Abstract
The effects of riparian vegetation and bottom substrate on water quality and macroinvertebrate communities were assessed by utilising data gathered during a 2004 reconnaissance of nine sites in the Otara Creek, New Zealand. The aim of the study was to use multivariate statistical techniques to better explain the specific environmental factors that drive biological communities in an environment. During the survey, macroinvertebrate assemblages were sampled along with water quality, bottom substrate, riparian vegetation and habitat assessments. Multivariate statistical analyses of habitat and channel characteristics, physical conditions and water quality data were used to determine the relationship between environmental variables and the macroinvertebrate community structure. Ordination of biological and environmental data showed a clear separation of bush from urban and pastures streams. The observed macroinvertebrates assemblage pattern was best correlated with a single variable, riparian width (r = 0.81), a combination of two variables: cobbles and riparian width (r = 0.88), three variables: boulders, nitrate and riparian width (r = 0.91) and five variables: habitat score, shade, boulders, nitrate and riparian width (r = 0.94). The BIO-ENV procedure showed that habitat and channel characteristics had the greatest relationship to macroinvertebrate community structure followed by water chemistry.
1. Introduction
The term riparian zone is defined as three-dimensional zones of direct interaction between terrestrial and aquatic ecosystems (Gregory et al. Citation1991), and it signifies a strip of land bordering a stream, lake or coast. It is a transitional zone between land and water possessing soils which are wet and sometimes inundated, as commonly found in floodplains and near the bottom of hillslopes adjoining streams. Such zones are recognised as being important in the management of water and ecological resources because most runoff must pass over, through or round the soil and vegetation of this zone before it can reach the adjacent water body. It acts as a buffer to moderate the adverse effects of land use and land management on the stream, lake or estuarine ecosystems. The buffer effect also works in the reverse direction, protecting the land from damage caused by floods by reducing water velocities when flows overtop banks. It also acts as nutrient sinks and reduces nutrient concentrations and sediments loads before reaching the stream channel. A number of studies have shown that vegetated buffers in general, and riparian forests in particular, are effective in filtering sediments, nutrients, pesticides, particulate organic matter and bacteria from farm and feedlot runoff (Baker et al. Citation2006; Carline and Walsh Citation2007; Lovett and Price 2007; Madden et al. Citation2007; Mankin et al. Citation2007; Costello and Lamberti Citation2008; Dosskey et al. Citation2008, Citation2010; Gorsevski et al. Citation2008; Horwitz et al. Citation2008; Ficetola et al. Citation2009; Ghermandi et al. 2009; Clinton et al. Citation2010; DeWalle Citation2010; Wilkerson et al. Citation2010.). Hence, the riparian zone can potentially have critical effects on the quality – and quantity to certain extent – of the water body to which it drains; and the life forms it harbours.
The light reaching the bed of streams is reduced by interception by the canopy of riparian shade plants, by topography and by adsorption and scattering (attenuation) within the water column (Collier et al. Citation1995a). This light is important for photosynthesis and animal vision. In small streams, the reduction in lighting by terrestrial shade from stream banks and riparian vegetation is usually more important than the light reduction (attenuation) that occurs within the water column (Westlake 1966). Where there have been substantial losses of indigenous forest cover and other tall vegetation, water temperature fluctuates more readily due to greater sunlight exposure on the stream (Quinn et al. Citation1992; Wilkerson et al. Citation2006). Temperature is a critical influence in aquatic ecosystems, affecting both the physical and biological characteristics of the stream. Higher stream temperatures can reduce the stream's oxygen carrying capacity, increase rates of organic decomposition and influence the rate at which nutrients are released from suspended sediments (Wilkerson et al. Citation2006). When water temperatures increase, it may become impossible for species that require lower temperatures to continue living in the stream, resulting in a shift in community structure to species that tolerate the increased temperatures (Wilkerson et al. Citation2006). Horwitz et al. (Citation2008) found that both species diversity and total numbers of benthic organisms decreased significantly as forest cover was removed from stream banks, primarily as a result of increasing water temperatures. Shading by trees can reduce the growth of instream vegetation (macrophytes and algae) and hence water temperature fluctuations (Dawson and Haslam Citation1993; Wilkerson et al. Citation2006). Shade also encourages growth of bryophytes (moses and liverworts) and shade-adapted vascular plants which provide important food sources and habitat for aquatic invertebrates in small streams (Suren 2000).
Terrestrially derived food sources are important to aquatic ecosystems because low nutrient and light levels usually restrict plant growth (Collier et al. Citation1995a). Allochthonous input of various forms of organic matter into stream ecosystems forms the basis of the detrital food web (Vannote et al. Citation1980; Thomas et al. Citation2003). Change in a catchment landuse, particularly moving from native forest to pasture and urban development can affect the type of organic matter present in a stream. The quantities of leaves and wood entering a stream will be reduced, and the food bases may shift from allochthonous to autochthonous (Winterbourn Citation1996). All these probably influence the success, survival and distribution of some macroinvertebrate species.
A diversity of plants work together to hold streambank soils in place and protect them from erosion and undercutting by floodwaters, transported woody debris, or ice jams. The deep, penetrating roots of sedges, rushes, grasses and other herbaceous plants provide structural support for streambanks, while the thicker, harder roots of woody plants protect streambanks against bank scouring by floods (Winward Citation2000). Collier et al. (Citation1995b) concluded that vegetation above the ground increases channel roughness and slows flood flows and traps fine sediments, while the roots below the ground increase bank stability in two ways: first, the root systems of trees and shrubs form an extensive and deep root system that stabilises banks which protect the soil against floodflows. Second, root mass and density increase the shear strength of soils and hence offer protection against gravity collapse of undercut banks. Uplands disturbance, especially the removal of riparian vegetation, may contribute to increased floodflows and destabilisation of the bed and banks. Newbold et al. (Citation1980) observed that scoured banks occurred in modified sites with no riparian vegetation and proposed that modified sites had significantly lower channel stability ratings.
In the previous paper (Shilla and Shilla Citation2011), we assessed the effects of land use on streams health by using macroinvertebrates at selected sites in the Otara Creek. In that paper, we showed that macroinvertebrate community and water chemistry parameters measured exhibited trends that were related to land use in some way. It was obvious that changes in these parameters were due to a variety of human disturbances. We did not develop detailed analyses of physical habitat, riparian vegetation or bottom substrate but presented only those data necessary to explain the patterns of vertebrate abundance. We now present data on the effects of riparian vegetation and bottom substrate on water quality and macroinvertebrate communities at selected sites in the Otara Creek.
