaliella for glycerol production impractical [Citation78]. Another osmoprotectant, betaine, is one of products that are rarely synthesized by microorganisms, Halobacillus dabanensis isolated from the Dead Sea was shown to increase the production of betaine and ectoine concentrations at high salinity [Citation79].
4.1.5. Extracellular polysaccharides
The production of exopolysaccharides by microorganisms has attracted interest because of their potential applications in biomedicine, emulsifiers, biosurfactants, bioadsorbents, bioflocculants, gelling agents, pharmacy, food, cosmetics, enhancers of immunomodulatory, anti-inflammatory and antioxidant activities. D. salina is known to synthesize considerable amounts of exopolysaccharides (944 mg L−1) at 5 M NaCl, whereas the minimum (56 mg L−1) was observed at 0.5 M salinity [Citation103].
Bioemulsifiers are active compounds on the surface that diminish the interfacial tension between immiscible liquids, resulting in the creation of stable emulsions. These bioemulsifiers are synthesized by diverse microorganisms, including halophiles, and have promising applications in environmental bioremediation and oil recovery [Citation43]. Halomonas produces extracellular polyanionic polysaccharides that act as emulsifier [Citation80]. Haloferax mediterranei secretes anionic exopolysaccharides. The sulfated acidic heteropolysaccharide Haloferax sp. has high viscosity at low concentrations, exhibits excellent rheological properties, and is resistant to pH and temperature extremes. They have potential applications in enhanced oil recovery [Citation104].
4.1.6. Antimicrobials
Antibiotics have transformed modern medicine. The need to discover new antibiotics exists due to the emergence of antibiotic resistance. Therefore, microorganisms are exploited continuously for the production of antibiotics [Citation105]. In addition to the use of antibiotics to treat infection, they are used in the production of biopharmaceuticals to prevent contamination and ensure the purity of products like insulin, monoclonal antibodies, and vaccines [Citation106]. Also, they are used in biotechnology to ensure the purity of DNA samples by preventing bacterial contamination that could interfere with PCR and other molecular techniques. Antibiotics are added to culture media and bioreactors to ensure their sterility and prevent contamination by unwanted microorganisms. Furthermore, antibiotics are essential components in genetic engineering. When a gene of interest is inserted into a host organism, an antibiotic resistance gene is co-inserted and only those cells that successfully incorporate the gene will survive in the presence of the antibiotic that is added to the culture media, allowing for easy selection of modified cells [Citation106].
Studies have also shown that Dead Sea mud can inhibit the viability of E. coli, S. aureus, Propionibacterium acnes, and Candida albicans, although the identity of the bacteria or archaebacteria producer has not been identified [Citation107]. In a second study, antibacterial activity was also shown by four isolates from Dead Sea mud against S. aureus and P. aeruginosa [Citation43]. In a third study, Bacillus persicusi 24 DMS showed antimicrobial activity against Corynebacterium diphtheria 51696 [Citation2]. Bacillus species DSM2 from the same location has activity against pathogenic fungi, including C. albicans ATCC 10,231 and A. brasiliensis ATCC 16,404 [Citation68].
The antimicrobial activity of Dead Sea archaea is still unexplored. It is well-known that halophilic archaea can produce halocins which are protein-based antimicrobial compounds [Citation43]. Another important aspect is the production of antiviral compounds by halophilic archaea. It has been reported that the C50 carotenoid, bacterioruberin, produced by the haloarchaeal species Natrialba sp. M6, isolated from El-Hamra Lake, Egypt had robust antiviral activity against hepatitis C and B viruses [Citation108]. Antiviral activity of Dead Sea halophiles needs further investigation.
4.1.7. Carotenoids
Carotenoids are tetraterpenoid compounds comprising 40 carbon atoms constructed from four terpene units, each containing 10 carbon atoms. These compounds naturally occur in plants and microorganisms, such as algae, certain bacteria, and some fungi. They are also prevalent in animals, contributing to distinct colors, such as pink hues in flamingos and salmon, as well as the red tint in cooked lobsters. There are two primary types of carotenoids: (1) carotenoids, which are devoid of oxygen atoms, encompass lycopene (found in tomatoes, contributing to their red color) and β-carotene (present in carrots, lending them their orange hue). (2) Xanthophylls containing oxygen atoms include lutein, canthaxanthin (responsible for the gold pigment in chanterelle mushrooms), zeaxanthin, and astaxanthin. Carotenoids serve as effective scavengers of free radicals, mitigating oxidative stress and the consequent cellular damage [Citation82].
Carotenoids have numerous applications as colorants in food products and cosmetics, feed additives for poultry, livestock, fish, and crustaceans, antioxidants, antitumor and heart disease prevention agents, and enhancers of in vitro antibody production. Hence, they are widely used in the food, medical, pharmaceutical, and cosmetic industries as dyes and functional ingredients [Citation109–111].
β-Carotene is a rich source of antioxidants and pro-vitamin A (retinol). Although β-carotene can be synthesized chemically, the chemical product differs from that extracted from algae and higher plants. The synthetic form is all-trans β-carotene, whereas algae produce a high percentage of 9-cis β-carotene, which is more effective as an antioxidant. Dunaliella spp., reported previously from the Dead Sea after heavy rainfall [Citation39], produce a large amount of β-carotene when grown under suitable conditions [Citation112–114]. A research group was able to extract β-carotene from D. salina isolated from the Dead Sea by ethanol extraction, and the enzymatic oxidation activity was tested using 15,15’- β-carotene dioxygenase. The resulting natural powder was more than 99% pure using the HPLO5 instrument at the research lab of Al-Hikma pharmaceutical Company [Citation81].
