?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Large-scale food fortification of vegetable oils with vitamin A has been implemented successfully for decades in numerous African and Asian countries, contributing demonstrably to reductions in vitamin A deficiency. For these programmes, reliable and validated analytical data are essential to demonstrate compliance with legal standards and fortification levels. Commonly, many analytical laboratories use a saponification method for the quantitative analysis of retinyl palmitate (the mostly used form of vitamin A for fortification) in fortified oils, which implies a multiple-step procedure with long analysis times and the potential risk of analyte loss. The aim of the present study was to develop and validate a direct High-performance Liquid Chromatography (HPLC) method that reduces these sample preparation steps, leading to the cost- and time-efficient quantification of retinyl palmitate in fortified oils. Oil samples are dissolved into the HPLC solvents, then injected directly into a common C18 column, and subsequently detected by a fluorescence detector. The limit of quantification (1.0 mg retinyl palmitate kg−1) and the working range of 1.0–100 mg retinyl palmitate kg−1 with a linearity of R2 = 0.9989 are appropriate to analyse fortified oil samples. The method also showed adequate precision (RSD between 1.1% and 3.1%) and recoveries (86–103%) at two different concentration levels. The accuracy of the direct HPLC method was additionally proven by the comparison of spiked samples with two external laboratories that used the saponification method. The robustness of the method was confirmed by the analysis of various spiked edible oils. The HPLC column is not deteriorated by the lipid matrix and shows excellent stability and long lifetime. Also, 9-cis-retinyl palmitate formed mainly by light exposure could be detected by this method. The direct HPLC method is a well-suited alternative to the saponification method for the rapid and reliable routine analysis of fortified oil samples.
Introduction
Vitamin A deficiency remains a leading public health problem in low- and middle-income countries (West Citation2003; WHO Citation2009; Muthayya et al. Citation2013; Stevens et al. Citation2015). Its health consequences are notably apparent and severe among infants, young children, and pregnant and lactating women. The most common cause of the vitamin A deficiency is insufficient dietary intake of vitamin A-rich foods, particularly food of animal origin and fruits and vegetables containing vitamin A precursors (carotenes).
To address the prevalent nutrient intake gaps in such settings, vitamin A has been successfully used to fortify vegetable oils, margarine, cereal flours, sugar, infant formula, milk, and milk products at the point of processing (Allen et al. Citation2006). This large-scale food fortification of staple foods has shown to be a cost-effective and sustainable strategy for substantially reducing micronutrient malnutrition (Allen et al. Citation2006). Vegetable oils are the most suitable vehicle for vitamin A fortification (Diosady and Krishnaswamy Citation2018) due to their high consumption in many African and Asian countries, the high fat-solubility of vitamin A, and the simplicity of the fortification technology needed. For decades, the more stable vitamin A ester retinyl palmitate has been successfully used as the predominant vitamin A fortificant in vegetable oils. The oil matrix protects retinyl palmitate against oxidation during storage and facilitates absorption by the human body (Souganidis et al. Citation2013). Today, the fortification of vegetable oils with vitamin A is mandatory in 27 and voluntary in 11 countries (mostly in Africa and Asia) in the range 6–55 mg retinol kg−1, mainly with retinyl palmitate as fortificant (GFDx. Global Fortification Data Exchange, Citation2021).
Food fortification programmes can only be successful and sustainable if food producers continuously assure the produced food meets the fortification standards, and regulatory monitoring or food control conducted at production level (external monitoring), borders (import monitoring), and retail stores (commercial monitoring) ensures all of the food supply complies with food legislation and standards (Allen et al. Citation2006; Luthringer et al. Citation2015; Rowe et al. Citation2018). Laboratory analysis plays an important role in measuring the micronutrient content and confirming the quality of fortified food. Only reliable and validated analytical data can serve as a sound basis to prove compliance with legal levels/standards and label declarations and recommend appropriate follow-up actions.
