?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A large number of insulation/dielectric failures in power systems are related to thermally-induced dielectrical breakdown, also known as ‘thermal breakdown’, at higher operating temperatures. In this work, the thermal breakdown behavior of typical silicone formulations, used as dielectrics in stretchable electronic devices, is analyzed at practically relevant operating temperatures ranging from 20°C to 80°C. An effective way of delaying the thermal breakdown of insulating materials is to blend micro- or nano-sized inorganic particles with high thermal conductivity, to dissipate better any losses generated during energy transduction. Therefore, two types of commercial silicone formulations, blended with two types of rutile hydrophobic, high-dielectric TiO2 fillers, are investigated in relation to their dielectric properties, namely, relative permittivity, the dissipation factor, and electrical breakdown strength. The breakdown strengths of these silicone composites are subsequently evaluated using Weibull analysis, which indicates a negative correlation between temperature and shape parameter for all compositions, thus illustrating that the homogeneity of the samples decreases in line with temperature, but the breakdown strengths nevertheless increase initially due to the trapping effect from the high-permittivity fillers.
GRAPHICAL ABSTRACT
1. Introduction
Given the rapid development in power electronics technology, the trend of electric appliances is toward high power density, high switching frequency, and miniaturization [Citation1], all of which may lead to overheating during operation [Citation2]. However, most electrically insulating materials are also thermally insulating, so any heat generated by an overloaded operation or intensive discharges will accumulate, resulting in a rise in the operating temperature of electric apparatus [Citation3]. A small difference in operating temperature has a significant impact on the reliable operation of an electric device [Citation4], and a large number of insulation failures in the power system are related to thermal breakdown [Citation5,Citation6].
Excellent dielectric elastomers based on silicone elastomers are being developed for use in high-voltage transducers [Citation7]. Pristine silicone elastomers also appear as potential candidates for power electronic device insulation [Citation8]. Silicone is a preferred material choice, due to its excellent intrinsic properties such as thermal stability, high dielectric breakdown strength, and relatively low dielectric and mechanical losses [Citation9,Citation10]. However, for many applications, the performance of dielectric elastomers is limited by the risk of failure, which is triggered by several factors [Citation11], amongst which thermal effects may strongly influence electrical breakdown strength, due to strong thermal insulation [Citation12].
Nowadays, composites comprising a polymer matrix and particles with a high dielectric constant have been extensively explored to serve as dielectric materials in high-energy-density devices [Citation13–Citation15]. The nanoscale dispersion of inorganic particles in a polymeric matrix has been proven very convenient, due to the positive reinforcing effect and specific properties that result when the particles are finely and homogeneously dispersed in the polymer matrix [Citation16,Citation17].
Titanium dioxide (TiO2) is a widely available, highly dielectric nanomaterial, which exhibits high thermal conductivity, excellent insulating properties, and matrix compatibility when fumed [Citation18]. TiO2 is thus a promising filler to improve the dielectric permittivity of silicone elastomers and can be used for a variety of applications in the form of additives to enhance their capability to store thermal energy as well as modify wear or frictional resistance [Citation19,Citation20]. The rutile form of TiO2 exhibits a higher dielectric constant (εr ~110) compared to the anatase TiO2 (εr ~45) and the brookite TiO2 (εr ~14) [Citation21,Citation22]. Therefore, high-permittivity rutile TiO2 is a better candidate for achieving higher nanocomposite permittivity and largely improves the dielectric constant of the silicones when blended with it [Citation23]. The presence of rutile TiO2 nanostructures is expected to influence the distribution of electrical field lines within the elastomer, due to the much higher relative permittivity than seen in the matrix [Citation24]. An optimal amount of TiO2 filler should be blended with silicones for best results as too low rutile TiO2 content cannot increase the dielectric permittivity of silicones significantly [Citation25]. On the contrary, excessively high rutile TiO2 loading results in aggregates, and thus other properties deteriorate sharply, due to poor film formation properties [Citation26]. The dispersion of rutile TiO2 nanostructures in transformer oils has been reported to enhance dielectric performance by capturing fast streamer electrons and transforming negatively charged but slower electrons accordingly, thereby leading to modified and improved breakdown characteristics [Citation27].