Astaxanthin is widely used in cosmetics [Citation115]. It is also produced by D. salina isolated from the Dead Sea [Citation82]. Astaxanthin exhibits up to 10 times stronger free radical scavenging activity compared to β-carotene. Unlike β-carotene, astaxanthin lacks pro-vitamin A activity, meaning that it is not converted into vitamin A in the human body. Astaxanthin plays a role in halting lipid peroxidation and enhancing the preventive capabilities of numerous other antioxidants [Citation116].
4.2. Environmental bioremediation
The unique metabolic capabilities of Dead Sea microbes can be harnessed for bioremediation. A halophilic archaeon, Haloferax volcanii D1227, isolated from the Dead Sea, was able to degrade mono-aromatic compounds, such as benzoate, cinnamate, and 3-phenylpropionate [Citation117–119]. The ability to degrade petroleum hydrocarbons opens a door for using halophiles in cleaning up oil spills in marine environments and contaminated soil [Citation99]. Moreover, the wastewater produced by the oil industry is saline wastewater contaminated with oil or natural gases. For every barrel of extracted oil, approximately 10 barrels of brackish or saline water with complex organic compositions are generated. Consequently, managing and disposing of these produced waters presents significant challenges owing to their high impact and toxicity on soils, vegetation, surface water, and shallow groundwater [Citation120]. More studies are needed to explore the potential of Dead Sea microbes in bioremediation.
4.3. Nanotechnology
The unique lipids and biopolymers produced by haloarchaea have the potential use in nanotechnology for the development of nanomaterials and drug delivery systems [Citation121]. Bioactive silver nanoparticles were synthesized using Haloferax volcanii. These particles demonstrated notable antibacterial activity [Citation122]. It has been shown that certain haloarchaeal species are capable of synthesizing nanoparticles during the process of heavy metal detoxification such as Haloferax sp. that can produce silver nanoparticles intracellularly [Citation123].
4.4. Biotechnology
Bacteriorhodopsin is a complex of a protein and chromophore (retinal) that acts as a light-driven proton pump. Its notable attributes, including high cyclicity, thermal stability, and quantum efficiency, have garnered substantial attention for biotechnological and photoelectrical applications [Citation124]. Consequently, it has applications in artificial retinas, photochromic data storage, holographic cameras, spatial light modulators, optical computing, optical sensors, neuroimaging, and light-sensitive switches [Citation62,Citation102]. Successful purification of the photoactive membrane protein bacteriorhodopsin from H. marismortui, an extremely halophilic archaeon isolated from the Dead Sea, in the 1930s [Citation125] was achieved using a simple, nontoxic, environmentally friendly, and easily scalable extraction process [Citation126]. Nevertheless, the industrial utilization of bacteriorhodopsin faces limitations owing to inadequate production yields, high production costs, and the necessity for time-consuming purification processes [Citation127]. While E. coli overexpressing bacteriorhodopsin is an industrially attractive host for the production of bacteriorhodopsin [Citation128,Citation129], a frequent consequence of overexpression of this protein in E. coli is the formation of insoluble inclusion bodies that require solubilization and reactivation [Citation129]. To date, the isolation and biochemical characterization of bacteriorhodopsins from halophilic archaea are limited to Halobacterium salinarum [Citation130], Haloarcula sp. IRU1 [Citation131] and Haloquadratum walsbyi [Citation124]. Despite the high demand of Bacteriorhodopsin both for research and technological applications, only small amounts at large price points are available due to the difficulty of H. salinarum cultivation and low bacteriorhodopsin yield. Currently, H. salinarum bacteriorhodopsin is commercially produced by Halotek company [Citation132].
4.5. Agricultural applications
Soil salinity is one of the major limiting factors for plant growth and productivity. Though unexploited yet, Dead Sea halophilic microbes can benefit agriculture by improving soil health and plant growth in saline soils. Halophilic microorganisms may contribute to soil fertility by producing extracellular polysaccharides, which improve soil aggregation, water retention, and aeration [Citation133]. Additionally, the metabolic activities of halophiles may play a role in nutrient cycling, enhancing the availability of essential elements like nitrogen and phosphorus [Citation134]. Certain halophilic archaea and bacteria produce antimicrobial compounds that can serve as biocontrol agents against plant pathogens [Citation135]. This reduces reliance on chemical pesticides and promotes more sustainable agricultural practices. Inoculating crops with these archaea can enhance their tolerance to salinity, thereby improving crop yields in salt-affected soils [Citation136]. Also, these archaea can directly promote plant growth by producing phytohormones such as indole-3-acetic acid (IAA), which stimulate root elongation and overall plant development [Citation137]. Additionally, halophilic archaea are capable of degrading organic pollutants and detoxifying heavy metals in contaminated soils. Their robust metabolic pathways allow them to thrive in extreme environments, making them valuable for bioremediation of polluted agricultural lands [Citation138]. Most importantly, genes from halophilic microorganisms can be engineered into plants for desiccation resistance [Citation139].
4.6. Satellite remote sensing systems
Dynamic spatiotemporal variations in carotenoid pigments can be traced through satellite remote sensing systems. For example, Halobacterium salinarium, produces red coloration due to the presence of β-carotene, bacterioruberins (C50 analogs of carotenoids), lycopene, and diphytanyl-glycerol pigments. Also, Haloferax volcanii possesses bacterioruberin, which confers pink hues to the Dead Sea [Citation99].
