ABSTRACT
Imprudent and superfluous use of antibiotics contributes to the selection of resistant bacteria, which is a large threat to human health. Therefore analytical procedures have been implemented in the poultry production sector to check if antibiotic treatments are registered, aiming to achieve more prudent use of antibiotics. These methods rely on the analysis of feathers, a matrix in which antibiotic residues persist. However, other routes besides direct administration, through which poultry feathers could contain antibiotic residues, should also be taken into account. In this research the vertical transmission from parent hen to broiler was investigated through a controlled animal study for the antibiotics enrofloxacin, doxycycline and sulfachlorpyridazine. Vertical transmission was observed for all antibiotics to both egg and egg shell. Also it is demonstrated that the transferred antibiotics from parent hen to chick are subsequently excreted via the chick’s droppings. Through this route, the broilers’ environment is contaminated. If eggs are hatched that were taken during treatment of the parent hen, this indirect route and/or the direct vertical transmission can eventually result in the detection of low concentrations of antibiotic residues in the broilers’ feathers at greater age: <50 µg kg−1 for freely extractable residues and <10 µg kg−1 for non-freely extractable residues. No antibiotics were detected in the broilers’ muscle or kidney from 4 weeks of age. This research provides relevant information regarding the possible amount of residues originating from vertical transmission when monitoring matrices such as feathers and broiler droppings in order to stimulate correct use and registration of antibiotics in the poultry sector.
Graphical abstract
Introduction
Antibiotics are of great importance to cure disease in humans and animals by killing or inhibiting the growth of bacteria. Therefore antibiotics are used worldwide to treat infections in both humans and animals. In animal husbandry, antibiotics are mainly used according to registration of specific formulations (veterinary drugs) for defined purposes and species. However, forbidden use cannot be excluded for growth-promoting purposes, treating animals at sub-therapeutic levels over a long period of time, or off-label and unnecessary use.
Imprudent and unnecessary use of antibiotics contributes to the selection of resistant bacteria. Over a million people die of an untreatable bacterial infection each year worldwide (WHO Citation2014). This includes 25.000 people from the European Union (EU) (European Commission Citation2015) and 23.000 people from the United States (US Department of Health and Human Services Citation2013).
In order to decrease the use of antibiotics, measures aiming for a more selective and prudent use of antibiotics were initiated. This includes the restricted use of last-resort antibiotics, such as (fluoro)quinolones and 3rd and 4th generation cephalosporins, in food producing animals and the registration of antibiotic use by the farmer under supervision of a veterinarian. These measures lead to a decrease of 58.4% in the use of antibiotics in animal husbandry in The Netherlands in 2015 compared to 2009. However, the decrease observed from 2013 to 2017 was minimal (RIVM Citation2018).
Products of animal origin in the EU and The Netherlands are monitored for antibiotic residues by enforcing maximum residue limits (MRL) (European Commission Citation1990, Citation2010). However, these monitoring methods proved not to be sufficient in enforcing correct registration of antibiotics administered as residues are usually relatively quickly excreted from these matrices (San Martín et al. Citation2007; Cornejo et al. Citation2011a; Berendsen et al. Citation2013). Consequently, there was a need for matrices in which antibiotics could be detected over a longer time span. For the monitoring of antibiotic use in poultry, the analysis of feathers provided a means to detect antibiotic residues with a long detection window, even after they were excreted fully from muscle and liver (San Martín et al. Citation2007; Cornejo et al. Citation2011b; Berendsen et al. Citation2013; Cornejo et al. Citation2017; Pokrant et al. Citation2018; Církva et al. Citation2019; Maddaleno et al. Citation2019). Using such a non-invasive method, antibiotic use during almost the whole lifespan of a broiler can be monitored, making it possible to better enforce registration of antibiotics use aiming for prudent application (Jansen et al. Citation2016, Citation2017).
Another non-invasive way to monitor antibiotic usage is through analysis of bird droppings. After administration of antibiotics, 30–90% is excreted as the parent compound. Monitoring studies reported high concentrations of antibiotics found in poultry, often finding multiple compounds in a single dropping sample (Zhao et al. Citation2010; Carballo et al. Citation2016). Therefore droppings analysis is useful to enforce policies, preventing illegal and off-label use of antibiotics.