2. Materials and methods
2.1. Study area
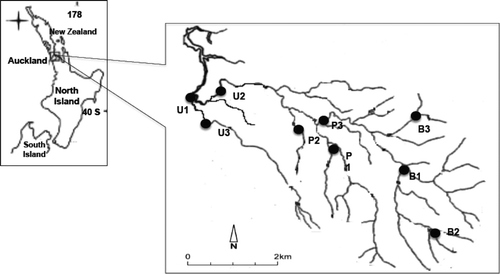
The Otara Creek catchment () is located mostly within the Otara Ward of the City of Manukau with a small portion of it in the Papatoetoe Ward and the upstream reaches within the Clevedon Ward. The catchment covers approximately 3500 hectares of land (Manukau City Council Citation2001). The catchment is bounded by the East Tamaki road to the west and north and the foot hills of the east of murphys road, while Redoubt road marks the southern boundary.
The Catchment has three other discrete sub-catchments, and these are the easternmost sub-catchment (Flat bush), northern section sub-catchment which consists of a number of discrete sub-catchments draining industrial areas and the southern section sub-catchment which is mainly residential in nature and has a small commercial area around the shopping centre. Land in the upper parts of the catchment between Chapel Road and the eastern boundary hills is still currently rural and largely in livestock farming including dairying, beef cattle, horses and sheep. Most of this part is zoned for the future east Tamaki/Flatbush Integrated Development Area. The area incorporates the aspect of intensive residential development in close proximity to shopping service with a Greenfield area. The lower part of the catchment has intensive industrial and commercial centres, for example, Otara Town Centre which is located south of Ngati Otara Park bounded by Bairds and Tamaki Roads. This is the most developed part of the catchment for industrial, commercial and residential uses and imposes a range of impacts on the Otara Creek. In this study, six sites which represent pastoral area and native bush are restricted to Flat bush sub-catchment and three urban sites are in the rest of the sub-catchments ().
Table 1. ANOVA results showing F-ratio (F) and significance value at p < 0.05 for the mean values of water quality across the land use at Otara Creek.
In order to locate the comparable sites within the Otara Creek catchment area that differed in the land-use type (native bush, pasture and urban), an extensive survey of stream was conducted before this study commenced. Areas upstream and downstream in the catchment were visited in January 2004 for comparison purpose and to locate the sites with different land use. Kiwi Metromap (1:50,000) was also used to find the comparable sites with different land use along the catchment. The sites were selected in such a manner to allow a research to contrast stream communities influenced by three major land-use types in the area while minimising differences in other factors including geology, landforms, elevation and gradient. A three-level nested design was employed in a sample collection with each land use represented by three sites and each site was represented by three sampling which reaches 30 m each. Field study was conducted on summer February 2004 and consisted of three sections, a description of habitat and channel condition, semi-quantitative sampling using a hand-net and measurements of physical–chemical parameters of water quality.
2.2. Sampling of macroinvertebrates
Benthic macroinvertebrates were collected at each site from multiple habitats, including trailing vegetation, bottom substrate, channels banks and underbank-cut, to get a comprehensive species list and relative abundance. Macroinvertebrate samples were collected using a 250-μm mesh kick net from each habitat and at 90 m reach at each site. The net was dipped into each habitat and scooped against the current. The sample was allowed to drain to get rid of mud and silt materials before transferring the rest of the contents into an empty plastic container and preserved with 70% alcohol for later identification. All samples were collected within the same day to minimise variations due to climatic and hydrological changes (NCDENR Citation2001). Sampling was not conducted during heavy rain and freshes.
In the laboratory, invertebrate samples were washed through a 500-μm mesh sieve. Large pieces of substrate and leaves were inspected for invertebrates, which were removed from the sample. Remaining invertebrates were empted into the white trays for sorting activities. Invertebrates were identified according to Winterbourn and Gregson (Citation1989). Macroinvertebrates were classified to lower taxonomic levels except for Ostracoda, Amphipoda, Oligochaeta and Nematoda which were not classified further.
2.3. Habitat and channel condition assessment
The channel and habitat condition is an important determinant of the ecology and instream values associated with aquatic habitats (NIWA 2000). Habitat assessment was carried out from visual estimates of instream aquatic habitat, bank stability and riparian characteristics within a 90 m reach of stream (Woodsmith et al. Citation2005). Habitat was assessed using a modified Auckland Regional Council habitat assessment method (Maxted et al. Citation2000). This assessment leads to a single score that can be used for comparative purposes. The maximum score is 140 (high-quality habitat), and the minimum is zero (low-quality habitat).
At each point where macroinvertebrate samples were taken, wetted channel width, water depth, bank height and undercut width were measured with a metre tape. The substrate compositions were taken at five points across the wetted channel and were estimated visually. The substrate composition in each site was categorised visually according to the Wentworth-lane scale (Wilding Citation1996) as follows: clay (firm); silt (< 0.5 mm); sand (< 2 mm); gravel (< 2–64 mm); cobble (65–256 mm); boulders (> 256 mm); bedrock; coarse particulate organic matter (CPOM); fine particulate matter (FPOM); woody debris; macrophytes and concrete.
Bank stability was assessed using Pfanckuch bank stability form (Maxted et al. Citation2000) based on observation at each site. The assessment leads to a single score that can be used for comparative purposes. The maximum score is 152, which indicates poor channel and bank stability, and the minimum score is 38, which indicates excellent bank and channel stability.
2.4. Water quality measurements
Water samples for the measurements of nitrate, sulphate and phosphate were collected from each site and kept in plastic bottles under low temperature (in a chilly bin) and transported to the laboratory freezer. All the water samples taken to the laboratory were analysed a day after the field study. Measurements of physico–chemical parameters were done in situ using water quality checker (Horiba U21XD, Korea) calibrated before each day of sampling (Barr and Rees Citation2003). Nitrate and phosphate were analysed using the HACH DR700 colorimeter and the reagents provided (Murphy and Riley Citation1962; Nelson Citation1987; APHA et al. Citation1995). The analysis of nitrate was given as nitrogen (N), the value of nitrogen obtained was multiplied by 4.4 to get the value for nitrate (NO3).
2.5. Data analysis
A multiple approach that comprises a number of biotic indices each with different discriminatory power to detect different environmental stresses and to enable a broad-scale biological assessment was used to analyse the data (Clarke and Gorley Citation2001). Non-metric Multi-dimensional Scaling (NMDS) was used for graphical representation of community relationship (Clarke Citation1993). All data were square root transformed and then tested for normality prior to analysis. KMO and Bartlett's test of sphericity were run using the statistical computer program SPSS (version 11.5) as a test for normality which produced p < 0.0001, KMO index = 0.803) showing that the requirements for multivariate normal were satisfied (Clarke and Warwick Citation2001).