5. Challenges facing industrial biotechnological use of microorganism and the advantage of using halophiles
Halotolerant microorganisms are vital contributors to food biotechnology, particularly to the manufacture of fermented foods and dietary supplements. Additionally, extremophiles find applications in diverse fields, such as the breakdown of various organic pollutants and generation of alternative energy [Citation102]. However, the current state of industrial biotechnology for the manufacturing of chemicals, biofuels, and biomaterials faces economic challenges compared with traditional chemical industries. Several factors contribute to the high production costs of bioprocessing. First, fermentative processes necessitate the use of pure microorganisms for effective bioproduction, and preventing contamination is crucial for successful fermentation. Achieving this requires sterilization of the fermentation media, air supply, and fermentation vessels with piping systems using high-temperature and high-pressure steam, thereby increasing energy consumption and process complexity. The use of halophilic organisms can mitigate the risk of contamination because many organisms are unable to thrive under such growth conditions [Citation140]. This capability facilitates contamination-free fermentation under non-sterile conditions [Citation140]. Second, many fermentation processes are conducted intermittently, as batch processes, to prevent contamination from prolonged cell growth, which diminishes the process efficiency compared to continuous processes. Halophilic bacteria have been successfully used for bioproduction under open and continuous fermentation conditions [Citation141]. Third, bioprocessing involves substantial freshwater usage for nutrient dissolution, cell growth, product purification, washing, and sterilization, exacerbating the global freshwater scarcity [Citation141] and this is not the case when using halophiles because of the lower demand for freshwater.
Conclusion
This study emphasizes the diversity of microorganisms living in Dead Sea lake, mud, sinkholes and underwater hot springs. However, the salinity of the Dead Sea is anticipated to rise in the coming years. This raises the question of its impact on the prokaryotic populations present at present. Gene banks are required to preserve these unique halophiles.
Studying microbial adaptations to increasingly harsh environments, along with seasonal and geographical variations in microbial communities, is crucial. The ability of these communities to shift with seasonal changes demonstrates their adaptability and resilience. The unique adaptations of microorganisms in the Dead Sea to fluctuating salinity and temperature offer valuable insights for biotechnological research, particularly in developing enzymes and compounds for industrial applications.
The investigation of the halophillicity of microorganisms offers important insights that could serve as the foundation for future biotechnological applications. Modern molecular biology and bioengineering approaches would make it easier to create biomolecules with commercial potential. Their capacity to endure elevated salinity levels, osmotic pressures, pH levels, and frequently a confluence of these parameters led to a paradigm-shifting realization that these organisms may be employed as likely candidates in diverse sectors. However, cost-effectiveness studies must precede commercial projects that utilize them.
In conclusion, the use of microorganisms isolated from the Dead Sea holds significant importance in biotechnology. The polyextremophiles found in the Dead Sea had adaptations to its highly saline, acidic, and radiation-rich conditions. The Dead Sea’s extremophiles haven’t been extensively studied compared to extremophiles from other hypersaline environments such as marine environments or alkaline hypersaline lakes. This means there’s a vast potential for discovering novel biomolecules with unique properties. In essence, the Dead Sea offers a treasure trove of extremophiles with the potential to revolutionize various fields thanks to their unique adaptations to a harsh and challenging environment.
Author contribution
MA: Introduction; HAD: Microbial diversity in the Dead Sea; SZ, MA: Potential biotechnological applications; MA: Challenges facing industrial biotechnological use of microorganisms and the advantages of using halophiles.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Ottom MA, Al-Shibli F, Atoum MS. The future of data storytelling for precipitation prediction in the Dead-Sea-Jordan using SARIMA model. Int J Membr Sci Techno. 2023;10(1):1159–19. doi: 10.15379/ijmst.v10i1.2794
- Al-Karablieh N. Antimicrobial activity of bacillus persicus 24-DSM isolated from Dead Sea mud. Open Microbiol J. 2017;11(1):372–383. doi: 10.2174/1874285801711010372
- Kreiger B. The Dead Sea and the Jordan River. Bloomington (IN): Indiana University Press; 2016.
- Oroud IM. The future fate of the Dead Sea: total disappearance or a dwarfed hypersaline hot lake? J Hydrol. 2023;623:129816. doi: 10.1016/j.jhydrol.2023.129816
- Lazar M, Siebert CJG. Out of sight, out of mind. Submarine springs in the Dead Sea—an underappreciated phenomenon. Geomorphology. 2023;436:436 108777. doi: 10.1016/j.geomorph.2023.108777
- Reznik IJ, Gavrieli I. Massive-scale dissolution, conveyance, and disposal of dead sea potash industry halite waste. Environ Sci Technol. 2023;57(22):8385–8395. doi: 10.1021/acs.est.3c01197
- Nazareth S, Gonsalves V, Nayak S. A first record of obligate halophilic aspergilli from the dead sea. Indian J Microbiol. 2012;52(1):22–27. doi: 10.1007/s12088-011-0225-z
- Abu-Qubu J, Merkel B, Dunger V, et al. Variation of the chemistry of the Dead Sea brine as consequence of the decreasing water level. Aquat Geochem. 2018;24(2):121–135. doi: 10.1007/s10498-018-9336-z
- Weber N, Antler G, Lazar B, et al. Hydrological and thermodynamic controls on late Holocene gypsum formation by mixing saline groundwater and Dead Sea brine. Geochim Cosmochim Acta. 2022;316:363–383. doi: 10.1016/j.gca.2021.10.002
- Oren A. Life in magnesium-and calcium-rich hypersaline environments: salt stress by chaotropic ions. In: Seckbach J, Oren A, Stan-Lotter H, editors. Polyextremophiles: life under multiple forms of stress. Netherland: Springer; 2013. p. 215–232.
- Hallsworth JE. Water is a preservative of microbes. Microb Biotechnol. 2022;15(1):191–214. doi: 10.1111/1751-7915.13980
- Ouillon R, Lensky NG, Lyakhovsky V, et al. Halite precipitation from double‐diffusive salt fingers in the Dead Sea: numerical simulations. Water Resour Res. 2019;55(5):4252–4265. doi: 10.1029/2019WR024818
- Zumdahl S, DeCoste D. Chemical principles.: Cengage learning. 2016.