The dielectric breakdown of silicones has already received much attention since their development for electrical insulation and their intensive use in industrial applications [Citation28]; however, for temperatures ranging above room temperature, very few examples have been investigated [Citation29]. Hence, for practical operating temperatures, an extended study seems necessary, and so a study of the influence of fillers on composite silicones is of primary importance, since it will help predict the dielectric breakdown field for wider temperatures. For commercial electronics, the operating temperature range is usually 0°C to 70°C [Citation30]. Outside of these temperatures, at either end of the scale, there may be some loss of function, or the device may stop working entirely [Citation31]. Silicones, however, should work down to at least −90°C, due to their low glass transition temperature, and they can therefore also be used in hostile conditions such as the Arctic.
In the present work, relative permittivity, the dissipation factor, the storage modulus, and the breakdown strength of rutile TiO2-filled silicone elastomers are measured, and temperature-dependent (20°C to 80°C) results are presented. The morphology of the elastomers – as characterized by scanning electron microscopy (SEM) – is also presented. A thorough analysis and discussion of the breakdown strength of the silicone composites, using the Weibull approach, is also presented herein.
2. Experimental
2.1. Materials
One room-temperature vulcanization (RTV) silicone elastomer (Elastosil® RT 625 A/B) and one liquid silicone rubber (LSR) (Elastosil® LR 3043/50) were both provided by Wacker Chemie AG. The mass mixing ratio of premixes A and B was 9:1 for Elastosil® RT 625 and 1:1 for Elastosil® LR 3043/50.
Two types of hydrophobic rutile titanium dioxide (TiO2) fillers with a modified polysiloxane surface (Hombitec® RM130 F and Hombitec® R420) were acquired from Sachtleben Chemie GmbH, Germany. Primary particle sizes of RM130 F and R420, as stated by the supplier, were 20 nm and 300 nm, respectively (Figure S1).
Solvent OS-20 (an ozone-safe volatile methylsiloxane (VMS) fluid) was purchased from Dow Corning, USA. OS-20 was not added to the pure RT 625 formulation, since its viscosity allows for coating without solvent. OS-20 additions to the high-viscosity LR 3043/50, and formulations with TiO2, varied in order to acquire appropriate viscosities for coating thin and homogeneous films.
2.2. Film preparation
Solvent OS-20 was mixed into commercial silicone premix A from the respective silicone elastomer, using a FlackTek Inc. DAC 150.1 FVZ-K SpeedMixer™ for 3 minutes at 3500 rpm. Next, 30 parts per hundred (phr) of TiO2 filler was mixed into the previous mixture for another 3 minutes at 3500 rpm in the speedmixer. Afterward, premix B was mixed into the previous mixture for the last speedmixer treatment of 3 minutes at 3500 rpm. It should be noted that 30 parts per hundred rubber (phr) TiO2 filler does not cause evident limitations in processing and film properties as previously reported [Citation32]. specifies the mass ratios of the components in the film mixtures. The uniform mixtures thus created were coated onto a glass substrate, using a film applicator (3540 bird, Elcometer, Germany) with a 150 µm gap-adjustable blade. The films were cured in an oven for 1 hour at 115°C, followed by post-curing for 4 hours at 200°C, to ensure that all volatiles have been removed efficiently and in order not to influence the results [Citation33]. For rheological and dielectric permittivity measurements, the thicknesses of the films were in the range 0.5–1.0 mm, and for the breakdown experiments they were in the range 54–106 μm.
Table 1. Mass ratios of the components in film mixtures*.
2.3. Characterization
2.3.1. Measurement of shear moduli
Rheological characterization of the prepared films was performed with a TA Instruments Discovery Hybrid Rheometer HR-1 set to 2% controlled strain mode, which is ensured to be within the linear viscoelastic regime by conducting initial strain sweeps. Measurements were performed with a parallel plate geometry of 25 mm at multiple temperatures (20, 30, 40, 50, 60, 70 and 80°C), with a normal force of ~7 N and in the frequency range 100–0.01 Hz.
2.3.2. Dielectric properties determination
Dielectric relaxation spectroscopy (DRS) was performed on a Novocontrol Alpha-A high-performance frequency analyzer (Novocontrol Technologies GmbH & Co) operating in the constant frequency 1 Hz at multiple temperatures, ranging from 20 to 200°C, and with an electrical field of ~1 V mm−1. The diameter of the tested samples was 25 mm, and their thicknesses were in the range 0.5 to 1 mm.