From the analytical point of view, the quantitative analysis of retinyl palmitate in fortified vegetable oils represents a general challenge given that the very lipophilic retinyl palmitate must be determined at mg kg−1 (ppm) concentrations in a lipid matrix, consisting mainly of various triglycerides. A saponification procedure (hereafter, called the saponification method) is commonly applied in analytical laboratories to remove the bulk lipid components before HPLC-UV analysis. During saponification with potassium hydroxide, the triglycerides are converted into the alkali salts of the fatty acids and glycerine, and retinyl palmitate into retinol. This chemical process converts the polarity of the oily matrix into an aqueous phase. To avoid isomerisation of retinyl palmitate and decomposition of the relatively unstable retinol during this vigorous saponification reaction, it is strongly recommended to perform the saponification under a nitrogen atmosphere and to add antioxidants. Retinol is then extracted in multiple steps by organic solvents; afterwards, the solvent phase is evaporated and dissolved in the HPLC solvents. Finally, retinol is determined by HPLC-UV. This saponification method is laid down with minor modifications in several international standards (AOAC International Citation1992a, Citation1992b, Citation2001; European Standard EN 12823-1:Citation2014; CitationAACC Method 86-06.01). However, it should be mentioned here that all these standard methods were developed and validated for several foods, but not for fortified vegetable oils. The saponification method is a multiple-step procedure with long overall analysis times and requires several manipulations, with the risk that the analyte is partially lost or degraded. Thus, this analytical method is quite vulnerable to errors and analyte loss causing potential between-laboratory variations and bias (Thiex et al. Citation1996). In addition, the procedure involves the use of caustic potassium hydroxide, which necessitates appropriate diligence and caution of the staff and a high amount of organic solvents is used, producing extensive quantities of chemical waste with substantial disposal and environmental issues.
A direct analysis of fortified oils without saponification was described in the scope of an assessment of a portable device and in stability studies of vitamins A and D in soybean oil (Renaud et al. Citation2013; Hemery et al. Citation2015). However, this direct method used a specific polymeric HPLC column and two mobile phase solvent mixtures, a binary and a ternary solvent mixture. The HPLC analysis time was 65 min, plus a 20-min column wash with ethyl acetate was added after the end of each sample to prevent fat deposition, followed by an intensive washing for extra 2 h after an analysis day. These long analysis times and washing procedures, together with a complex mobile phase system, indicate a problematic oil deposition on the HPLC column by this method producing general instability of the analysis. Another validation study of the above-mentioned portable device used a direct HPLC-UV method (Rohner et al. Citation2011), but no validation data are available for this method.
It is well known that all-trans-retinyl palmitate isomerises in the absence of antioxidants by heat and light to various cis isomers, mainly 9- and 13-cis-retinyl palmitate (Schwartz Citation1987; Murphy et al. Citation1988; Kurzer et al. Citation2014). According to several studies, 13-cis-retinly palmitate is formed primarily during heat treatment and 9-cis-retinly palmitate by visible/UV light exposure (Schwartz Citation1987; Murphy et al. Citation1988; Kurzer et al. Citation2014). The cis isomers have a lower bioactivity in comparison to all-trans-retinyl palmitate (Weiser and Somorjai Citation1992). In literature (Mulry et al. Citation1983; Gaylord et al. Citation1986; Murphy et al. Citation1988), the complete separation of all-trans and cis isomers of retinyl palmitate is reported on silica gel normal phase HPLC columns. The analysis of the two relevant cis isomers is included in the direct HPLC method specified here.
In this study, a direct HPLC method was developed and comprehensively validated for a cost- and time-efficient analysis of retinyl palmitate in fortified edible oils which does not require lengthy sample preparation nor additional washing procedures of the HPLC column. The aim of the study is to provide analytical laboratories a rapid method, which is well suited to the reliable routine analysis of large numbers of fortified oil samples. Also, the analysis of relevant cis retinyl palmitate isomers is described in this study.
Material and methods
Chemicals and reagents
LC-grade methanol was obtained from Honeywell/Riedel-de Haen (Seelze, Germany), and LC-grade methyl tert-butyl ether (MTBE) was obtained from Th. Geyer (Renningen, Germany). Ethanol (96%) was purchased from Carl Roth (Karlsruhe, Germany), and butylated hydroxytoluene (BHT) was purchased from Sigma-Aldrich (Munich, Germany).