- Sirota I, Enzel Y, Mor Z, et al. Sedimentology and stratigraphy of a modern halite sequence formed under Dead Sea level fall. Sedimentology. 2021;68(3):1069–1090. doi: 10.1111/sed.12814
- Yakushev EV, Andrulionis NY, Jafari M, et al. How climate change and human interaction alter chemical regime in salt lakes, case study: lake urmia, aral sea, the Dead Sea, and lake Issyk-kul. Lake urmia: a hypersaline waterbody in a Drying. Climate: Springer; 2022. p. 275–296.
- El-Hasan T, Abu-Jaber N. Geochemistry, mineralogy and origin of the shallow water sediments collected along the eastern shore of the northern part of the Dead Sea. Carbonates And Evaporites. 2019;34(3):975–985. doi: 10.1007/s13146-018-0453-y
- Grant W, Daniel RM, Finney JL, et al. Life at low water activity. Philos Trans R Soc London Ser B Biol Sci. 2004;359(1448):1249–1267. doi: 10.1098/rstb.2004.1502
- Ventosa A, de la Haba RR, Sanchez-Porro C, et al. Microbial diversity of hypersaline environments: a metagenomic approach. Curr Opin Microbiol. 2015;25:80–87. doi: 10.1016/j.mib.2015.05.002
- Adar O, Groner E, Natan GB. Colonization of a new habitat: the case of the Dead Sea sinkholes—preliminary observations. Negev Dead Sea Arava Stud. 2014;6:74–89.
- Oren A. Salts and brines. In: Whitton B, and Potts M, editors. The ecology of cyanobacteria. The Netherlands: Kluwer Academic Publishers; 2000. p. 281–306.
- Anton J, Rossello-Mora R, Rodriguez-Valera F, et al. Extremely halophilic bacteria in crystallizer ponds from solar salterns. Appl environ microbiol. 2000;66(7):3052–3057. doi: 10.1128/AEM.66.7.3052-3057.2000
- Jacob JH, Hussein EI, Shakhatreh MAK, et al. Microbial community analysis of the hypersaline water of the Dead Sea using high-throughput amplicon sequencing. Microbiol Open. 2017;6(5):e00500. doi: 10.1002/mbo3.500
- Kushner DJ, Kamekura M. Physiology of halophilic eubacteria. In: Rodriguez-Valera F, editor. Halophilic bacteria. Vol. 1. Boca Raton (FL): CRC Press Inc.; 1988. p. 109–138.
- Al’abri K. Use of molecular approaches to study the occurrence of extremophiles and extremodures in non-extreme environments. UK: University of Sheffield; 2011.
- Lane DJ, Pace B, Olsen GJ, et al. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci. 1985;82:6955–6959.
- Mbata T. Isolation of fungi in hyper saline Dead Sea water. Sudan J Public Health. 2008;3:172.
- Buchalo AS, Nevo E, Wasser SP, et al. Fungal life in the extremely hypersaline water of the Dead Sea: first records. In: Proceedings of the royal society B-Biological Sciences; London; 1998; 265. p. 1461–1465.
- Baker BJ, De Anda V, Seitz KW, et al. Diversity, ecology and evolution of archaea. Nat Microbiol. 2020;5(7):887–900. doi: 10.1038/s41564-020-0715-z
- Maignien L, Parkes RJ, Cragg B, et al. Anaerobic oxidation of methane in hypersaline cold seep sediments. FEMS Microbiol Ecol. 2013;83(1):214–231. doi: 10.1111/j.1574-6941.2012.01466.x
- Dugan HA, Bartlett SL, Burke SM, et al. Salting our freshwater lakes. Proc Natl Acad Sci. 2017;114:4453–4458.
- Fedotov A, Phedorin M, Enushchenko I, et al. Drastic desalination of small lakes in East Siberia (Russia) in the early twentieth century: inferred from sedimentological, geochemical and palynological composition of small lakes. Environ Earth Sci. 2013;68(6):1733–1744. doi: 10.1007/s12665-012-1864-z
- Zahran HJB. Soils fo. Diversity, adaptation and activity of the bacterial flora in saline environments. Biol Fertili Soils. 1997;25(3):211–223. doi: 10.1007/s003740050306
- Huang J, Yang J, Jiang H, et al. Microbial responses to simulated salinization and desalinization in the sediments of the qinghai–tibetan lakes. Front Microbiol. 2020;11:1772. doi: 10.3389/fmicb.2020.01772
- Horneck G, Baumstark-Khan C. Astrobiology: the quest for the conditions of life. Berlin/Heidelberg (Germany): Springer Science & Business Media; 2012.
- Zhang G, Chen W, Xie HJGRL. Tibetan plateau’s lake level and volume changes from NASA’s ICESat/ICESat‐2 and landsat missions. Geophysical Research Letters. 2019;46(22):13107–13118. doi: 10.1029/2019GL085032
- Häusler S, Weber M, de Beer D, et al. Spatial distribution of diatom and cyanobacterial mats in the Dead Sea is determined by response to rapid salinity fluctuations. Extremophiles: life under extreme conditions. Extremophiles. 2014;18(6):1085–1094. doi: 10.1007/s00792-014-0686-1
- Ionescu D, Zoccarato L, Cabello-Yeves PJ. Extreme fluctuations in ambient salinity select for bacteria with a hybrid “salt-in”/”salt-out” osmoregulation strategy. Front Microbiomes. 2024;2:1329925. doi: 10.3389/frmbi.2023.1329925
- Jacob JH. Classification of halophilic heterotrophic bacteria thriving in the Jordanian Dead Sea littoral zone. J of Biol Sci. 2012;12(4):246. doi: 10.3923/jbs.2012.246.252
- Oren A, Gurevich P, Anati DA, et al. A bloom of dunaliella parva in the Dead Sea in 1992: biological and biogeochemical aspects. Hydrobiologia. 1995;297(3):173–185. doi: 10.1007/BF00019283
- Oren A. The dying Dead Sea: the microbiology of an increasingly extreme environment. Lakes & Reservoirs. 2010;15(3):215–222. doi: 10.1111/j.1440-1770.2010.00435.x
- Barinova SS, Tsarenko PM, Nevo E. Algae from experimental pools on the Dead Sea coast, israel. Isr J Plant Sci. 2004;52(3):265–275. doi: 10.1560/V889-764E-MCDY-NPDP
- Mack EE, Mandelco L, Woese CR, et al. Rhodospirillum sodomense, sp. nov. a Dead Sea rhodospirillum species. Arch Microbiol. 1993;160(5):363–371. doi: 10.1007/BF00252222
- Satbhai A, Kasodekar A, Pachuau L, et al. Isolation of halophiles from the Dead Sea and exploring their potential biotechnological applications. Int J Curr Microbiol App Sci. 2015;1–17.