Because of the potential of these matrices to detect antibiotic use over a long time span, other pathways (besides direct treatment) through which antibiotics might enter the animal should be taken into account. It is known that after treatment of laying hens, antibiotic residues are transferred to eggs (Yoshimura et al. Citation1991; Gorla et al. Citation1997; Roudaut and Garnier Citation2002; Cornejo et al. Citation2011a; Bilandžić et al. Citation2015; Gajda and Posyniak Citation2015). For parent hens the antibiotics transfer to eggs is expected to occur as well. It might even be possible that these residues subsequently transfer to the new-born chick and are detectable in the next generation broiler. However, to what extent vertical transmission of antibiotic residues from parent hens to broilers occurs has not been studied before.
To our best knowledge, no literature is available regarding the vertical transmission of antibiotics from parent hen to broiler. In this study, the transmission from parent hen to eggs, which was frequently reported, is only the first step. We selected critically important antibiotic classes according to the World Organisation for Animal Health (OIE Citation2019) and took into account the classification of critically important antibiotics for human health according to the World Health Organisation (WHO Citation2019). Among these we chose antibiotic classes having different chemical properties and of which is known that the likelihood of transfer from parent hen to broiler occurs. Last, within the selected antibiotic classes we selected the compounds that a most frequently applied. Sulfachlorpyridazine was selected as it is the most commonly used sulphonamide within breeding establishments. Based on several studies (Yoshimura et al. Citation1991; Gorla et al. Citation1997; Roudaut and Garnier Citation2002; Cornejo et al. Citation2011a; Bilandžić et al. Citation2015; Gajda and Posyniak Citation2015), sulphonamides were indicated to have the largest risk of vertical transmission. Beside sulphonamides, tetracyclines are often used in the poultry sector and as their chemical properties are very different compared to the sulphonamides, they might show very different behaviour. Therefore doxycycline, which is the most often used tetracycline in poultry breeding, was investigated as well. Finally, enrofloxacin is a fluoroquinolone which is listed as a third choice antibiotic on the WHO list of last resort antibiotics; however it is still used in the poultry sector. Furthermore, residues of enrofloxacin have been detected before using feather analysis (Jansen et al. Citation2017). Therefore enrofloxacin was also selected.
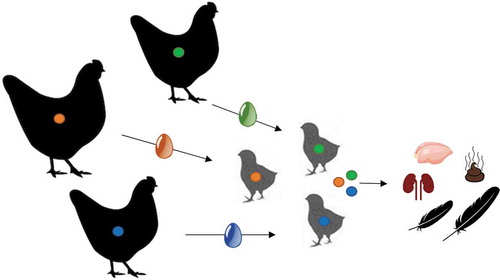
In this research the vertical transmission from parent hen to broiler was investigated for the antibiotics enrofloxacin, doxycycline and sulfachlorpyridazine. Even though the transmission of antibiotic residues to eggs has frequently been reported, we included this step in our study to determine the total amount of antibiotics that could theoretically be transferred to the chick. Additionally, we analysed the egg shell as these are a waste stream of the process under investigation. The further vertical transmission to the hatched broilers was investigated by analysis of the chick droppings and the broilers’ breast muscle, kidney, tail feathers and wing feathers. Additionally the local environment was monitored by means of swab sampling at different time points. This research yielded new insights on the possible origin of residues in broiler production.
Materials and methods
Chemicals
Methanol (MeOH) and acetonitrile (ACN), both UPLC grade, were purchased at Biosolve (Valkenswaard, The Netherlands). Acetic acid, formic acid, trifluoroacetic acid (TFA), ammonia (25%), ammonium formate, citric acid monohydrate, disodium hydrogen phosphate dehydrate, sodium ethylenediamine tetra acetate (Na2EDTA), sodium chloride and hexane were purchased at VWR International (Darmstadt, Germany). Lead acetate, disodium tetraborate was purchased at Sigma-Aldrich (St. Louis, Mo, United States). Throughout all experiments, where water is mentioned it refers to Milli-Q water which was prepared having a resistance of at least 18.2 MΩ cm−1.
McIlvain-EDTA buffer was prepared by dissolving 74.4 g of Na2EDTA in 500 mL of a 0.1 M citric acid solution and 280 mL of a 0.2 M phosphate buffer solution. Citric acid solution or phosphate buffer was added until the solution was adjusted to a pH of 4.0. The total volume was then adjusted to 2 L.
Reference standards
The reference standards sulfachlorpyridazine (SCP), doxycycline (DC) and enrofloxacin (ERF) were purchased at Sigma-Aldrich. Demeclocycline (DMC) was purchased at USP (Rockville, MD, United States), SCP-13C6 and ERF-d5 were purchased at Witega (Berlin, Germany) and DC-d3 at Toronto Research Chemicals (North York ON, Canada).