The analytical standard all-trans-retinyl palmitate (CAS: 79–81-2) was supplied by LGC Standards (Wesel, Germany). 9-cis-retinyl palmitate and 13-cis-retinyl palmitate were obtained from Toronto Research Chemicals, TRC (Toronto, Canada).
Edible oil samples
All edible oil samples for the validation studies were purchased from local supermarkets in Bremen, Germany, and were tested for the absence of all-trans-retinyl palmitate before spiking.
Apparatus
All equipments listed below were provided by Thermo Scientific, Dreieich, Germany. Analyses were performed using an integrated Accela HPLC system comprising Thermo Accela 1250 HPLC pump, an autosampler including injector and column oven, and a Thermo Surveyor fluorescence detector (FLD) with λEx = 325 nm, λEm = 480 nm. The reversed phase (RP) HPLC column was a Hypersil Gold 3 µm, 150 × 4.6 mm i.d. The software Chrom-Quest, version 5.0 was used to process the obtained data and to control the HPLC system.
A UV-VIS photometer Genesys 10S was employed for the measurement of the purity and concentration of the analytical standard all-trans-retinyl palmitate.
Standard solutions
Stock solution S0 of all-trans-retinyl palmitate (500 mg L−1)
Note: Retinyl palmitate is sensitive to (UV) light and oxidation. All laboratory operations were performed in the absence of light using amber glassware, or glassware protected with aluminium foil, while BHT is added to protect against oxidisation.
We weighed 50 mg (± 0.1 mg) of retinyl palmitate as a solid (e.g. at fridge temperature) in a 100-ml volumetric flask and dissolved and diluted to volume with ethanol. After determination of retinyl palmitate concentration by photometry, we added ca 100 mg BHT as antioxidant and kept in dark and in freezer at −18°C. The stock solution S0 is stable for 3 months.
Photometric concentration and purity test of the stock solution
It is recommended to measure the concentration and purity of the stock solution by photometry (AOAC International Citation1992b; Thiex et al. Citation1996; AOAC International Citation2001, Citation2012; Commission Regulation No 152/2009, Annexe IV), in particular, for new standard substances or after longer storage period of the standard substance (>3 months). Briefly, the stock solution is diluted with 2-propanol (or hexane or ethanol) and the UV spectrum of the solution measured in 10-mm quartz cuvettes against 2-propanol (or hexane or ethanol) between 300 and 400 nm. The extinction maximum must be between 325 and 327 nm. The retinyl palmitate content is calculated by the extinction at 326 nm, according to the given references.
Calibration standard solutions
Reference solution Ref1 of all-trans-retinyl palmitate (5 mg L−1):
100 µl stock solution S0 were pipetted into a 10-ml volumetric flask by a variable micropipette and filled up with methanol.
Reference solution Ref2 of all-trans-retinyl palmitate (2.5 mg L−1):
500 µl Ref1 and 500 µl methanol were pipetted by a variable micropipette in an amber glass vial and shaken.
Reference solution Ref3 of all-trans-retinyl palmitate (0.5 mg L−1):
100 µl Ref1 and 900 µl methanol were pipetted by a variable micropipette in an amber glass vial and shaken.
The calibration standard solutions Ref1, Ref2, and Ref3 were prepared fresh before each testing sequence.
Sample preparation
1.00 g (± 0.01 g) of oil (homogenised by thorough manual stirring or swirling) and ca 10 mg BHT were filled into a 20 ml amber (or aluminium-foiled protected) volumetric flask, dissolved with 9 ml MTBE and mixed. The solution was diluted to 20 ml with methanol and mixed again. This procedure can be performed also in a graduated centrifuge tube. The solution is centrifuged when particulates or suspended solids are observed. An aliquot of the clear solution was filled in an amber glass vial and analysed directly by HPLC.
Chromatographic analysis and quantification
The injector volume was 20 µl; the analytical column was kept at 30°C. The mobile phase solvent system comprised methanol and MTBE at a flow rate of 1.0 ml min−1. The following routine gradient profile was used: 0–10 min: 0% MTBE; 10.1–14 min: 100% MTBE; 14.1–19 min: 0% MTBE. An alternative gradient programme was used for complex oil samples with matrix interferences: 0–2 min: 5% MTBE; 2.1–10 min: 5–20% MTBE; 10.1 − 13 min: 100% MTBE; 13.1–20 min: 5% MTBE.