- Thomas C, Ionescu D, Ariztegui D, et al. Archaeal populations in two distinct sedimentary facies of the subsurface of the Dead Sea. Mar Genomics. 2014;17:53–62. doi: 10.1016/j.margen.2014.09.001
- Ionescu D, Siebert C, Polerecky L, et al. Microbial and chemical characterization of underwater fresh water springs in the Dead Sea. PLOS ONE. 2012;7(6):e38319. doi: 10.1371/journal.pone.0038319
- Volcani B. Studies on the microflora of the Dead Sea. PhD Thesis in Hebrew Jerusalem, Israel: The Hebrew University; 1940.
- Khatibi SMH, Vahed FZ, Sharifi S, et al. Osmolytes resist against harsh osmolarity: something old something new. Biochimie. 2019;158:156–164. doi: 10.1016/j.biochi.2019.01.002
- Oren A. Bacteriorhodopsin-mediated CO 2 photoassimilation in the Dead Sea1. Limnol Oceanography. 1983;28(1):33–41. doi: 10.4319/lo.1983.28.1.0033
- Khlaifat A, Al-Khashman O, Qutob H. Physical and chemical characterization of dead sea mud. Mater Charact. 2010;61(5):564–568. doi: 10.1016/j.matchar.2010.02.015
- Hamawi R. Diversity of UV resistance in the halophilic archaea. 2018.
- Cui H-L, Dyall-Smith ML. Cultivation of halophilic archaea (class halobacteria) from thalassohaline and athalassohaline environments. Mar Life Sciamp; Technol. 2021;3(2):243–251. doi: 10.1007/s42995-020-00087-3
- Sun Y, Liu Y, Pan J, et al. Perspectives on cultivation strategies of archaea. Microb Ecol. 2020;79(3):770–784. doi: 10.1007/s00248-019-01422-7
- in’t Zandt MH, van den Bosch TJ, Rijkers R, et al. Co-cultivation of the strictly anaerobic methanogen Methanosarcina barkeri with aerobic methanotrophs in an oxygen-limited membrane bioreactor. Appl Microbiol Biotechnol. 2018;102(13):5685–5694. doi: 10.1007/s00253-018-9038-x
- Scheller S, Yu H, Chadwick GL, et al. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science. 2016;351:703–707. doi: 10.1126/science.aad7154
- Ej Z, Toledo G, Rappé M. Cultivating the uncultured. Proc Natl Acad Sci, USA. 2002;99(24):15681–15686. doi: 10.1073/pnas.252630999
- Ingham CJ, Sprenkels A, Bomer J, et al. The micro-Petri dish, a million-well growth chip for the culture and high-throughput screening of microorganisms. Proc Natl Acad Sci. 2007;104:18217–18222.
- Kaeberlein T, Lewis K, Epstein SS. Isolating“uncultivable” microorganisms in pure culture in a simulated natural environment. Science. 2002;296(5570):1127–1129. doi: 10.1126/science.1070633
- Nissenbaum A. The microbiology and biogeochemistry of the Dead Sea. Microb Ecol. 1975;2(2):139–161. doi: 10.1007/BF02010435
- Bodaker I, Beja O, Sharon I, et al. Archaeal diversity in the Dead Sea: microbial survival under increasingly harsh conditions. Nat Resour And Environ Issues. 2009;15:25.
- Oren A. Life in hypersaline environments. In: Their world: a diversity of microbial environments. Berlin/Heidelberg (Germany): Springer; 2016. p. 301–339.