Stock solutions of SCP, DC and their internal standards SCP-13C6, DC-d3 and DMC were prepared in MeOH at a concentration of 1000 mg L−1. ERF and ERF-d5 were prepared in 5% ammonia/95% MeOH at a concentration of 100 mg L−1. Mixed spike solutions of standards or internal standards were prepared in MeOH.
Animal study
Following ethical approval, a parallel animal study was conducted with SCP, DC and ERF. These antibiotics are all critically important antibiotics for animal health, represent different antibiotic groups and cover the spectrum from very persistent to mobile (Berendsen et al. Citation2018). During this study, only the parent hens were treated. The experiment consisted of two phases. In phase 1, eggs were collected from the treated parent hens. In phase 2 the eggs were hatched and matrices of the broilers were investigated. The most commonly applied formulations of the selected antibiotics used in The Netherlands were selected and treatment was carried out according to registration.
Phase 1
In phase 1, 30 parent hens (Ross 308) and three roosters were randomly divided over three cages, which corresponds to ten hens and one rooster per treatment. The feed and water systems of these three cages were fully separated. The birds had unrestricted access to antibiotic-free drinking water and commercial feed. After a week of acclimatisation, antibiotics were administered via the drinking water during 5 consecutive days.
Treatment with SCP was carried out using Cosumix plus (REG NL 5388, Elanco GmbH, Utrecht, The Netherlands). Animals got 30 mg of SCP-Na per kg bodyweight per day. Treatment of DC was carried out using 50% WSP/Doxylin® 50% WSP (REG NL 8753, Dopharma, Raamsdonksveer, The Netherlands). Animals received 25 mg DC hyclate per kg body weight per day. Treatment of ERF was carried out using Enrox 10% oraal (REG NL 10409, Krka – Farma, Zagreb, Croatia). Animals got 10 mg ERF per kg bodyweight per day.
In order to check what amount of antibiotic residues transfers from parent hen to egg 2 eggs were sampled one day before treatment (day 0), daily during treatment (day 1–5) and daily until 4 days after treatment (day 6–9). In order to study the vertical transmission, for each treatment, the remaining eggs on the last 3 days of the treatment (day 3–5) and 1 day after treatment (day 6) were selected for hatching. These specific eggs were chosen for hatching as a worst case scenario: because the highest antibiotic residue concentrations were expected in these eggs. Eggs for hatching were also taken 4 days prior to the start of the treatment as a negative control. The eggs selected for hatching were placed in an incubator, checked for fertilisation and hatched.
Phase 2
In phase 2, after hatching, broilers were placed by treatment group (of the parent hens) in clean cages in a different housing from phase 1 to prevent contamination. Before placing the animals in the new cages, swab samples were taken of the wall, feeder and poultry drinker. Additionally droppings were sampled from the chicks before placing them in the cages. Animals were vaccinated against Newcastle disease (NCD). After placing the animals, droppings and swab sampling was continued. Samples were taken 1 day after placing the broilers and once every week thereafter.
Animals were kept in the cages until a maximum of 6 weeks of age. For each group three animals were slaughtered at 4, 5 and 6 weeks of age. Samples of breast muscle, kidney, tail feathers and wing feathers were collected from these animals.
Sample preparation
Eggs were stored at 4°C before blending. Breast muscle and kidney were homogenised using a food processor. Samples were stored at −18°C prior to analysis. Droppings samples were homogenised by mixing using a wooden spatula before weighing. Egg shells and the part of the feather samples meant for analysis of the non-freely bound residues, were washed using water and afterwards ground using a ball mill (Type MM301, Retsch Haan, Germany). Other feathers were analysed without prior processing.
Sample extraction
Of the blended egg samples, droppings samples, breast muscle and kidney samples, 2 grams were weighed into a 50 mL polypropylene (PP) tube (Greiner Bio-One, Alphen aan de Rijn, The Netherlands). For swabs, the entire cotton pad was placed into a 50 mL PP-tube. Internal standard solution was added, samples were mixed and left at room temperature during 20 minutes. To the egg, breast muscle and kidney samples, 5 mL McIlvain-EDTA buffer was added. To the droppings and swab samples, 4 mL McIlvain-EDTA buffer and subsequently 1 mL of ACN was added. All samples were extracted during 15 minutes using a rotating head-over-head apparatus (Heidolph REAX-2, Schwabach, Germany). Before centrifuging, 2 mL of lead acetate solution was added to the egg and droppings samples to remove excessive proteins. All samples were then shaken thoroughly and centrifuged during 10 minutes at 3500 g (Biofuge Stratos centrifuge, Heraeus instruments, Germany). The supernatant was decanted into a new 50 mL PP-tube. Egg and droppings samples were then diluted with 10 mL respectively 13 mL of a 0.2 M EDTA-solution before continuing to SPE clean-up. The extraction procedure was repeated once more and the extracts combined for breast muscle, kidney and egg samples before continuing to SPE clean-up.