Quantification was performed on peak area basis against external calibration of the standard solutions Ref1, Ref2, and Ref3. Retinyl palmitate was identified by the comparison of retention times to the external standards.
The results in mg retinyl palmitate kg−1 can be converted by factor 0.546 to mg retinol kg−1 (e.g. 1 mg retinyl palmitate kg−1 is equivalent to 0.546 mg retinol kg−1) and by factor 1818 to IU kg−1 (IU: international units) (e.g. 1 mg retinyl palmitate kg−1 is equivalent to 1818 IU kg−1).
Method validation
The linearity of the method was determined by quantifying reference solutions with the following concentrations: 0.05, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 mg retinyl palmitate L−1. The limit of quantification (LoQ) was calculated using the peak area of a spiked sunflower oil, with signal-to-noise (S/N) ratio of 10. The repeatability was determined with six spiked sunflower oil samples each at levels 5 and 50 mg retinyl palmitate kg−1, at the same day by the same technician. Similarly, the reproducibility was checked on six different days for each of the same two concentration levels (n = 6) by three different technicians, with each technician analysing both concentrations on two different days. The spiked samples of the repeatability and reproducibility studies were also used for the calculation of the recovery at the concentration levels 5 and 50 mg retinyl palmitate kg−1. The method was compared with the conventional saponification analysis using spiked sunflower oil samples at two different concentration levels by two external private laboratories in Germany, applying the European Standard EN 12823–1:2014. Robustness of the method and matrix effects were studied with spiked groundnut oil, soya bean oil, coconut oil, rapeseed oil, and palm oil at level 50 mg retinyl palmitate kg−1. The variance of the retention times (RTs) was calculated for a sequence of 40 fortified oil samples.
Cis isomers of retinyl palmitate
The 9- and 13-cis-retinyl palmitate isomers together with the all-trans-isomer were analysed as standard solutions in methanol by the described HPLC method.
Results
Validation of the developed direct HPLC method included linearity, working range, LoQ, precision, measurement uncertainty, accuracy, and robustness.
The linearity was determined in the range of 0.05–5 mg retinyl palmitate L−1 with the correlation coefficient R2 = 0.9989. This linearity range corresponds with the applied sample preparation to a working range of 1.0–100 mg retinyl palmitate kg−1 (equivalent 0.546–54.6 mg retinol kg−1). In general, this working range is appropriate to analyse fortified oil samples. There may be limited instances in some countries where overfortified samples, according to their national fortification standards, can be outside the working range. In these cases, the final solution for HPLC analysis should be diluted or the original oil sample weight must be reduced in a repeated analysis.
Based on the S/N ratio, the LoQ of the method is 1.0 mg retinyl palmitate kg−1 (equivalent to 0.546 mg retinol kg−1), which is sufficient to monitor the fortification of edible oil samples (the lowest concentration of the nationally regulated fortification ranges is 6 mg retinyl palmitate kg−1 (GFDx. Global Fortification Data Exchange, Citation2021).
For the evaluation of the precision, the repeatability and reproducibility were determined at two different concentration levels. The results are presented in . The relative standard deviations (RSDs) for repeatability and reproducibility at both concentrations were calculated between 1.1% and 3.1%. Based on the Horwitz equation (Horwitz Citation1982a, Citation1982b), AOAC International (Citation2019), FDA (Citation2019), and Codex Alimentarius (Citation2013) guidelines for the validation of chemical methods present acceptable RSDs for within-laboratory repeatability are at 10 and 100 ppm of 6% and 4%, respectively, and for within-laboratory reproducibility at the same concentrations 11% and 8%, respectively. In comparison with these values, the method shows a good precision.