2.3.3. Electrical breakdown strength determination
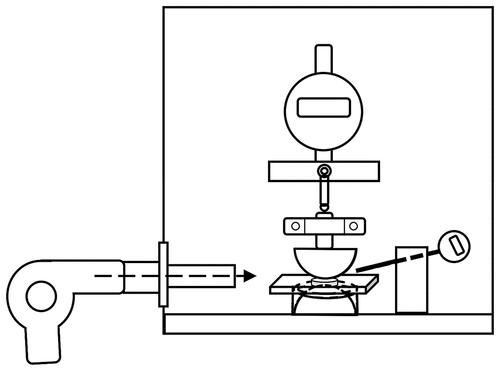
Electrical breakdown tests were performed on an in-house-built device based on international standards (IEC 60,243–1 (1998) and IEC 60,243–2 (2001)) [Citation34]. Film thicknesses were measured through optical microscopy (LEICA DM LB, Germany) of cross-sectional cuts. The distance between the spherical electrodes was set accordingly, using a micrometer stage and a gauge. An indent of less than 5% of sample thickness was then applied to ensure that the spheres are in contact with the sample. The polymer film was slid between the two spherical electrodes (diameter of 20 mm), and the breakdown strength was measured at the point of contact by applying a stepwise increasing voltage (50–100 V step−1) at a rate of 0.5–1 steps s−1. Each sample was subjected to 20 breakdown measurements at multiple temperatures between 20 and 80°C, and the temperature was controlled by a hot air gun (Metabo, Germany) and a thermometer (Ebro TDC 110) with ±1°C accuracy. An average of the recorded breakdown values was given as the breakdown strength of the sample. illustrates the setup for electrical breakdown measurement.
2.3.4. Scanning electron microscopy (SEM)
The morphology of the films was investigated with an FEI Quanta 200 ESEM scanning electron microscope, equipped with a field emission gun. The films were coated in 2 nm-thick gold by means of a sputter coater (Cressington, model 208 HR) under vacuum conditions and a current of 40 mA for 5 seconds. The sample surface was detected with a secondary electron detector (ETD SE) for an incident electron beam of spot 3.5 accelerated to 15–20 keV.
2.3.5. Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) of the films was performed on a Discovery TGA from TA Instruments in a nitrogen atmosphere with a heating rate of 10°C min−1 from RT to 900°C.
3. Results and discussion
3.1. Morphological analysis
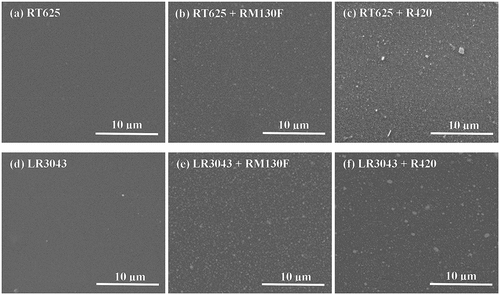
In order to investigate the structural morphology of the distribution of high loading TiO2 fillers in the silicone matrix, SEM images of the surface of the investigated elastomers are illustrated in . The morphologies of RT625 elastomers with TiO2 particles are shown in ). Following TiO2 addition, no obvious aggregation of particles is observed by SEM imaging, thereby indicating a homogeneous dispersion of both types of TiO2 in the RT625 matrix. The SEM spectra in ) show a minor aggregation of the particles in the LR3043 elastomers. The filler distribution in the matrix will directly influence the dielectric behavior of the resulting samples, as discussed in the following sections.
It is commonly known that high dielectric permittivity can be achieved by adding a high-volume fraction of inorganic particles to silicone elastomers [Citation35]. However, this approach undoubtedly results in a sharp decrease in dielectric breakdown strength if the density of cracks and void defects increases as a result of particle agglomeration [Citation36].
3.2. Dielectric properties
Recent studies show that composites with enhanced dielectric permittivity can be obtained by enlarging the particle-polymer interfacial area, as a result of promoting polarization at the interfaces [Citation37]. Choosing an appropriate filler content and particle size is critical in terms of optimizing the dielectric compatibility of the composites [Citation1]. However, decreasing the particle size does lead to difficulties when processing due to increased viscosity.