Feather samples were analysed using the method described by Jansen et al. (Jansen et al. Citation2017). For feathers, both the freely-extractable and non-freely extractable residues were determined. In brief: 0.125% TFA in MeOH solution was added to the (ground) samples. Samples were then extracted during 1 hour in a water bath at 45°C (not-freely extractable residues) or using a rotating head-over-head apparatus at room temperature (freely-extractable residues). Samples were then diluted using McIlvain-EDTA buffer and centrifuged. The extraction procedure was then repeated once more. For non-freely extractable residues, SPE clean-up was carried out right after centrifuging, without repeating the extraction procedure.
For egg shell samples, a method derived from the procedure applied for non-freely extractable residues in feathers was carried out. Samples were washed using water instead of extraction solvent before grinding with a ball mill. Of the ground egg shell powder, 1 g was weighed into a 50 mL PP-tube. A larger volume of 10 mL of 0.125% TFA in MeOH solution was used to extract the samples. Extraction was carried out twice using a water bath at 45°C during 30 minutes. The 0.125% TFA in MeOH was evaporated at 40°C after centrifuging and the remaining extract was diluted using McIlvain-EDTA buffer before SPE clean-up.
Sample clean-up
For egg, a 3 mL OASIS™ HLB (60 mg) reversed-phase polymeric SPE cartridge (Waters, Manchester, UK) was conditioned using 3 mL of MeOH and 3 mL of McIlvain-EDTA buffer subsequently. For all the other matrices a 6 mL Strata™-X (200 mg) reversed-phase polymeric SPE cartridge (Phenomenex, Torrance, CA, USA) was conditioned with 5 mL of MeOH and 5 mL of McIlvain-EDTA buffer subsequently. The extract(s) were decanted entirely onto the cartridge and were passed through using a vacuum manifold. The cartridge was then rinsed with 3 mL and 5 mL of water respectively for egg and the other matrices. Residues were eluted using 3 mL of MeOH for egg or 5 mL of MeOH for all other matrices. Samples were then evaporated at 40 °C under a gentle nitrogen stream until dry.
Egg, breast muscle and kidney samples were reconstituted in 200 µL of MeOH. Droppings and swab samples were reconstituted in 100 µL of MeOH and diluted using 400 µL of water. Feather and egg shell samples were reconstituted in 200 µL of MeOH and diluted using 300 µL of water. All samples were centrifuged at 2800 g and transferred to UPLC vials.
LC-MS analysis
Analysis of all matrices was carried out using a Acquity UPLC System (Waters). The mobile phases used were 2 mM ammonium formate and 0.16% FA in water (Solvent A) and 2 mM ammonium formate and 0.16% FA in MeOH (Solvent B). The gradient used at a flow of 0.4 mL min−1 was: 0–1.0 min, 0% mobile phase B, 1.0–2.5 min, linear increase to 25% B, 2.5–5.4 linear increase to 70% B, and 5.4–5.5 min linear increase to 100% with a final hold of 1.0 min. The system returned to its initial conditions within 0.1 min with a final equilibration time of 0.9 min, resulting in a total run of 7.5 min. The injection volume was 5 μL.
Separation was carried out according to these published methods (Berendsen et al. Citation2015; Jansen et al. Citation2017). An Acquity HSS-T3 C18 analytical column of 2.1 × 100 mm, 1.7 μm (Waters) placed in a 40°C column oven was used for chromatographic separation for all matrices except droppings. For droppings analysis, separation was done using a Kinetex C18 2.1 × 100 mm 1.7 µm analytical column (Phenomenex) instead (Berendsen et al. Citation2015). The gradient used at a flow rate of 0.3 mL min−1 for droppings analysis was: 0–0.5 min, 1% B, 0.5–5.0 min, a linear increase to 100% B with a final hold of 1.0 min and an equilibration time of 3.5 min. The injection volume was 10 µL.