Two-way analysis of similarities (ANOSIM) was used to test whether there were any significant differences between the streams in three land uses and the site groups within those land uses (Clarke and Warwick Citation2001). In order to see which environmental variables were responsible for macroinvertebrates variation among the studied sites, BIOta ENVironmental (BIOENV) matching was preferred (Clarke and Warwick Citation2001).
3. Results
3.1. Physical conditions and water quality
Seven water-quality parameters were measured and compared from each land use (). Temperatures varied according to land uses showing daily maximum temperatures that were 5–6°C higher in urban and pasture streams than in bush streams. In general, urban streams had the highest average temperatures, followed by pasture streams and then bush streams. The differences were statistically significant between the bush and the remaining land uses (). Stream pH readings did not differ significantly among the land uses (p > 0.05), indicating that the stream water was neither acidic nor alkaline. Conductivity values varied according to land use. Generally, urban streams had higher mean conductivity values than pasture and bush streams. The trend was urban > pasture > bush (). Turbidity was the highest in pasture streams, with intermediate conditions in the urban streams, while bush had the lowest turbidity (). One-way analysis of variance (ANOVA) revealed significant differences in turbidity between the urban, pasture and bush streams (p < 0.05). However, there was no significant difference in turbidity between streams found in pasture and urban sites (p > 0.05).
There was a decreasing concentration of dissolved oxygen among the three land uses from bush toward urban (). One-way ANOVA showed significance difference (p < 0.05) in dissolved oxygen concentration across land uses. Total phosphate was significantly higher in pasture streams compared to urban and bush streams (p < 0.05). Nitrate concentration was significantly different across all three land-use streams (p < 0.05) and followed similar trend as total phosphate. The high nitrogen in pasture streams may originate from animal waste and increased fixation of nitrogen by clover in pasture, which suggests a strong land-use effect with high nitrogen input to pasture streams.
3.2. Macroinvetrebrate communities
Because the habitat and channel conditions as well as bottom substrate variables showed the high correlations with macroinvertebrate community, scores from these habitat indices were used along with species data in Multi-dimensional Scaling (MDS) where sites were assigned a code of one through three. Using Bray-Curtis similarity of square root transformed macroinvertebrate abundance data, MDS was used to illustrate that sites with similar habitat and channel conditions as well as bottom substrate contain similar macroinvertebrate species community composition. Ordination of biological and environmental data showed a clear separation of bush from urban and pastures streams (a and b; ).
Figure 2. Non-metric multidimensional scaling ordination of macroinvertebrates and environmental parameters. (a) Ordination of macroinvertebrate community structure at all sites. (b) Same ordination but for environmental parameters at all sites in Otara Creek.
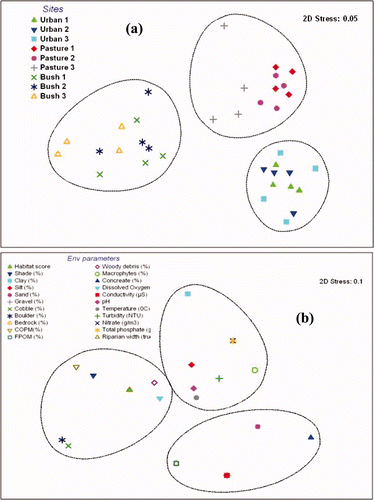
Table 2. Non-metric multi-dimensional scaling ordination axes showing correlation statistics for macroinvertebrate groups.
Cluster analysis clearly separated bush streams from urban and pastures streams (a). Axis 1 in b was associated with higher counts of Mollusca (e.g. Potamopyrgus), Annelida (e.g. Oligochaeta), Odonata (Xanthocnemis), Diptera (Tanytarsus), Crustacea (e.g. Isopoda), to the right hand side of the axis, which clustered urban and pasture streams. Ephemeroptera (e.g. Deleatidium, Zephlebia, Austroclima and Acanthophlebia), Trichoptera (e.g. Polyplectropus, Hydrobiosis, Psilochorema, Orthopysche, Oxyethira and Picnocentria) and Coleoptera (e.g. Scritidae and Elmidae) were associated with bush streams at the left hand side of the axis. Axis 2 on the other hand was associated with higher counts of Crustacea (e.g. Isopoda) to the top-right side of the axis and Annelida (e.g. Oligochaeta) to the bottom-right side of the axis. Pollution tolerant organisms from taxa such as Mollusca, Annelida and Crustacea tended to increase across axis 1 towards the right side along an environmental gradient to urban and pasture streams. The opposite was the case for pollution sensitive organisms (e.g. organisms belonging to EPT taxa).
3.3. Habitat and channel characteristics
3.3.1. Bottom substrate composition across land uses and seasons
In the bush streams cobbles, boulders, coarse particulate organic matter (CPOM) and wood debris dominated the bed substrate (), while clay, macrophytes and silt were dominant substrates in the pasture streams. Sand, gravel, bedrock and concrete dominated most of the urban streams. All bed substrates were significantly different among all land uses (ANOVA, p < 0.05) ().
Figure 3. Percentage of bottom substrates classified into 12 categories for the streams of Otara Creek in three land uses.
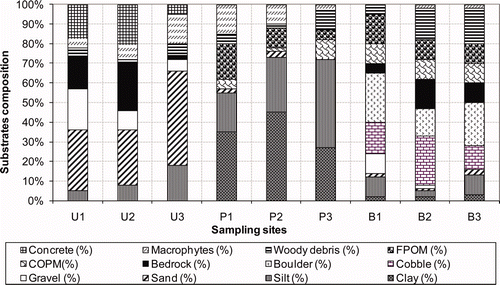
Table 3. ANOVA for bottom substrate composition across land-use showing degree of freedom (df), F-ratio (F) and significance value at p < 0.05.
3.3.2. Channel characteristic and habitat score across land uses
The mean values of habitat score and percentage shade across land use were higher in bush streams (92.33 ± 8.05 for habitat score and 79.50 ± 8.90 for percentage shade), intermediate in pasture streams (61.25 ± 9.78 for habitat score and 27.67 ± 9.02 for percentage shade) and lowest in urban streams (53.00 ± 3.41 for habitat score and 11.85 ± 5.50 for percentage shade) (). The mean value for the Pfankuch bank stability decreased across land use from urban streams (109.00 ± 11.85), pasture streams (89.00 ± 10.48) and bush streams (72.00 ± 2.15). Under-bank cut on both sides of the stream channel were much deeper in bush sites than in urban and pasture streams. The mean bank-height (both sides) was higher in bush streams followed by urban and lowest in pasture streams.