Table 1. Results of repeatability and reproducibility studies at two different concentration levels
Accuracy of the method was assessed by recovery studies. The mean recoveries at 5 mg retinyl palmitate kg−1 (n = 12) and 50 mg retinyl palmitate kg−1 (n = 12) were 94% (range 86–100%, RSD = 5.2%) and 98% (range 90.4–103.2%, RSD = 4.01%), respectively (). In addition, the results of spiked oil samples were compared by external validation with those of two external laboratories which used the saponification method. For sample 1, a result of 12.1 mg retinyl palmitate kg−1 was found by the direct method, and 9.8 and 9.5 mg retinyl palmitate kg−1 by two external laboratories; for sample 2, the direct method found 40.1 mg retinyl palmitate kg−1 and the external laboratories 33.7 and 31.1 mg retinyl palmitate kg−1. The results showed a relatively good comparability; however, it seems that the results by saponification are systematically lower than those by the direct method.
Table 2. Results of recovery studies at two different concentration levels
The measurement uncertainty of the method was calculated from a QC chart used during the analysis of oil samples. The expanded measurement uncertainty with a coverage factor k = 2 was 10%.
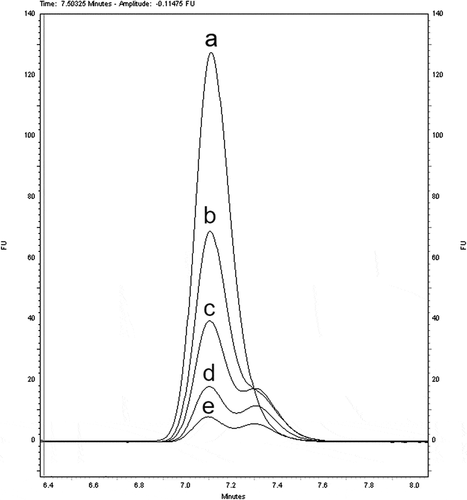
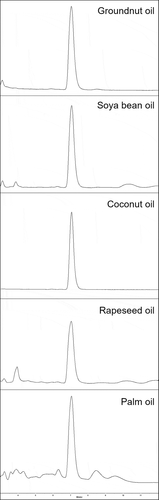
To demonstrate the robustness of the direct method, various vegetable oil samples typically used for oil fortification were spiked and analysed. The chromatograms are presented in ; the corresponding recoveries are given in the caption. The mostly symmetric retinyl palmitate peak in the chromatograms of the different oils and the blank chromatograms indicated no interferences by the matrix. Also, the corresponding recoveries are in the range of the validation study. The recovery for rapeseed oil is slightly lower and the peak form broader; it is unknown whether this is, in general, typical for rapeseed oils or only for the oil sample used for the validation study. If there are higher matrix inferences observable in the chromatogram, a slightly modified gradient can be used (all chromatograms in this publication were run with the routine gradient profile). The retention time of the HPLC analysis was very stable with low variance during routine operation; for instance, during testing in a total of 40 various samples of fortified vegetable oils with different matrix and from different origin in a time frame of 2 days, the RSD of the retention time of retinyl palmitate was 0.3%. A general trend to shorter retention times was not observed. Several hundreds of oil samples with different origin and matrix could be tested with this method in routine operation by exclusively the same HPLC column, illustrating that the lipid portion of the solutions injected did not deteriorate the HPLC column efficiency. Washing procedures are not necessary after either each sample or each sequence.
Figure 1. HPLC-FLD chromatograms of various vegetable oils, spiked at level 50 mg retinyl palmitate kg−1 (recovery given in paratheses): groundnut oil (101%), soya bean oil (97%), coconut oil (106%), rapeseed oil (87%), and palm oil (104%).
All validation data are summarised in .
Table 3. Summary of the validation data
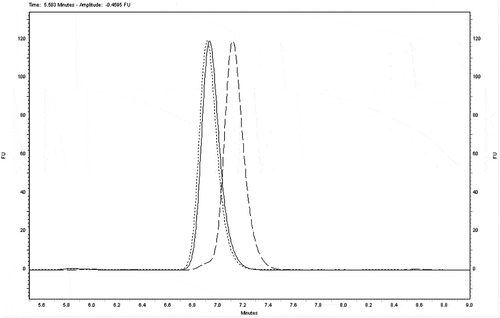
In addition, the 9- and 13-cis-retinyl palmitate isomers together with the all-trans-isomer were also analysed as standard solutions (). 13-cis-Retinyl palmitate is not separated (with a resolution factor Rs = 0.08 and separation factor α = 1.004) and 9-cis-retinyl palmitate is half separated (Rs = 0.70 and α = 1.037) from all-trans-retinyl palmitate on the RP HPLC column. In a study, an all-trans-retinyl palmitate standard solution (without added BHT) was exposed to the light of a halogen lamp for 4, 8, 24, and 48 h and subsequently analysed (), in order to observe photoisomerisation effects.