- Oren A. Glycerol metabolism in hypersaline environments. Environ Microbiol. 2017;19(3):851–863. doi: 10.1111/1462-2920.13493
- Häusler S, Noriega‐Ortega BE, Polerecky L, et al. Microenvironments of reduced salinity harbour biofilms in Dead Sea underwater springs. Environ Microbiol Rep. 2014;6(2):152–158. doi: 10.1111/1758-2229.12140
- Hannigan RE, Sholkovitz ER. The development of middle rare earth element enrichments in freshwaters: weathering of phosphate minerals. Chem Geol. 2001;175(3–4):495–508. doi: 10.1016/S0009-2541(00)00355-7
- Oren A. Molecular ecology of extremely halophilic archaea and bacteria. FEMS Microbiol Ecol. 2002;39(1):1–7. doi: 10.1111/j.1574-6941.2002.tb00900.x
- Bodaker I, Sharon I, Suzuki MT, et al. Comparative community genomics in the Dead Sea: an increasingly extreme environment. Isme J. 2010;4(3):399–407. doi: 10.1038/ismej.2009.141
- Alpert P, Shafir H, Issahary DJCC. Recent changes in the climate at the Dead Sea–a preliminary study. Clim Change. 1997;37:513–537. doi: 10.1023/A:1005330908974
- Wilhelm SW, LeCleir GR, Bullerjahn GS, et al. Seasonal changes in microbial community structure and activity imply winter production is linked to summer hypoxia in a large lake. FEMS Microbiol Ecol. 2014;87(2):475–485. doi: 10.1111/1574-6941.12238
- Obeidat M. Isolation and characterization of extremely halotolerant bacillus species from Dead Sea black mud and determination of their antimicrobial and hydrolytic activities. Afr J Microbiol Res. 2017;11(32):1303–1314. doi: 10.5897/AJMR2017.8608
- Al-Quadan F, Akel H, Natshi R. Characteristics of a novel, highly acid-and thermo-stable amylase from thermophilic bacillus strain HUTBS62 under different environmental conditions. Ann Microbiol. 2011;61(4):887–892. doi: 10.1007/s13213-011-0210-0
- Oren A. A thermophilic amyloglucosidase from halobacterium sodomense, a halophilic bacterium from the Dead Sea. Curr Microbiol. 1983;8(4):225–230. doi: 10.1007/BF01579551
- Malik AD, Furtado IJ. Isolation of halomicroarcula pellucida strain GUMF5, an archaeon from the Dead Sea-Israel possessing cellulase. 3 biotech. 3 Biotech. 2022;12(1):26. doi: 10.1007/s13205-021-03090-2
- Pundak S, Eisenberg H. Structure and activity of malate dehydrogenase from the extreme halophilic bacteria of the Dead Sea: 1. Conformation and interaction with water and salt between 5 M and 1 M NaCl concentration. Eur J Biochem. 1981;118(3):463–470. doi: 10.1111/j.1432-1033.1981.tb05542.x
- Madern D, Zaccai G. Molecular adaptation: the malate dehydrogenase from the extreme halophilic bacterium salinibacter ruber behaves like a non-halophilic protein. Biochimie. 2004;86(4–5):295–303. doi: 10.1016/j.biochi.2004.04.004
- Izotova LS, Strongin AY, Chekulaeva LN, et al. Purification and properties of serine protease from halobacterium halobium. J Bacteriol. 1983;155(2):826–830. doi: 10.1128/jb.155.2.826-830.1983
- Han J, Lu Q, Zhou L, et al. Molecular characterization of the phaEC hm genes, required for biosynthesis of poly (3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl environ microbiol. 2007;73(19):6058–6065. doi: 10.1128/AEM.00953-07
- Alsafadi D, Ibrahim MI, Alamry KA, et al. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci Total Environ. 2020;737:139716. doi: 10.1016/j.scitotenv.2020.139716
- Ike A, Murakawa T, Kawaguchi H, et al. Photoproduction of hydrogen from raw starch using a halophilic bacterial community. Journal of Biosci And Bioeng. 1999;88(1):72–77. doi: 10.1016/S1389-1723(99)80179-0
- Lentzen G, Schwarz T. Extremolytes: natural compounds from extremophiles for versatile applications. Appl Microbiol Biotechnol. 2006;72(4):623–634. doi: 10.1007/s00253-006-0553-9
- Amasha RH. Use of nmr to determine compatible solutes in halophilic bacteria isolated from highly saline areas. Pharmacophore. 2018;9:50–60.
- Davis JS, Coogan AL, editors. Importance of microorganisms in solar salt production In: Proceedings of the 4th Symposium on Salt vol. 1. Northern Ohio geological society. Cleveland; 1974. p. 369–372.
- Emeish S. Producing natural smart biopharmaceuticals from the microalgae living in the dead sea utilizingphototube bioreactor. In: The Eighth Jordan International Chemical Engineering Conference (JIChEC 2017); Amman, Jordan; 2017 Nov 7–9, 2017.
- Al-Muhteseb SI, Emeish S. Producing natural mixed carotenoids from dunaliella salina. J Nat Sci Res. 2015;5:53–59.
- Bhatt BM, Trivedi UB, Patel KC. Extremophilic amylases: microbial production and applications. In: Arora N, Mishra J Mishra V, editors. Microbial enzymes: roles and applications in industries. Singapore: Springer Singapore; 2020. p. 185–205.
- El-Fallal A. Starch and microbial α-amylases: from concepts to biotechnological applications. In: Dobara MA, editor. Carbohydrates—comprehensive studies on glycobiology and glycotechnology. Rijeka (Croatia): IntechOpen; 2012. Chapter 21.
- Yadav KK, Patil PB, Kumaraswamy HH, et al. Ligninolytic microbes and their role in effluent management of pulp and paper industry. In: Kashyap B, Solanki M, Kamboj D Pandey A, editors. Waste to energy: prospects and applications. Singapore: Springer Singapore; 2020. p. 309–350.