Figure 4. Comparison of habitat and channel characteristics as a function of land use in Otara Creek.
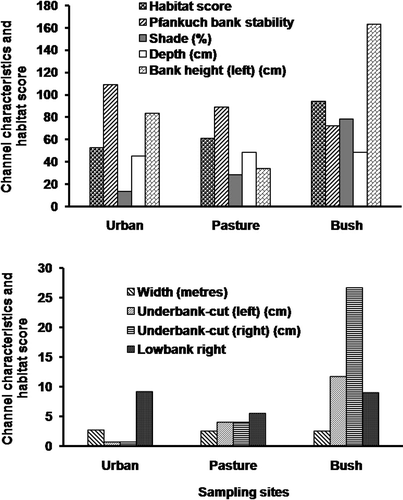
Significance difference (one-way ANOVA, p < 0.05) for all of the above variables (except channel width and depth) occurred across land use. Temporal mean values of the above variables were stable, and therefore, no significant seasonal differences were detected for all of the above variables ().
Table 4. ANOVA results for channel characteristics and habitat score across land-use showing degree of freedom (df), F-ratio (F) and significance value at p < 0.05.
3.3.3. Riparian vegetation composition and characteristics
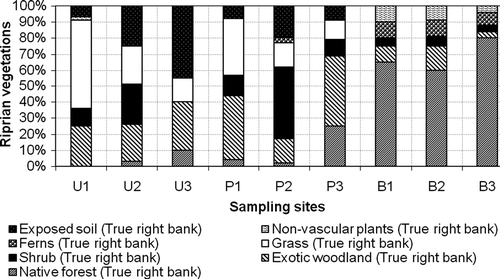
Assessment of riparian vegetation was undertaken to determine riparian vegetation composition and buffer widths. The results show that riparian vegetation composition varied significantly (one-way ANOVA, p < 0.05) () among land uses. Native forest accounted for an average of 68% of the bush sites. The mean values of riparian vegetation composition decreased to 10.33% and 4.33% for pasture and urban streams, respectively. Mean values of exotic woodland and scrubs present in the riparian vegetation were higher in pasture followed by urban streams and were lowest in bush streams. Exposed soil and grasses were a characteristic of urban streams, intermediate in pasture streams and lowest in bush streams (). Both canopy height and riparian width were higher in bush streams, intermediate in pasture streams and lowest in urban streams.
3.4. Linkage of macroinvertebrate assemblages to environmental variables (BIOENV)
A step was allowed for the BIOENV program to select the environmental variables responsible for macroinvertebrates variation among the studies sites. Correlation of biota and environmental variables revealed groups of environmental variables considered responsible for macroinvertebrates community patterns amongst the three stream types (). The observed macroinvertebrates assemblage pattern was best correlated with a single variable, riparian width (r = 0.81), followed by temperature (r = 0.79), boulders (r = 0.74), conductivity (r = 0.72), cobbles (r = 0.70), shade (r = 0.61), turbidity (r = 0.57), nitrate (r = 0.55), wood debris (r = 0.41), COPM (r = 0.36), temperature (r = 0.39), Habitat score (r = 0.32) and dissolved oxygen (r = 0.15).
Table 5. Results of the multivariate Spearman rank correlation of environmental data to benthic community data using BIOENV showing the importance of a single variable as well as a combination of two, three and five environmental variables.
A combination of two variables which best correlated with the biota assemblages were cobbles and riparian width (r = 0.88), while a combination of three environmental variables which best correlated with the biota assemblages were boulders, nitrate and riparian width (r = 0.91). A combination of five environmental variables which best explained the changes in the macroinvertebrate assemblages between sites were habitat score, shade, boulders, nitrate and riparian width (r = 0.94) (). Bottom substrate, water quality and habitat conditions play a vital role in shaping the distribution of macroinvertebrates in ecosystems. The importance and relationships between these variables and macroinvertebrate communities are discussed in the next section.
Generally, stream shade, riparian width, temperature, conductivity, turbidity, nitrates, dissolved oxygen and large-sized bottom substrates have been found to strongly correlate with macroinvertebrate distributions observed for all seasons. Such variables as elevated temperature, high concentrations of nitrate, conductivity and turbidity have been associated with urban and pasture development. Large-sized bottom substrates and shade were well reflected to the benthic community assemblages found in bush streams.
The most abundant macroinvertebrate communities groups that showed a significant relationship with environmental variables (e.g. substrate size) were Ephemeroptera, Trichoptera, Coleoptera, Oligochaeta, Snails (primarily, Potarmopyrgus) and Crustacea (Isopoda and Ostracoda) (Data not shown). Ephemeroptera, Trichoptera and Coleoptera were most commonly associated with coarse substrates (higher substrates index), high habitat score, higher proportions of shade and riparian width and high dissolved oxygen. The other three groups were generally found in urban and pasture streams with finer mud/silt and sand, low riparian shading and riparian width (Table 5).
4. Discussion
4.1. Relationship between habitat, channel condition and land use
The distribution of taxa within an area reflects the concordance of habitat conditions and habitat requirements of organisms. The influence of benthic habitat substrates on the distribution and diversity of macroinvertebrates in aquatic systems has been associated with substrate particle size in stream habitats (Riley et al. Citation2003), spatial heterogeneity of habitat in littoral zones (Parkyn et al. Citation2003; Quinn et al. Citation2004) and the substrate preference of organisms in aquatic systems (Collier and Kelly Citation2006).
Based on the analysis of macroinvertebrate communities using BIOENV procedure, the current study has demonstrated that bed substrate and channel characteristics were among the most important factors directly controlling macroinvertebrates distribution and abundance in the streams studied. The bed substrate of streams draining bush sites was characterised by cobbles, boulders, coarse particulate organic matter (COPM) and wood debris. On the other hand, streams draining pasture streams were characterised by bed substrate with higher content of macrophytes, clay and silt. Sand, silt, gravel, bedrock and concrete were not uncommon among beds of streams draining urban streams. Quinn et al. (Citation1997) and Scarsbrook et al. (Citation2000) in their studies reported the similar results where they found higher COPM in forest streams and native bush sites than in pasture streams.