Detection of all antibiotics in all matrices except egg was carried out using a Sciex (Framingham, MA, USA) Q-trap 6500 mass spectrometer. The operating parameters for this system were: capillary voltage 2.0 kV, cone voltage 25 V, source offset 20 V, source temperature 150°C, desolvation temperature 550°C, cone gas flow 150 L h−1, and desolvation gas, 600 L h−1. Data processing was done using MultiQuant 3.0.2 software. For analysis of egg, a Micromass Quattro Ultima Pt with ESI interface (Waters) was used. Operating parameters for this system; capillary voltage 2.8 kV, cone voltage 65 V, source temperature 120°C, desolvation temperature 450°C, cone gas flow 120 L h−1 and desolvation gas 700 L h−1. Both systems operated in positive ion mode. The detection mode used was selected reaction monitoring (SRM) with collision-induced dissociation (CID) using argon as the collision gas. Data processing was done using MassLynx 4.2 software (Waters). Transitions and parameters of the compounds are presented in for both systems used.
Table 1. Transitions and compound specific parameters like cone voltage, collision energy (CE), declustering potential (DP), entrance potential (EP) and collision cell exit potential (CXP) for LC-MS analysis on both the Micromass Quattro Ultima Pt as the Qtrap-6500.
Method validation
The methods for analysis of breast muscle, kidney, egg, droppings (Berendsen et al. Citation2015) and feathers (Jansen et al. Citation2017) were validated according to 2002/657/EC (European Commission Citation2002) at relevant levels. Quantification was carried out using the above mentioned internal standards and matrix matched calibration curves. The concentration at which SCP and ERF could still be confirmed in breast muscle, kidney, egg and droppings was 1 µg kg−1 and for DC this was slightly higher: 5 µg kg−1. In feathers, this limit was determined to be 2 µg kg−1 for all compounds. The method for quantification of feathers was used for the quantification of egg shell as both are very difficult materials to work with and need a strong extraction solvent. Egg shell was quantified by preparing a matrix matched calibration curve using blank egg shell, resulting in a coefficient of correlation of 0.999. Retention time and ion ratio criteria as described in 2002/657/EC (European Commission Citation2002) were applied and all reported concentrations complied with these criteria, confirming the identity of the compound. The lowest concentration at which the compounds could be confirmed in egg shell was based on the lowest spiked concentration. For DC this was 10 µg kg−1 and for SCP and ERF 1 µg kg−1.
Results and discussion
Animal study
The first phase of the animal study went according to plan. A sufficient number of eggs were produced to start the second phase; 26 eggs as control, 24 for treatment with DC, 23 for treatment with ERF and 26 for treatment with SCP. Unfortunately, not all eggs were fertilised by the roosters, especially for the antibiotics DC (48%) and SCP (58%). This considerably reduced the number of chicks to start phase 2. Either the roosters in both of these cages were relatively inactive or DC and SCP treatment decreases the fertility rate. As a consequence, for DC only 1 animal was taken for slaughter at week 5 in this second phase. For ERF and SCP, 3 animals were available for each time point. The number of available animals was sufficient to obtain a good indication of the possible vertical transfer of antibiotic residues from parent hen to broiler. However, the numbers are somewhat limited for obtaining statistically significant depletion kinetic data, but even though the data give information about depletion, this was not the goal of this study.
Eggs and eggshells
Egg samples were taken on the day before commencing the treatment (day 0), daily during the treatment (day 1–5) and at the 4 days after the end of the treatment (day 6–9). Every day 2 eggs per treatment group were taken for analysis. Note that the goal was not to specifically study the transfer of the antibiotics to eggs, but to estimate the residue levels in the eggs used for hatching. Therefore the eggs were analysed as a whole, instead of separating egg yolk and egg white. Note that the number of egg samples per time point (n = 2) used in this study should be taken into account when estimating possible residue kinetics behind the transfer of ERF, DC or SCP from parent hens to eggs and eggs to broilers.
Also the egg shells after hatching were available. It was decided to also analyse these as egg shells have the potential to be used as a non-invasive matrix and, to our knowledge, no information is present in literature on the antibiotic residue levels in egg shells. The detected concentrations for SCP, DC and ERF in egg and egg shell are presented in .
Figure 1. Detected residues of SCP, DC and ERF in egg (dark green) and egg shells (light green) of animals treated during day 1 – day 5. Vertical lines indicate the standard deviation of the concentrations found in the two samples taken (biological variation).