Discussion
In this study, an HPLC method was developed to directly analyse retinyl palmitate in fortified oils, without a laborious and time-consuming sample preparation. The oil sample was simply dissolved into the HPLC mobile phase solvents and injected directly into the chromatographic system. This dilution preparation before injection requires only about 10–15 min; however, for the entire saponification procedure, an average time of 2.5 h per sample can be estimated (on the basis of the AOAC method 2001.13 (AOAC International Citation2001) mostly used by the laboratories). This time for the saponification procedure can be shortened to some extent when samples are prepared in parallel. A very common C18 RP column was used for the separation. Also, RP HPLC columns with other dimensions might be applicable for this method; however, retention times will change and potential matrix effects might occur, which requires a change of the gradient programme. For the detection, a fluorescence detector was chosen which is more sensitive than a UV detector and, in general, less sensitive to matrix interferences. Analytical methods which directly inject portions of lipids into the HPLC system involve, in general, the risk that the separation efficiency and the lifetime of the HPLC column deteriorate. The validation study proved that the lipid portion did not affect the separation power and lifetime of the HPLC column. This was achieved by the choice of the mobile phase solvent system methanol/MTBE and the specific gradient programme. Additional washing procedures after each sample or sample sequence were not necessary and the total HPLC analysis is finished after 20 min. The method was fully validated and also compared with parallel results of two external laboratories, which used the saponification method. The results showed a relatively good comparability; however, it seems that the results by saponification are systematically lower than those by the direct method. This might be explained by the potential loss or degradation of retinyl palmitate or retinol during the vigorous saponification process. In addition, the results depend upon the quantification procedure, whether a retinol or saponified retinyl palmitate standard is used. A loss of approximately 10% of retinol was also observed by saponification of eel muscle, compared with analysis without saponification (Ishimaru et al. Citation2017). It was considered that this loss was caused by the decomposition of retinol during the heat process of saponification. Finally, the direct HPLC method is included in the Intertek Food Services laboratory’s scope of accreditation by the German accreditation body DAkkS (Deutsche Akkreditierungstelle GmbH).
Also, the analysis of the cis retinyl palmitate isomers by the described method was studied. It was found that 13-cis-retinyl palmitate is not separated, and 9-cis-retinyl palmitate is half-separated from all-trans-retinyl palmitate on the RP HPLC column. A photoisomerisation experiment indicated the depletion of all-trans-retinyl palmitate and simultaneously the isomerisation to 9-cis-retinyl palmitate in the HPLC chromatograms. Thus, the influence of light on all-trans-retinyl palmitate can also be detected by the direct HPLC method.
Conclusions
A direct HPLC method, without sample preparation, has been developed for a cost- and time-efficient analysis of all-trans-retinyl palmitate in fortified edible oils. The method does not require the saponification process and the need of polarity-inversion of the oily matrix, thus limiting the degradation of the heat- and light-sensitive retinyl palmitate. The rapid analysis with fewer work steps considerably minimises the risk of analyte loss or decomposition. Consequently, the method is less vulnerable to different systematic and random errors, and thus more reliable. The method is not only faster and more reliable but also more environmentally friendly by using fewer solvents and chemicals, and by producing less chemical waste with its disposal implications. Furthermore, the method showed appropriate sensitivity, precision, and accuracy during validation. A fluorescence detector is used, which is more sensitive than a UV detector and less sensitive to matrix interferences. The method’s working range is well suitable for the analysis of fortified oil samples. In this context, the robustness of the method could be proven by analysing different edible oils that are typically used for fortification. The RP HPLC column is not deteriorated by the lipid matrix, does not need any washing procedures, and proved to have an excellent stability and long lifetime. In addition, 9-cis-retinyl palmitate as photo isomerisation product of all-trans-retinyl palmitate and as an indicator for the effect of light during oil storage and analysis can also be detected by this method. The simplicity of sample preparation combined with the speed of the HPLC analysis makes this method well suited for the rapid and reliable routine analysis of a large number of fortified oil samples.