Large size and varieties of substrate (e.g. woody debris, boulders and particulate organic matter), observed in bush streams, play many roles in streams, including habitat formation, food source, sites for egg laying and pupation (Harmon et al. Citation1986; Benke and Wallace Citation1990; Mosley 1992). Varieties of substrate type and size in most of the bush streams in the current study could be associated with the observed high abundance of Ephemeroptera (e.g. Deleatidium spp.), Acanthophlebia and Trichoptera (e.g. Polyplectropus and Triplectides) taxa in such streams. High abundance of P. antipodarum, Oligochaeta and Ostracoda reflected lack of heterogeneous substrate and poor habitat quality observed in urban and pasture streams. The pollution sensitive taxa (Ephemeroptera, Trichoptera and Coleoptera) negatively correlated with the fine sediment particles (e.g. clay and silt) while positive correlations were found between those taxa and cobbles, boulders, COPM and wood debris. Positive correlation was also found between P. antipodarum and macrophyte. Oligochaeta and Ostracoda correlated with clay and silt as was expected (a and b; ). These results are consistent with those of Weatherhead and James (Citation2001) and Jowett et al. (Citation1991) who found association of EPT taxa with habitats characterised of cobbles and boulders.
Feeding mode, water quality requirements and biotic interaction of invertebrates are deemed to be amongst the determinants of preference of invertebrates to substrate size (Quinn and Hickey Citation1990a, Rounick and Winterbourn Citation1983). Webster (Citation1987) reported that large particles provide a more stable habitat for both periphyton and invertebrates, and that they trap and retain more coarse particulate organic matter. Quinn and Hickey (Citation1990b) added that this may account for the strong preference of the shredders (e.g. Trichopterans – Triplectides spp.) for large cobbles and boulders. Parker (Citation1989) on the other hand reported that smaller interstitial spaces of gravel beds are expected to retain more fine particulate organic matter, which according to Quinn and Hickey (Citation1990a) may account for the greatest abundance of detrital-feeding Oligochaetes and Nematodes in rivers.
Habitats such as coarse substrates, COPM, cobbles and wood debris which were a characteristic of bush streams were reduced in urban and pasture streams probably as a result of the removal of riparian vegetation. Such changes may cause reduction in input of organic matter, reduction in bank stability, increase in sediment loadings and scouring of the streams. The presence of macrophytes in pasture streams compared to the occurrence of wood debris in bush streams indicates a shift in relative importance of allochthonous and autochthonous food and nutrient sources for the biota in the respective streams. A shift from allochtonous food source to autochthonous food together with other factors in the pasture and urban streams may have contributed to the observed differences in stream macroinvertebrate communities across land use.
Apart from bed substrate and macrophyte discussed above, other physical parameters information such as channel morphology, bank stability and riparian vegetation and amount of shade were used to link and support chemical and biological data with land use. Among these, riparian width and stream shade had strongest influence in determining the macroinvertebrate communities’ composition pattern in Otara Creek. Higher in-stream temperatures were observed in some pasture and urban streams and are associated with the lower riparian shade and too narrow riparian width compared to bush streams. The strong correlation between shade and macroinvertebrate communities indicates that shade also had a strong influence on the macroinvertebrate distribution. For example, several species of mayfly, stonefly, and caddisfly, which are sensitive to high temperature, are poorly represented in unshaded pasture and urban streams (Quinn and Hickey Citation1990a). Riparian width was also found to strongly influence the macroinvertebrate communities. Bush streams had wider riparian strips compared to pasture and urban streams. The width of vegetated riparian zone determines to a large extent the reduction in diffuse silt and nutrient concentrations reaching the stream. More vegetation can take up larger quantities of silt. Although there are no magic numbers in sustaining the natural functions of stream (Wilding Citation1996), the required width of riparian zones will vary depending upon the aquatic characteristics to be managed (Collier et al. Citation1995a) and should also increase in direct proportion to the size of the area contributing runoff, sediment and nutrients, and the intensity of cultural activities and disturbances in the uplands such as agriculture, forestry, or suburban or urban development (Leung Citation1999).
4.2. Effects of bottom substrate and riparian vegetation on water quality and macroinvertebrate communities
The first dimension of Multi Dimensional Scaling (MDS) ordinations appeared to represent a biologically meaningful gradient attributing the lowest values to the urban and pasture streams, whereas the highest values were consistently recorded for bush sites. As seen from the taxa abundance plots, a shift in community composition from pollution-intolerant to pollution-tolerant was apparent in the streams studied. Large proportions of taxa found in bush sites were absent in the urban and pasture streams. Although there was a close association between the macroinvertebrate communities collected from certain areas of pasture and bush, separation of the sites was clear and without an overlap. This may be explained by the fact that pasture sites had dense riparian vegetation on both sides and the streams were well shaded, which suggest that the stream has a better quality of habitat. The stream had also high abundance of trichoptera (Polyplectropus spp. and Triplectides obsoleta).
It is likely that these taxa benefit from the presence of wide riparian vegetation. However, part of bush sites comprised predominantly crustaceans and snails which were also more abundant in pasture and urban sites, respectively. The macroinvertebrate communities’ composition of this site suggested that the site is not completely free of human disturbance. The reason for this is probably that Murphys Road runs beside the stream and there is also a large stormwater pipe discharging into the stream. Therefore, despite its appearance as a relatively unimpacted, part of bush site is subject to a number of pressures that result in its relatively degraded state.
The presence of pollution-tolerant macroinvertebrates in urban and pasture streams could be attributed to a lack of riparian vegetation alongside these streams, which through reduction of leaf litter input and light penetration to streams changed the energy base of the ecosystem. This has been addressed in other studies (Quinn et al. Citation1992; Collier et al. Citation1995a, 1995b; Quinn Citation2000) as a determinant of impacts of riparian grazing on the invertebrates in small streams. Amphipods feed on fine detritus and are more abundant in the presence of macrophytes, as reported by James et al. (Citation1998), and macrophytes usually prefer to grow in an open, unshaded streams trapping fine particulate matter. The mollusc P. antipodarum appeared to reflect stream temperature, as reported by Quinn et al. (Citation1997) and be moderately tolerant to higher temperatures (up to 28°C). Oligochaetes are deposit feeders and need fine detritus, hence their abundance in urban and pasture streams where there was high proportion of the fine bottom substrates.
Bush streams that supported the rich and most sensitive macroinvertebrate communities were characterised as having intact riparian zones, high dissolved oxygen concentrations, low water temperatures and a high percentage of coarse substrates (cobbles, boulders, coarse organic particulate matter and wood debris). Biotic indices were highly correlated with all of these environmental variables, indicating that as physical and chemical conditions have become impaired by increased land-use intensity across the streams (as seen in urban and pasture streams), macroinvertebrate communities also have been compromised.