- Bhardwaj N, Kumar B, Agrawal K, et al. Green biomimetic synthesis of Ag–TiO2 nanocomposite using origanum majorana leaf extract under sonication and their biological activities. Bioresour Bioprocess. 2021;8(1):1–34. doi: 10.1186/s40643-020-00357-z
- Sochaj AM, Świderska KW, Otlewski J. Current methods for the synthesis of homogeneous antibody–drug conjugates. Biotechnol Adv. 2015;33:775–784. doi: 10.1016/j.biotechadv.2015.05.001
- Mevarech M, Eisenberg H, Neumann E. Malate dehydrogenase isolated from extremely halophilic bacteria of the Dead Sea. 1. Purification and molecular characterization. Biochemistry. 1977;16(17):3781–3785. doi: 10.1021/bi00636a009
- Cendrin F, Chroboczek J, Zaccai G, et al. Cloning, sequencing, and expression in Escherichia coli of the gene coding for malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui. Biochemistry. 1993;32(16):4308–4313. doi: 10.1021/bi00067a020
- Chandra P, Enespa SR, Arora PK. Microbial lipases and their industrial applications: a comprehensive review. Microb Cell Fact. 2020;19(1):1–42. doi: 10.1186/s12934-020-01428-8
- Song P, Zhang X, Wang S, et al. Microbial proteases and their applications. Front Microbiol. 2023;14:1236368. doi: 10.3389/fmicb.2023.1236368
- Mokashe N, Chaudhari B, Patil U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int j biol macromol. 2018;117:493–522. doi: 10.1016/j.ijbiomac.2018.05.217
- Poidevin L, MacNeill SA. Biochemical characterisation of LigN, an NAD±dependent DNA ligase from the halophilic euryarchaeon Haloferax volcanii that displays maximal in vitro activity at high salt concentrations. BMC Mol Biol. 2006;7(1):1–14. doi: 10.1186/1471-2199-7-44
- Aparici-Carratalá D, Esclapez J, Bautista V, et al. Archaea: current and potential biotechnological applications. Res Microbiol. 2023;174(7):104080. doi: 10.1016/j.resmic.2023.104080
- Koller M. Polyhydroxyalkanoate biosynthesis at the edge of water activitiy-haloarchaea as biopolyester factories. Bioengineering. 2019;6(2):34. doi: 10.3390/bioengineering6020034
- Yue H, Ling C, Yang T, et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant halomonas campaniensis LS21 grown in mixed substrates. Biotechnol Biofuels. 2014;7(1):1–12. doi: 10.1186/1754-6834-7-108
- Ben Abdallah M, Chamkha M, Karray F, et al. Microbial diversity in polyextreme salt flats and their potential applications. Environ Sci Pollut Res. 2024:1–35. doi: 10.1007/s11356-023-31644-9
- Pramanik A, Mitra A, Arumugam M, et al. Utilization of vinasse for the production of polyhydroxybutyrate by Haloarcula marismortui. Folia Microbiol (Praha). 2012;57(1):71–79. doi: 10.1007/s12223-011-0092-3
- Dutta B, Bandopadhyay R. Biotechnological potentials of halophilic microorganisms and their impact on mankind. Beni-Suef Univ J Basic Appl Sci. 2022;11(1):75. doi: 10.1186/s43088-022-00252-w
- Srivastava RK. Bio-energy production by contribution of effective and suitable microbial system. Mater Sci Energy Technol. 2019;2(2):308–318. doi: 10.1016/j.mset.2018.12.007
- Nieto J, Vargas C. Synthesis of osmoprotectants by moderately halophilic bacteria: genetic and applied aspects. Recent Res Developments In Microbiol. 2002;6:403–418.
- Margesin R, Schinner F. Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles. 2001;5(2):73–83. doi: 10.1007/s007920100184
- Mishra A, Jha B. Isolation and characterization of extracellular polymeric substances from micro-algae dunaliella salina under salt stress. Biores Technol. 2009;100(13):3382–3386. doi: 10.1016/j.biortech.2009.02.006
- Kanekar P, Kanekar S, Kelkar A, et al. Halophiles–taxonomy, diversity, physiology and applications. In: Satyanarayana T, and Johri B, editors. Microorganisms in environmental management: microbes and environment. Netherlands: Springer; 2012. p. 1–34.
- Cook MA, Wright GD. The past, present, and future of antibiotics. Sci, trans med. 2022;14(657):eabo7793. doi: 10.1126/scitranslmed.abo7793
- Kapoor D, Sharma P, Sharma MM, et al. Microbes in pharmaceutical industry. In: Sharma S, Sharma N, Sharma M, editors. Microbial diversity, interventions and scope. 1st ed. Singapore: Springer; 2020. p. 259–299.
- Ma’or Z, Henis Y, Alon Y, et al. Antimicrobial properties of Dead Sea black mineral mud. Int J Dermatol. 2006;45(5):504–511. doi: 10.1111/j.1365-4632.2005.02621.x
- Hegazy GE, Abu-Serie MM, Abo-Elela GM, et al. In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci Rep. 2020;10(1):5986. doi: 10.1038/s41598-020-62663-y
- Vilchez C, Forjan E, Cuaresma M, et al. Marine carotenoids: biological functions and commercial applications. Mar Drugs. 2011;9(3):319–333. doi: 10.3390/md9030319
- Naziri D, Hamidi M, Hassanzadeh S, et al. Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from urmia lake. Adv Pharmceutical Bull. 2014;4:61–67.
- Mata-Gomez LC, Montanez JC, Mendez-Zavala A, et al. Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact. 2014;13(1):12. doi: 10.1186/1475-2859-13-12
- Abusara N, Emeish S, Sallal A. The effect of certain environmental factors on growth and β-carotene production by Dunaliella sp. isolated from the Dead Sea. Jordan J Biol Sci. 2011;4:29–36.
- Raja R, Hemaiswarya S, Rengasamy R. Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biotechnol. 2007;74(3):517–523. doi: 10.1007/s00253-006-0777-8
- Ben-Amotz A, Avron M. The biotechnology of mass culturing dunaliella for products of commercial interest. Algal And Cyanobacterial Biotechnol. 1989;7:90–114.
- Lima SGM, Freire MCLC, VdS O, et al. Astaxanthin delivery systems for skin application: a review. Mar Drugs. 2021;19(9):511. doi: 10.3390/md19090511
- Dose J, Matsugo S, Yokokawa H, et al. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int J Mol Sci. 2016;17(1):103. doi: 10.3390/ijms17010103
- Orellana SC, Pohlschroder M, Grossman MJ, et al. Biodegradation of aromatic compounds by a halophilic archaeon isolated from the Dead Sea. Chem Eng Trans. 2012;27:13–18.