Acknowledgments
Mduduzi Mbuya and Penjani Mkambula are gratefully acknowledged for their contributions to the conceptualization of the project and critical comments to the drafts of the manuscript. The authors also thank Maike Rohde for the skilful sample preparation and HPLC analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AACC Method 86-06.01. American Association of Cereal Chemists (AACC) approved methods of analysis, 11th Edition, 2009, Cereals & Grains Association. AACC Method 86-06.01: analysis of vitamins A and E by high-performance liquid chromatography, St. Paul (MN).
- Allen L, de Benoist B, Dary O, Hurrell R. 2006. Guidelines on food fortification with micronutrients. Geneva (Switzerland): World Health Organization, Food and Agricultural Organization of the United Nations; p. 1–376.
- AOAC International. 1992a. Official methods of analysis of AOAC International (2008). Rockville (MD): AOAC International. Official Method 992.04: Vitamin A (retinol isomers) in milk and milk-based infant formula.
- AOAC International. 1992b. Official methods of analysis of AOAC International (2005). Rockville (MD): AOAC International. Official Method 992.06: Vitamin A (retinol) in milk-based infant formula.
- AOAC International. 2001. Official methods of analysis of AOAC International (2011), AOAC International, Rockville (MD, USA). Official Method 2001.13: Vitamin A (retinol) in foods.
- AOAC International. 2012. Official methods of analysis of AOAC International (2016). Rockville (MD): AOAC International. Official Method 2012.10: Simultaneous determination of vitamins E and A in infant formula and adult nutritionals.
- AOAC International. 2019. Appendix K: guidelines for dietary supplements and botanicals, Part 1 AOAC guidelines for single-laboratory validation of chemical methods for dietary supplements and botanicals, 2019. Rockville (MD): AOAC International. [accessed 2021 Aug 18]. http://www.eoma.aoac.org/app_k.pdf.
- Codex Alimentarius. 2013. Codex Alimentarius Commission, procedural manual, 21. Edition, Principles for the establishment of codex methods of analysis. [accessed 2021 Aug 18]. http://www.fao.org/3/a-i3243e.pdf.
- Diosady LL, Krishnaswamy K. 2018. Micronutrient fortification of edible oils. In: Venkatesh Mannar MG, Hurrell R, editors. Food fortification in a globalized world. Cambridge (MA): Academic Press; p. 167–174.
- European Standard EN 12823-1:2014. Foodstuffs – determination of vitamin A by high performance liquid chromatography – part 1: measurement of all-E-retinol and 13-Z-retinol. European Committee for Standardization (CEN), Brussels (Belgium).
- [FDA] Food and Drug Administration 2019. Guidelines for the validation of chemical methods in food, feed, cosmetics, and veterinary products. 3. Edition. U.S. Food and Drug Administration, Foods Program [accessed 2021 Aug 18]. https://www.fda.gov/media/81810/download.
- Gaylord AM, Warthesen JJ, Smith DE. 1986. Effect of fluorescent light on the isomerization of retinyl palmitate in skim milk. J Food Sci. 51(6):1456–1458. doi:https://doi.org/10.1111/j.1365-2621.1986.tb13833.x.
- [GFDx] Global Fortification Data Exchange. 2021. [accessed 2021 Aug 9]. https://fortificationdata.org/.
- Hemery YM, Fontan L, Moench-Pfanner R, Laillou A, Berger J, Renaud C, Avallone S. 2015. Influence of light exposure and oxidative status on the stability of vitamins A and D3 during the storage of fortified soybean oil. Food Chem. 184:90–98. doi:https://doi.org/10.1016/j.foodchem.2015.03.096.
- Horwitz W. 1982a. Evaluation of analytical methods used for regulation of foods and drugs. Anal Chem. 54:67 A–76 A. doi:https://doi.org/10.1021/ac00238a002.
- Horwitz W. 1982b. Evaluation of analytical methods used for regulation. J AOAC. 65:525–530. doi:https://doi.org/10.1093/jaoac/65.3.525.