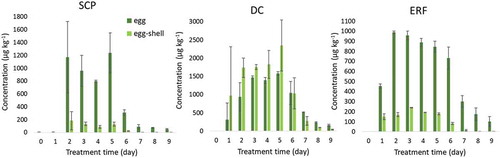
After commencing the treatment, residues in egg increase rapidly. For all treatments, residues are detectable from at least 1 day after the start of the treatment (day 2 in ). The concentration found stabilises during the treatment and decreases afterwards as expected. Most important for this specific study is that the antibiotic concentration in the eggs that were hatched were approximately 800 µg kg−1 for SCP, 1400 µg kg−1 for DC and 900 µg kg−1 for ERF.
Additionally to egg, the egg shells were analysed. All antibiotics seem to be transferred to egg shell as well. For SCP, residues in egg shell are about 10 times lower compared to egg. For ERF transmission to egg shell seems to be slightly higher, where concentrations in egg shell are a factor 5 lower compared to egg. Interestingly, DC concentrations found in egg shell were higher than in egg (about 30%). ERF, and especially DC are known to bind to divalent metal ions, such as calcium, which is one of the main constituents of egg shell. This explains the observations. Note that in some poultry production systems, the animals are hatched in the animal housing and the egg shells are consumed by the chicks as a calcium source. This could result in an additional transfer of antibiotics. Also these results indicate that egg shell is a useful non-invasive matrix which could be used to detect the application of antibiotics in parent hens and probably also in laying hens.
Droppings
Droppings samples were taken from the hatching trays, one day after placing the chicks in their cages and then weekly until slaughter. The concentrations of SCP, ERF and DC were analysed in the respective droppings samples and are presented in .
Figure 2. Detected concentration SCP (yellow), DC (black) and ERF (blue) in broiler droppings (to scale).
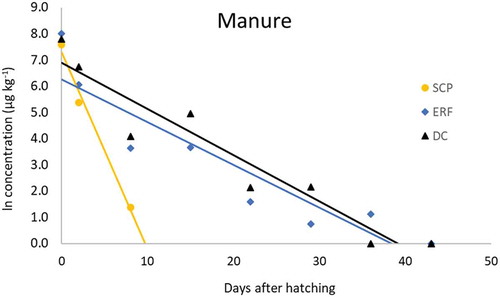
The highest concentration was found on the first sample day, which was 2.0 mg kg−1 for SCP, 2.4 mg kg−1 for DC and 3.0 mg kg−1 for ERF. All residues decrease exponentially in time, of which SCP showed the most rapid excretion. After 8 days SCP was not detectable any more. Both DC and ERF were detected up to 30 days after hatching. The slow degradation of DC is a common observation, which is likely to be the result of adsorption and desorption to for example bone material (Körner et al. Citation2001; Odore et al. Citation2015). For ERF no data is available on these kinds of processes.
In conclusion, antibiotic residues were, as a result of vertical transmission, present in broiler droppings and thus in the cage environment. As a result, especially the persistent antibiotics ERF and DC remain in the cage environment for a relatively long time.
Environmental samples
During the entire phase 2 of the animal experiment, swabs were taken (walls, the poultry drinker and feeder) in order to monitor the presence of antibiotic residues in the environment. One day after the start of phase 2, in all cages residues were detected of the antibiotic with which the parent hens of the respective groups were treated. These low levels of residues after placement of the birds demonstrate that some vertical transmission occurs, most likely through droppings. Additionally, some residues were detected in swab samples taken before the start of phase two. For instance the poultry drinker located in the cage of the SCP group, showed relatively high levels of ERF. This did not influence the final outcomes of the study, as we focussed on the specific antibiotics used to treat the parent hens of the individual groups. Small contaminations detected using the swab samples might also have occurred due to contamination during sampling.
Tissue
At 4, 5 and 6 weeks after hatching, muscle and kidney samples were taken from broilers. Although eggs contained significant concentrations of antibiotics, none of these samples contained any detectable residues of SCP, ERF or DC. Clearly vertical transmission cannot result in maximum residue limit violations in the broiler at slaughter.
Feathers
At 4, 5 and 6 weeks of age, feathers of 3 broilers were sampled, except for DC at week 5, where only 1 broiler could be sampled. The feathers were first analysed for freely extractable residues, these results are presented in .
Table 2. Detected (freely extractable) concentration of SCP, DC and ERF in tail and wing feathers of broilers of the 3 respective antibiotic treatments at 4, 5 and 6 weeks of age. All signals below 2.0 µg kg−1 were marked as not detected (nd) based on validation results. For doxycycline only 1 sample was taken at week 5, as not enough broilers were available.