One aspect of disturbance which was not measured directly in this study, yet is known to significantly affect both the form and function of streams, was hydrologic modification by urban and pasture-related land use. Urban development, in particular, significantly alters stream hydrology (Booth and Jackson Citation1997). Water velocity, depth and seasonal flow patterns are important factors that influence species distribution and life-cycle activities of stream organisms. Stream velocity affects oxygen levels, the retention of organic materials and the ability of aquatic organisms to move up and down the stream. Loss of riparian vegetation (as result of urbanisation and pasture development) can affect streams and aquatic communities by increasing the intensity and frequency of flood events and the degree and duration of low-flow conditions during droughts. Increased impervious areas within a catchment can result in less rainfall infiltration and an increase in storm water run-off. An example of this phenomenon is in the lower reaches of Otara Creek, where there is a high level of imperviousness and a high proportion of industrial, commercial and residential properties which have impacted the natural character of the stream.
4.3. Linkage of macroinvertebrate assemblages to environmental variables
The BIOENV procedure (Clarke and Warwick Citation1994) was used to identify which underlying environmental variable best correlated with the observed community patterns. The variety of factors believed to influence macroinvertebrate communities (e.g. temperature, conductivity, dissolved oxygen, turbidity and nutrient) that differed between the streams in the different land uses makes it difficult to establish the exact causes of changes. However, correlation of environmental variables with macroinvertebrate communities (using BIOENV procedure) indicated that conductivity, temperature, nitrate, phosphate and dissolved oxygen were particularly important in the distribution of macroinvertebrates across the land use.
It is obvious that changes in these parameters can be attributed to a variety of human disturbances (e.g. inputs of contaminants into the streams). In some urban and pasture reaches, the loss of riparian vegetation greatly reduced shading and the capability of trapping non-point source pollutants. This has consequently resulted in more run-off and contaminants draining into the watercourses, deteriorating the stream health and conditions. This is reflected in the low abundance and perhaps absence of more sensitive taxa at the pasture and urban streams. Pollution-sensitive taxa such as mayflies Deleatidium spp. and Zephlebia prefer a larger substrate size with high interstitial oxygen levels (Jowett and Richardson Citation1990) and shaded streams with lower in-stream temperature. The blanketing effect of filamentous algae (observed in pasture) streams, combined with higher suspended sediment concentrations may have reduced interstitial oxygen levels making these streams unfavourable for sensitive species. Less sensitive species (P. antipodurum and Oligochaeta) which were more abundant in urban and pasture streams are generally known to prefer smaller substrate sizes (Jowett et al. Citation1991) and unshaded reaches (Towns 1985). Change in catchment land use, particularly moving from native forest to pasture and urban development can affect the type of organic matter present in a stream. The quantities of leaves and wood entering a stream will be reduced, and the food bases may shift from allochthonous to autochthonous (Winterbourn et al. Citation2006). This was observed in the current study in that pasture and urban streams had less organic particulate matter and woody debris compared to bush streams.
5. Conclusion
The riparian vegetation provides bank stability, clean substrates, adequate canopy cover for temperature control, refugia during floods and organic matter to the stream. Thus, the riparian vegetation indirectly contributes to good water quality and healthy macroinvertebrate community. However, the scores of riparian vegetation is not an adequate measurement for statistical analyses, because it is qualitative in nature. Further study of correlations between quantitative riparian measures and macroinvertebrate structure and function using a large dataset should be conducted to improve this analysis. Because riparian vegetation plays a role in increasing biodiversity and serves to provide habitat for native fauna, continuous research and monitoring of riparian vegetation (i.e. wide mapping) should be maintained. The loss of riparian vegetation through clearing, livestock grazing or recreational uses, can contribute to the loss of these benefits and the decrease of the overall condition of the stream. Physical data parameters such as riparian zone width and sediment deposition are very important in studying riparian and aquatic ecosystems, and thus, the proper equipment is needed to more accurately measure these parameters. It is as well important to understand associations between biota and the bottom substrate in order to parse out reasons responsible for structuring benthic macroinvertebrate communities.
Acknowledgements
The author wishes to express sincere thanks to the New Zealand Government for financing this work through the NZAID Scholarship. The author is also thankful to Dr Ian Boothroyd and his team (School of Geography and Environmental Science, Auckland University, New Zealand) for their help, advice and support while conducting this study.
References
- APHA , AWWA and WEF . 1995 . Standard methods for the examination of water and wastewater , 19th ed. , Washington, DC, USA : America Public Health Association, American Water Works Association, Water and Environment Federation .
- Baker , M E , Weller , D E and Jordan , T E . 2006 . Improved methods for quantifying potential nutrient interception by riparian buffers . Landscape Ecol , 21 ( 8 ) : 1327 – 1345 .
- Barr , N G and Rees , T AV . 2003 . Nitrogen status and metabolism in the green seaweed Enteromorpha intestinalis: an examination of three natural populations . Mar Ecol Progr Ser , 249 : 133 – 144 .
- Benke , A C and Wallace , J B . 1990 . Wood dynamics in coastal plain blackwater stream . Can J Fish Aquat Sci , 47 : 92 – 99 .
- Booth , D B and Jackson , C R . 1997 . Urbanization of aquatic systems-degradation thresholds, stormwater detention, and limits of mitigation . J Am Water Resour Assoc , 33 ( 5 ) : 1077 – 1090 .
- Carline , R F and Walsh , M C . 2007 . Responses to riparian restoration in the Spring Creek watershed, central Pennsylvania . Restor Ecol , 15 ( 4 ) : 731 – 742 .
- Clarke , K R . 1993 . Non-parametric multivariate analyses of changes in community structure . Aust J Ecol , 18 : 117 – 143 .
- Clarke , K R and Gorley , R N . 2001 . PRIMERv5: user manual/tutorial , Plymouth , , UK : PRIMER-E .
- Clarke , K R and Warwick , R M . 1994 . Change in marine communities: an approach to statistical analysis and interpretation , Plymouth , , UK : Plymouth Marine Laboratory .
- Clarke , K R and Warwick , R M . 2001 . Change in marine communities: an approach to statistical analysis and interpretation , 2nd ed. , Plymouth , , UK : PRIMER-E .
- Clinton , B D , Vose , J M , Knoepp , J D , Elliott , K J , Reynolds , B C and Zarnoch , S J . 2010 . Can structural and functional characteristics be used to identify riparian zone width in southern Appalachian headwater catchments? . Can J Forest Res , 40 : 235 – 353 .