- Ishimaru M, Haraoka M, Hatate H, Tanaka R. 2017. High-performance liquid chromatography with fluorescence detection for simultaneous analysis of retinoids (Retinyl Palmitate, Retinyl Acetate, and Free Retinol) and α-, β-, γ-, and δ-Tocopherols in foods. Food Anal Method. 10:92–99. doi:https://doi.org/10.1007/s12161-016-0553-z.
- Kurzer AB, Dunn ML, Pike OA, Eggett DL, Jefferies LK. 2014. Antioxidant effects on retinyl palmitate stability and isomerization in nonfat dry milk during thermally accelerated storage. Int Dairy J. 35:111–115. doi:https://doi.org/10.1016/j.idairyj.2013.11.001.
- Luthringer CL, Rowe LA, Vossenaar M, Garrett GS. 2015. Regulatory monitoring of fortified foods: identifying barriers and good practices. Glob Health Sci Pract. 3(3):446–461. doi:https://doi.org/10.9745/GHSP-D-15-00171.
- Mulry MC, Schmidt RH, Kirk JR. 1983. Isomerization of retinyl palmitate using conventional lipid extraction solvents. J AOAC Int. 66(3):746–750. doi:https://doi.org/10.1093/jaoac/66.3.746.
- Murphy PA, Engelhardt R, Smith SE. 1988. Isomerization of retinyl palmitate in fortified skim milk under retail fluorescent lighting. J Agric Food Chem. 36:592–595. doi:https://doi.org/10.1021/jf00081a046.
- Muthayya S, Rah JH, Sugimoto JD, Roos FF, Kraemer K, Black RE. 2013. The global hidden hunger indices and maps: an advocacy tool for action. PlOS One. 8(6):e67860. doi:https://doi.org/10.1371/journal.pone.0067860.
- Renaud C, Berger J, Laillou A, Avallone S. 2013. Quantification of vitamin A in fortified rapeseed, groundnut and soya oils using a simple portable device: comparison to high performance liquid chromatography. Int J Vitam Nutr Res. 83(2):122–128. doi:https://doi.org/10.1024/0300-9831/a000154.
- Rohner F, Frey SK, Mothes R, Hurtienne A, Hartong S, Bosso PE, Bui M, Schweigert FJ, Northrop-Clewes C. 2011. Quantification of vitamin A in palm oil using a fast and simple portable device: method validation and comparison to high-performance liquid chromatography. Int J Vitam Nutr Res. 81(5):335–342. doi:https://doi.org/10.1024/0300-9831/a000081.
- Rowe LA, Luthringer CL, Garrett GS. 2018. Regulatory monitoring of mandatory fortification programs. In: Venkatesh Mannar MG, Hurrell R, editors. Food fortification in a globalized world. Cambridge (MA): Academic Press; p. 283–290.
- Schwartz SJ. 1987. Cis/trans isomerization of retinyl palmitate in foods. J Agric Food Chem. 35:748–751. doi:https://doi.org/10.1021/jf00077a025.
- Souganidis E, Laillou A, Leyvraz M, Moench-Pfanner R. 2013. A comparison of retinyl palmitate and red palm oil β-carotene as strategies to address vitamin A deficiency. Nutrients. 5(8):3257–3271. doi:https://doi.org/10.3390/nu5083257.
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, Danaei G, Li G, White RA, Flaxman SR, et al. 2015. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Global Health. 3(9):e528–e536. doi:https://doi.org/10.1016/S2214-109X(15)00039-X.
- Thiex N, Smallidge R, Beine R. 1996. Sources of error in vitamin A analysis. J AOAC Int. 79(6):1269–1276. doi:https://doi.org/10.1093/jaoac/79.6.1269.
- Weiser H, Somorjai G. 1992. Bioactivity of cis and di-cis isomers of vitamin A esters. Int J Vitam Nutr Res. 62(3):201–208.
- West KP Jr. 2003. Vitamin A deficiency disorders in children and women. Food Nutr Bull. 24(4):S78–S90. doi:https://doi.org/10.1177/15648265030244S104.
- [WHO] World Health Organization. 2009. Global prevalence of vitamin A deficiency in populations at risk 1995-2005. WHO global database on vitamin A deficiency. Geneva, World Health Organization.