For SCP, freely extractable residues are especially found on the tail feathers compared to the wing feathers. For DC and ERF no difference between tail feathers and wing feathers is observed. All feathers from the control group were negative except in three cases, in which low levels of residues were detected, all below 10 µg kg−1. Freely extractable residues can originate from direct vertical transmission during egg incubation or through indirect exposure after transmission via the chicks’ droppings. To discriminate external exposure from actual vertical transmission (direct or through droppings picking) the amount of non-freely extractable residues was determined in selected antibiotic containing feather samples. Non-freely extractable residues are the residues which remain after washing the feathers using extraction solvent and which can only be extracted after grinding (14). A comparison of the freely and non-freely extractable residues is presented in .
Table 3. Comparison of freely and not-freely extractable residues in feathers.
The results show that most of the residues can be washed off: they were most likely present on the outside of the feathers. For the selected animal from the control group the detected residues proved to be freely extractable and therefore it is concluded these residues originated from an external source (e.g. contamination during slaughter). For the selected feather samples of the antibiotic treated groups that contained freely extractable residues, in only five cases, non-freely extractable residues were detected. These belong to all antibiotics groups and to both the wing and the tail feathers. These residues can be the result of direct vertical transmission or by oral intake by broilers pecking their own droppings which are contaminated due to the treatment of their parent hen. However, as the mechanisms of displacement of residues in chicken are not well understood, it cannot be excluded that non-freely extractable residues originate from external exposure, e.g. from contaminated droppings or the cage environment.
In this study, after treatment of the parent hens, the broilers’ tail feathers contain < 50 µg kg−1 and the wing feathers <10 µg kg−1 freely extractable antibiotic residues. Only in a few samples very low levels of non-freely extractable residues were found (< 10 µg kg−1) were detected. If vertical transmission from parent hen to broiler chicken feathers occurs, it is clearly only at very low levels.
In conclusion, in untreated broilers from 4 weeks of age, low levels of residues can be detected in the feathers. These residues can be the result of direct vertical transmission, oral exposure of the broilers by pecking their own contaminated droppings or external contact with the contaminated droppings or environment. These routes of exposure should be considered when low levels of antibiotic residues (freely or non-freely extractable) are detected in feathers.
Acknowledgments
We thank Maria Groot and Sherine Gasho-Asaad for their assistance in the animal study.
Disclosure statement
The authors declare to have no potential conflict of interest.
Additional information
Funding
References
- Berendsen BJA, Bor G, Gerritsen HW, Jansen LJM, Zuidema T. 2013. The disposition of oxytetracycline to feathers after poultry treatment. Food Addit Contam A. 30(12):2102–2107.
- Berendsen BJA, Lahr J, Nibbeling C, Jansen LJM, Bongers IEA, Wipfler EL, van de Schans MGM. 2018. The persistence of a broad range of antibiotics during calve, pig and broiler droppings storage. Chemosphere. 204:267–276.
- Berendsen BJA, Wegh RS, Memelink J, Zuidema T, Stolker LAM. 2015. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta. 132:258–268.
- Bilandžić N, Božić Đ, Kolanović BS, Varenina I, Cvetnić L, Cvetnić Ž. 2015. Distribution of sulfamonomethoxine and trimethoprim in egg yolk and white. Food Chem. 178:32–37.
- Carballo M, Aguayo S, González M, Esperón F, De la Torrre A. 2016. Environmental assessment of tetracycline’s residues detected in pig slurry and poultry droppings. J Environ Prot (Irvine, Calif). 07:82–92.
- Církva A, Málková I, Rejtharová M, Vernerová E, Hera A, Bureš J. 2019. Residue study of nitroimidazoles depletion in chicken feathers in comparison with some other selected matrixes. Food Addit Contam Part A Chem Anal Control Exposure Risk Assess. 36(8):1206–1217.
- Cornejo J, Lapierre L, Iraguen D, Cornejo S, Cassus G, Richter P, San Martín B. 2011a. Study of enrofloxacin and flumequine residues depletion in eggs of layinghens after oral administration. J Vet Pharmacol Ther. 35(1):67–72.
- Cornejo J, Lapierre L, Iragüen D, Pizarro N, Hidalgo H, San Martín B. 2011b. Depletion study of three formulations of flumequine in edible tissues and drug transfer into chicken feathers. J Vet Pharmacol Ther. 34(2):168–175.
- Cornejo J, Pokrant E, Riquelme R, Briceño C, Maddaleno A, Araya-Jordán C, San Martín B. 2017. Single-laboratory validation of an LC-MS/MS method for determining florfenicol (FF) and florfenicol amine (FFA) residues in chicken feathers and application to a residue-depletion study. Food Addit Contam Part A. 34(4):469–476.