- Collier , K J , Copper , A B , Davis-Colley , R J , Rutherford , J C , Smith , C M and Williamson , R B . 1995a . Managing riparian zones: a contribution to protecting New Zealand's rivers and streams. Volume 1: , Vol. 1 , Guidelines. Wellington , , New Zealand : Department of Conservation .
- Collier , K J , Cooper , A B , Davis-Colley , R J , Rutherford , J C , Smith , C M and Williamson , R B . 1995b . Managing riparian zones: a contribution to protecting New Zealand's rivers and streams. Volume 2: , Vol. 2 , Guidelines. Wellington , , New Zealand : Department of Conservation .
- Collier , K J and Kelly , J . Patterns and trends in the ecological condition of Waikato streams based on the monitoring of aquatic invertebrates from 1994 to 2005 . Environment Waikato Technical Report 2006/04 . pp. 28
- Costello , D and Lamberti , G . 2008 . Non-native earthworms in riparian soils increase nitrogen flux into adjacent aquatic ecosystems . Oecologia , 158 ( 3 ) : 499 – 510 .
- Dawson , F H and Haslam , S M . 1993 . The management of river vegetation with particular reference to shading effects of marginal vegetation . Landscape Plan , 10 : 147 – 169 .
- DeWalle , D R . 2010 . Modeling stream shade: riparian buffer height and density as important as buffer width . J Am Water Resour Assoc , 46 ( 2 ) : 323 – 333 .
- Dosskey , M G , Helmers , M J and Eisenhauer , D E . 2008 . A design aid for determining width of filter strips . J Soil Water Conserv , 63 ( 4 ) : 232 – 241 .
- Dosskey , M G , Vidon , P , Gurwick , N P , Allan , C J , Duval , T P and Lowrance , R . 2010 . The role of riparian vegetation in protecting and improving chemical water quality in streams . J Am Water Resour Assoc , 46 ( 2 ) : 261 – 277 .
- Ficetola , G F , Padoa-Schioppa , E and De Bernardi , F . 2009 . Influence of landscape elements in riparian buffers on the conservation of semiaquatic amphibians . Conserv Biol , 23 ( 1 ) : 114 – 123 .
- Ghermandi , A , Vandenberghe , V , Benedetti , L , Bauwens , W and Vanrolleghem , P A . 2009 . Model-based assessment of shading effect by riparian vegetation on river water quality . Ecol Eng , 35 ( 1 ) : 92 – 104 .
- Gorsevski , P , Boll , J , Gomezdelcampo , E and Brooks , E S . 2008 . Dynamic riparian buffer widths frompotential non-point source pollution areas in forested watersheds . Forest Ecol Manage , 256 : 664 – 673 .
- Gregory , S V , Swanson , F J , McKee , W A and Cummins , K W . 1991 . An ecosystem perspective of riparian zones . Bioscience , 41 ( 8 ) : 540 – 551 .
- Harmon , M E , Franklin , J F , Swanson , F J , Sollins , P , Gregory , J D , Lattin , J D , Anderson , N H , Cline , S P , Aumen , N G Sedell , J R . 1986 . Ecology of coarse woody debris in temperate ecosystems . Adv Ecol Res , 15 : 133 – 302 .
- Horwitz , F J , Johnson , T E , Overbeck , P F , O'Donnell , T K , Hession , W C and Sweeney , B W . 2008 . Effects of riparian vegetation and watershed urbanization on fishes in streams of the mid-Atlantic Piedmont (USA) . J Am Water Resour Assoc , 44 ( 3 ) : 724 – 741 .
- James , M R , Weatherhead , M , Stanger , C and Graynoth , E . 1998 . Macroinvertebrate distribution in the littoral zone of Lake Coleridge, South Island, New Zealand – effects of habitat stability, wind exposure, and macrophytes . NZ J Mar Freshw Res , 32 : 287 – 305 .
- Jowett , I G and Richardson , J . 1990 . Microhabitat preferences of benthic invertebrates in a New Zealand river and the development of in-stream flow-habitat models for Deleatidium spp . NZ J Mar Freshw Res , 24 : 19 – 30 .
- Jowett , I G , Richardson , J , Biggs , B JF , Hickey , C W and Quinn , J W . 1991 . Microhabitat preferences of benthic invertebrates and the development of generalized Deleatidium spp. habitat suitability curves, applied to four New Zealand rivers . NZ J Mar Freshw Res , 25 : 187 – 199 .
- Leung , Y C . Study on the production and application of biodiesel fuel in reducing the emissions from diesel vehicles in Hong Kong . Workshop on Air Pollution in Pearl River Delta. Hong Kong: Hong Kong University of Science and Technology . pp. 2
- Lovett , S , Price , P and editors . 2007 . Principles for riparian lands management , Canberra : Land and Water Australia .
- Madden , S S , Robinson , G R and Arnason , J G . 2007 . Spatial variation in stream water quality in relation to riparian buffer dimensions in a rural watershed of eastern New York state . Northeastern Naturalist , 14 ( 4 ) : 605 – 618 .
- Mankin , K R , Ngandu , D M , Barden , C J , Hutchinson , S L and Geyer , W A . 2007 . Grass-shrub riparian buffer removal of sediment, phosphorus, and nitrogen from simulated runoff . J Am Water Resour Assoc , 43 ( 5 ) : 1108 – 1116 .
- Manukau City Council . 2001 . Comprehensive Otara Creek catchment study and management plan options. Manukau , 77 New Zealand : Manukau City Council .
- Maxted , J R , Barbour , M T , Gerritsen , J , Poretti , V , Primrose , N , Silvia , A , Penrose , D and Renfrow , R . 2000 . Assessment framework for mid-Atlantic coastal p ain streams using benthic macroinvertebrates . J North Am Benthol Soc , 19 : 128 – 144 .
- Mosley , M P . 1992 . “ River morphology ” . In Water of New Zealand , Edited by: Mosely , M P . 285 – 304 . Wellington , , New Zealand : New Zealand Hydrological Society .
- Murphy , J and Riley , J P . 1962 . A modified single solution method for the determination of phosphate in natural waters . Anal Chim Acta , 27 : 31 – 36 .
- National Institute of Water and Atmospheric Research (NIWA) . 2003 . Climate statistics, national climate database , Wellington , , New Zealand : NIWA .
- NCDENR . 2001 . Standard operating procedure for benthic macroinvertebrates , Raleigh NC : Biological Assessment Unit, Environmental Sciences Branch, Water Quality Section, Division of Water Quality, North Carolina Department of Environment and Natural Resources .
- Nelson , N S . 1987 . An acid-persulfate digestion procedure for determination of phosphorus in sediments . Commun Soil Sci Plant Anal , 18 ( 4 ) : 359 – 369 .