- Cuadros-Orellana S, Pohlschröder M, Durrant LR. Isolation and characterization of halophilic archaea able to grow in aromatic compounds. Int Biodeterior Biodegrad. 2006;57(3):151–154. doi: 10.1016/j.ibiod.2005.04.005
- Emerson D, Chauhan S, Oriel P, et al. Haloferax sp. D1227, a halophilic archaeon capable of growth on aromatic compounds. Arch Microbiol. 1994;161(6):445–452. doi: 10.1007/BF00307764
- Bhattacharjee A, Sengupta A, Basu S, et al. Role of halophiles in xenobiotic bioremediation. In: Rodriguez-Couto S, Shah MP, editors. Development in wastewater treatment research and processes. Elsevier; 2022. p. 45–60.
- Romero EL, Morilla MJ. Ether lipids from archaeas in nano-drug delivery and vaccination. Int J Pharmaceut. 2023;634:122632. doi: 10.1016/j.ijpharm.2023.122632
- Costa MI, Álvarez‐Cerimedo MS, Urquiza D, et al. Synthesis, characterization and kinetic study of silver and gold nanoparticles produced by the archaeon Haloferax volcanii. J Appl Microbiol. 2020;129(5):1297–1308. doi: 10.1111/jam.14726
- Abdollahnia M, Makhdoumi A, Mashreghi M, et al. Exploring the potentials of halophilic prokaryotes from a solar saltern for synthesizing nanoparticles: the case of silver and selenium. PLOS ONE. 2020;15(3):e0229886. doi: 10.1371/journal.pone.0229886
- Lobasso S, Lopalco P, Vitale R, et al. The light-activated proton pump bop I of the archaeon haloquadratum walsbyi. Photochem Photobiol. 2012;88(3):690–700. doi: 10.1111/j.1751-1097.2012.01089.x
- Oren A, Ginzburg M, Ginzburg BZ, et al. Haloarcula marismortui (Volcani) sp. nov. nom. rev. an extremely halophilic bacterium from the Dead Sea. Int J Systematic Bacteriol. 1990;40(2):209–210. doi: 10.1099/00207713-40-2-209
- Alsafadi D, Khalili FI, Juwhari H, et al. Purification and biochemical characterization of photo-active membrane protein bacteriorhodopsin from Haloarcula marismortui, an extreme halophile from the Dead Sea. Int J Biol Macromol. 2018;118:1942–1947. doi: 10.1016/j.ijbiomac.2018.07.045
- Shiu P-J, Chen H-M, Lee C-K. One-step purification of delipidated bacteriorhodopsin by aqueous-three-phase system from purple membrane of halobacterium. Food Bioprod Process. 2014;92(2):113–119. doi: 10.1016/j.fbp.2014.01.003
- Nekrasova OV, Wulfson AN, Tikhonov RV, et al. A new hybrid protein for production of recombinant bacteriorhodopsin in Escherichia coli. J Biotechnol. 2010;147(3–4):145–150. doi: 10.1016/j.jbiotec.2010.03.019
- Hsu M-F, Yu T-F, Chou C-C, et al. Using Haloarcula marismortui bacteriorhodopsin as a fusion tag for enhancing and visible expression of integral membrane proteins in Escherichia coli. PLOS ONE. 2013;8(2):e56363. doi: 10.1371/journal.pone.0056363
- Kalenov SV, Baurina MM, Skladnev DA, et al. High-effective cultivation of Halobacterium salinarum providing with bacteriorhodopsin production under controlled stress. J Biotechnol. 2016;233:211–218. doi: 10.1016/j.jbiotec.2016.07.014
- Taran M, Monazah A, Asadi N. Production of a novel biomacromolecule for nanodevices from glycerol as carbon source in different conditions. Int J Biol Macromol. 2011;49(5):955–957. doi: 10.1016/j.ijbiomac.2011.08.014
- Gupta MN, editor. Some key topics in chemistry and biochemistry for biotechnologists. CRC Press; 2023.
- Arora NK, Fatima T, Mishra J, et al. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J Adv Res. 2020;26:69–82. doi: 10.1016/j.jare.2020.07.003
- Sharma A, Vaishnav A, Jamali H, et al. Halophilic bacteria: potential bioinoculants for sustainable agriculture and environment management under salt stress. In: Choudhary D, Varma A, Tuteja N, editors. Plant–microbe interaction: an approach to sustainable agriculture. Singapore: Springer; 2016. p. 297–325.
- Ould Ouali K, Houali K, Cruz C, et al. Halophilic plant growth-promoting rhizobacteria as producers of antifungal metabolites under salt stress. Agronomy. 2024;14(4):845. doi: 10.3390/agronomy14040845
- Saghafi D, Delangiz N, Lajayer BA, et al. An overview on improvement of crop productivity in saline soils by halotolerant and halophilic PGPRs. 3 biotech. 3 Biotech. 2019;9(7):261. doi: 10.1007/s13205-019-1799-0
- Naitam MG, Ramakrishnan B, Grover M, et al. Rhizosphere-dwelling halophilic archaea: a potential candidate for alleviating salinity-associated stress in agriculture. Front Microbiol. 2023;14:1212349. doi: 10.3389/fmicb.2023.1212349
- Naitam MG, Kaushik R. Archaea: an agro-ecological perspective. Curr Microbiol. 2021;78(7):2510–2521. doi: 10.1007/s00284-021-02537-2
- Kouhen M, García-Caparrós P, Twyman RM, et al. Improving environmental stress resilience in crops by genome editing: insights from extremophile plants. Crit Rev Biotechnol. 2023;43(4):559–574. doi: 10.1080/07388551.2022.2042481
- Yin J, Chen JC, Wu Q, et al. Halophiles, coming stars for industrial biotechnology. Biotechnol Adv. 2015;33(7):1433–1442. doi: 10.1016/j.biotechadv.2014.10.008
- Zhang X, Lin Y, Chen GQ. Halophiles as chassis for bioproduction. Adv Biosyst. 2018;2(11):1800088. doi: 10.1002/adbi.201800088