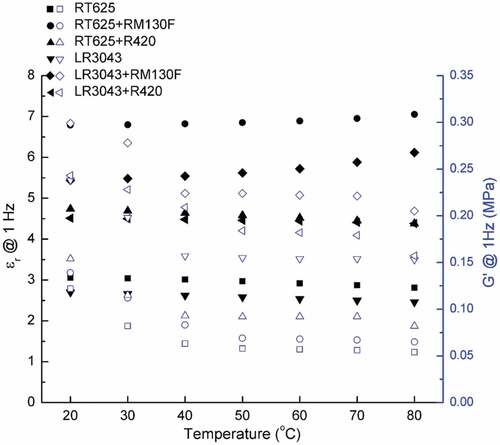
The smaller particles provide larger effective areas of the interfaces between fillers and polymer matrix, resulting in the higher dielectric properties attributed to the significant increment of the interfacial polarization. ) presents the variation in relative permittivity (εr) as a function of the frequency at 20°C, for all the examined systems. As seen in the curve, εr decreases in line with increasing frequency, as expected, thus reflecting the leveling off of polarization, since dipoles fail to follow the rapid alternation of the field [Citation38]. The results show a clear increase in the relative permittivity of the composite films, with TiO2 loading within the measured frequency range. Rutile TiO2 filler has much higher permittivity (εr ~110) compared to that of pure silicone (εr ~2.8) [Citation39], and thus it increases the overall permittivity of the composite. It is to be noted that commercial silicone formulations used in the present study are loaded with silica fillers for reinforcement (Figure S2) and their influence on dielectric permittivity is negligible [Citation7]. The dielectric permittivity of pure PDMS or PDMS reinforced with silica is in the range of 2–3 [Citation7].
Figure 3. Relative permittivity and electric loss tangent of the RT625 and LR3043 films, without and with 30phr TiO2 particles, as a function of investigated frequency at 20°C.
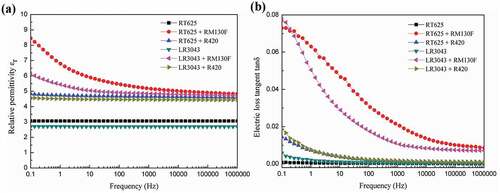
The relative permittivity of the filled elastomer is strongly influenced by polarization at the interface between PDMS and the TiO2 nanoparticles [Citation40]. With the smaller RM130 F particles, the interfacial area between them and the polymer matrix is enhanced, compared to the larger R420-filled samples. Consequently, the presence of more interfacial polarization in the RM130 F-filled elastomers results in higher permittivity compared to the R420-filled elastomers.
) shows the dissipation factor, i.e. the dielectric loss tangent (tanδ), of the investigated elastomers versus frequency at 20°C. The dissipation factor of pure RT625 is independent of the frequency; however, the dissipation factor of other samples decreases in line with an increase in frequency between 10−1 Hz and 106 Hz. The dissipation factor decreases dramatically at low frequencies, whereas its descending rate becomes relatively small and reaches a plateau at around 1 Hz, 100 Hz, and 105 Hz for pure LR3043, R420-filled and RM130 F-filled elastomers, respectively. Moreover, the influence of small RM130 F fillers on the dissipation factor is obvious and much higher than big R420 fillers. It is observed that at a certain low frequency 0.1–100 Hz, the small RM130 F-filled elastomers show a much higher loss tangent, compared to the big R420-filled elastomers, due to the fact that as particle size decreases, the interfacial interaction between the particles increases, leading to a higher dissipation factor. Consequently, this high interfacial interaction weakens the dispersion and packing of the filler particles within the compounds, thus increasing the size of pores and agglomerates and ultimately leading to an increase in dielectric loss.
As already noted, relative permittivity εr is proportional to polarization and is the result of orienting molecular and/or macromolecular dipoles parallel to the field, since at low temperatures the slow process of interfacial polarization does not come into play and thus can be neglected [Citation14]. Therefore, this behavior is more pronounced at higher temperatures and within the low-frequency region, as can illustrated in , and Table S1 (SI) shows the data. The relative permittivity of pure silicone and R420-filled elastomers decreases in line with an increase in temperature – as explained by the Debye model shown in EquationEquation (1)(1)
(1) [Citation41]:
Figure 4. Relative permittivity (ɛr@1 Hz) and storage modulus (G’@1 Hz) of the RT625 and LR3043 films, without and with 30phr TiO2 particles, as a function of the investigated temperature.