- Newbold , J D , Erman , D C and Roby , K B . 1980 . Effects of logging on macroinvertebrates in streams with and without buffer strips . Can J Fish Aquat Sci , 37 : 1076 – 1085 .
- Parker , M S . 1989 . Effect of substrate composition on detritus accumulation and macro invertebrate distribution in a southern Nevada desert stream . SW Nat , 34 : 181 – 187 .
- Parkyn , S M , Davies-Colley , R J , Halliday , N J , Costley , K J and Croker , G F . 2003 . Planted riparian buffer zones in New Zealand: do they live up to expectations? . Restor Ecol , 11 ( 4 ) : 436 – 447 .
- Quinn , J M . 2000 . “ Effects of pastoral development ” . In New Zealand stream invertebrates: ecology and implications for management , Edited by: Collier , K J and Winterbourn , M J . 208 – 229 . Christchurch , , New Zealand : New Zealand Limnological Society .
- Quinn , J M , Boothroyd , L KG and Smith , B J . 2004 . Riparian buffers mitigate effects of pine plantation logging on New Zealand streams. 2: invertebrate communities . Forest Ecol Manage , 191 : 129 – 146 .
- Quinn , J M , Copper , A B , Davis-Colley , R J , Rutherford , J C and Williamson , R B . 1997 . Land-use effects on habitat, water quality, periphyton, and benthic invertebrates in Waikato, New Zealand, hill-country streams . NZ J Mar Freshwat Res , 31 : 579 – 597 .
- Quinn , J M and Hickey , C W . 1990a . Characterisation and classification of benthic invertebrates communities in 88 New Zealand rivers in relation to environmental factors . NZ J Mar Freshwat Res , 24 : 387 – 409 .
- Quinn , J M and Hickey , C W . 1990b . Magnitude of effects of substrate particle size, recent flooding, and catchment development on benthic invertebrates in 88 New Zealand Rivers . NZ J Mar Freshwat Res , 24 : 411 – 427 .
- Quinn , J M , Williamson , R B , Smith , R K and Vickers , M L . 1992 . Effects of riparian grazing and channelization on streams in Southland, New Zealand. 2. Benthic invertebrates . NZ J Mar Freshwat Res , 26 : 259 – 269 .
- Riley , R H , Townsend , C R , Niyogi , D K , Arbuckle , C A and Peacock , K A . 2003 . Headwater stream response to grassland agricultural development in New Zealand . NZ J Mar Freshwat Res , 37 : 389 – 403 .
- Rounick , J S and Winterbourn , M J . 1983 . The formation, structure, and utilization of stone surface organic layers in two New Zealand streams . Freshwat Biol , 13 : 57 – 72 .
- Scarsbrook , M R , Boothroyd , I KG and Quinn , J M . 2000 . New Zealand's national river water quality network: long-term trends in macroinvertebrate communities . NZ J Mar Freshwat Res , 34 ( 2 ) : 289 – 302 .
- Shilla , D J and Shilla , D A . 2011 . The effects of catchment land use on water quality and macroinvertebrate assemblages in Otara Creek, New Zealand . Chem Ecol , 27 ( 5 ) : 445 – 460 .
- Suren , A M . 2000 . “ Effects of urbanization ” . In Zealand stream invertebrates: ecology and implications for management , Edited by: Collier , K J , Winterbourn , M J and New . 260 – 288 . Christchurch , , New Zealand : New Zealand Limnological Society .
- Thomas , H , Christian , B , Gerhard , J H , Wolfgang , W and Fritz , S . 2003 . Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: the importance of hydrological connectivity . Freshwat Biol , 48 : 220 – 232 .
- Towns , D R . Life history patterns and their influence on monitoring invertebrate communities . Biological monitoring in freshwaters: Proceedings of a Seminar . Nov 21–23 1984 . Edited by: Pridmore , R D and Cooper , A B . pp. 225 – 239 . Hamilton : Water and Soil Miscellaneous Publication .
- Vannote , R L , Minshall , G W , Cummins , K W , Sedell , J R and Cushing , C E . 1980 . The river continuum concept . Can J Fish Aquat Sci , 37 : 130 – 137 .
- Weatherhead , M A and James , M R . 2001 . Distribution of macroinvertebrates in relation to physical and biological variables in the littoral zone of nine New Zealand lakes . Hydrobiologia , 462 : 115 – 129 .
- Webster , J R . 1987 . The role of benthic macroinvertebrates in detritus dynamics of Streams: a computer simulation . Ecol Monogr , 53 : 383 – 404 .
- Westlake , D F . 1966 . “ The light climate for plants in rivers ” . In Light as an ecological factor , Edited by: Bainbridge , R , Evans , GC and Rackham , O . 99 – 119 . Oxford : Blackwell .
- Wilding , T K . 1996 . Effects of urban development on small stream ecosystems of Auckland, and the influence of riparian vegetation [MSc Thesis] , Auckland : University of Auckland .
- Wilkerson , E , Hagan , J M , Siegel , D and Whitman , A A . 2006 . The effectiveness of different buffer widths for protecting headwater stream temperature in Maine . Forest Sci , 52 ( 3 ) : 221 – 231 .
- Wilkerson , E , Hagan , J M and Whitman , A A . 2010 . The effectiveness of different buffer widths for protecting water quality and macroinvertebrate and periphyton assemblages of headwater streams in Maine, USA . Can J Fish Aquat Sci , 67 ( 1 ) : 177 – 190 .
- Winterbourn , M J . 1996 . Effects of land development on benthic stream communities . NZ Agric Sci , 19 : 115 – 118 .
- Winterbourn , MJ and Gregson , KLD . 1989 . Guide to the aquatic insects of New Zealand , 2nd ed. , Vol. 9 , 96 Auckland , , New Zealand : Bulletin of the Entomological Society of New Zealand .
- Winterbourn , M J , Gregson , K LD and Dolphin , C H . 2006 . Guide to the aquatic insects of New Zealand , 4th ed. , Vol. 14 , Auckland , , New Zealand : Bulletin of the Entomological Society of New Zealand .
- Winward , A H . 2000 . Monitoring the vegetation resources in riparian areas. General Technical Report RMRS-GTR-47 , 49 Ogden, UT : United States Department of Agriculture-Forest Service, Rocky Mountain Research Station .
- Woodsmith , R D , Noel , J R and Dilger , M L . 2005 . An approach to effectiveness monitoring of floodplain channel aquatic habitat: channel condition assessment . Landscape Urban Plan , 72 : 177 – 204 .