- European Commission. 1990. Council regulation (EEC) 2377/90 of 26 June 1990 laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off J Eur Union. L224:1–136.
- European Commission. 2002. Commission Decision 2002/657/EC implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, 2002. Off J Eur Union. L221:8–36.
- European Commission. 2010. Commission Regulation EU/37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin, 2010. Off J Eur Union. L15:1–72.
- European Commission. 2015. Factsheet AMR: A major European and global challenge. [accessed 2019 Jun]. https://ec.europa.eu/health/amr/antimicrobial-resistance_en.
- Gajda A, Posyniak A. 2015. Doxycycline depletion and residues in eggs after oral administration to laying hens. Food Addit Contam Part A. 32(7):1116–1123.
- Gorla N, Chiostri E, Ugnia L, Weyers A, Giacomelli N, Davicino R, Garcı́a Ovando H. 1997. HPLC residues of enrofloxacin and ciprofloxacin in eggs of laying hens. Int J Antimicrob Agents. 8(4):253–256.
- Jansen LJM, Bolck YJC, Berendsen BJA. 2016. Feather segmentation to discriminate between different enrofloxacin treatments in order to monitor off-label use in the poultry sector. Anal Bioanal Chem. 408(2):495–502.
- Jansen LJM, Bolck YJC, Rademaker J, Zuidema T, Berendsen BJA. 2017. The analysis of tetracyclines, quinolones, macrolides, lincosamides, pleuromutilins, and sulfonamides in chicken feathers using UHPLC-MS/MS in order to monitor antibiotic use in the poultry sector. Anal Bioanal Chem. 409(21):4927–4941.
- Körner U, Kuhne M, Wenzel S. 2001. Tetracycline residues in meat and bone meals. Part 1: methodology and examination of field samples. Food Addit Contam. 18(4):293–302.
- Maddaleno A, Pokrant E, Yanten F, San Martin B, Cornejo J. 2019. Implementation and validation of an analytical method for lincomycin determination in feathers and edible tissues of broiler chickens by liquid chromatography tandem mass spectrometry. J Anal Methods Chem. 2019:8.
- Odore R, De Marco M, Gasco L, Rotolo L, Meucci V, Palatucci AT, Rubino V, Ruggiero G, Canello S, Guidetti G, et al. 2015. Cytotoxic effects of oxytetracycline residues in the bones of broiler chickens following therapeutic oral administration of a water formulation. Poult Sci. 94(8):1979–1985.
- [OIE] World Organization for Animal Health. 2019. OIE list of antimicrobial agents of veterinary importance. [accessed 2020 Jan]. https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_OIE_List_antimicrobials_July2019.pdf.
- Pokrant E, Medina F, Maddaleno A, San Martín B, Cornejo J. 2018. Determination of sulfachloropyridazine residue levels in feathers from broiler chickens after oral administration using liquid chromatography coupled to tandem mass spectrometry. PloS one. 13(7):e0200206–e0200206.
- Rijksinstituut voor Volksgezondheid en Milieu RIVM. 2018. NethMap 2018: consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands/MARAN 2018: monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2017.
- Roudaut B, Garnier M. 2002. Sulphonamide residues in eggs following drug administration via the drinking water. Food Addit Contam. 19(4):373–378.
- San Martín B, Cornejo J, Iragüen D, Hidalgo H, Anadón A. 2007. Depletion study of enrofloxacin and its metabolite ciprofloxacin in edible tissues and feathers of white leghorn hens by liquid chromatography coupled with tandem mass spectrometry. J Food Prot. 70(8):1952–1957.
- US Department of Health and Human Services. 2013. Antibiotic resistance threats in the United States.
- [WHO] World Health Organization. 2014. Antimicrobial resistance: global report on surveillance 2014. ISBN: 978-92-4-156474-8.
- [WHO] World Health Organization. 2019. Critically important antimicrobials for human medicine, 6th revision. ISBN 978-92-4-151552-8.
- Yoshimura H, Osawa N, Rasa FSC, Hermawati D, Werdiningsih S, Isriyanthi NMR, Sugimori T. 1991. Residues of doxycycline and oxytetracycline in eggs after medication via drinking water to laying hens. Food Addit Contam. 8(1):65–69.
- Zhao L, Dong YH, Wang H. 2010. Residues of veterinary antibiotics in droppingss from feedlot livestock in eight provinces of China. Sci Total Environ. 408(5):1069–1075.