where is the dielectric constant at high frequency,
is vacuum permittivity, N is dipole density, and
is the dipole moment. In Table S2 (SI),
values for the silicones used in this study are calculated using EquationEquation (1)
(1)
(1) . The calculated
values are very similar to the measured
values. Using the Debye model, one considers that dipole moments
have no interaction with their near neighbors [Citation41]; however, RM130 F-filled elastomers do not obey the simple Debye model. As the temperature increases, the contribution of TiO2 to the permittivity of the composite improves, and so as a result free molecular mobility with increasing temperature is at least partially removed. Thus, small RM130 F particles are pulled apart from each other, and large dipoles are formed, which can be attributed to the accumulation of unbound charges at the interface between the matrix and the filler, causing an increase in polarization and dielectric permittivity. However, particle-to-particle interactions of large R420 particles compete with particle-macromolecule interactions, thus impeding dipole orientation and resulting in unchanged values for dielectric permittivity.
The storage modulus (G’) versus the temperature of the investigated films is also shown in and Table S1. It is evident that the G’ decreases in line with an increase temperature for all of the samples examined, which suggests that as temperature rises, more free volume in the network is created, and hence the G’ of the resulting elastomers reduces. On the other hand, the decrease in G’ for samples with fillers is lower than for unfilled samples, which indicates that the fillers enhance the compact nature of the network. The influence of temperature on the permittivity and modulus of the investigated elastomers will eventually affect dielectric breakdown strength, as discussed below.
3.3. Electrical breakdown strength
TiO2 particles form excellent electron traps, due to the convergence of local field lines, which appear because of the high dielectric constant of TiO2. This phenomenon is beneficial for increasing the breakdown strength of the resulting elastomers [Citation42]. The smaller RM130 F particles possess larger values in terms of specific surface area, which allows for scavenging, thus causing the filled elastomers to exhibit further enhanced breakdown strength [Citation43], as seen in the RT625+ RM130 F samples in .
Figure 5. Electrical breakdown strengths of the RT625 and LR3043 films, without and with 30phr TiO2 particles, as a function of the investigated temperature. Associated uncertainties in the experiments are shown in the corresponding error bars.
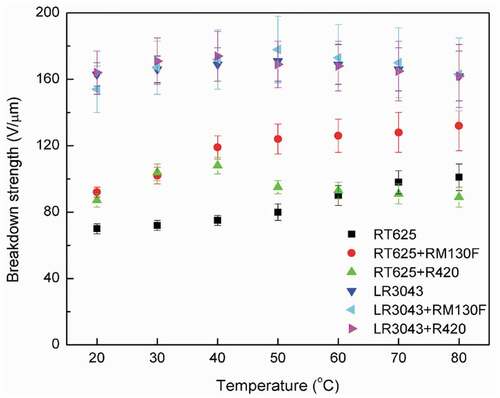
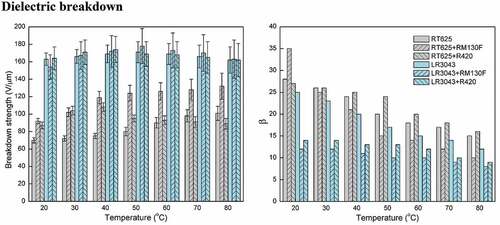
Experimental results reveal that the increase in temperature leads to further enhancement in the breakdown strength of RM130 F-filled elastomers compared to ones at room temperature. The maximum magnitude of the breakdown strength is observed as 132 V/µm for RT625+ RM130 F at 80°C, which, for this corresponding enhancement, is 43% higher compared to the breakdown value of 92 V/µm at 20°C. At elevated operating temperatures, the thermal conductivity and thermal degradation of the silicone dielectric are crucial in determining the point of thermal breakdown [Citation44]. Furthermore, the maximum breakdown strengths for the RT625+ R420, LR3043+ RM130 F, and LR3043+ R420 systems are observed to be 108 V/µm (40°C), 178 V/µm (50°C), and 174 V/µm (40°C), respectively (), while the maximum corresponding enhancements for the same are found to be 24 %, 16 %, and 6 %, respectively (). As shown in , the TiO2-filled RT625 elastomers have higher breakdown strength than pure RT625 at temperatures ≤ 60°C. It is worth noting that the breakdown strength of RT625+ RM130 F elastomers increases in line with increasing temperature, which indicates that the elastomers containing well-dispersed TiO2 particles possess enhanced breakdown strength. However, the breakdown strength of RT625+ R420 elastomers decreases in line with increasing temperature at > 60°C. Similarly, and Table S3 (SI) highlight that with increasing temperature, the breakdown strength of the LR3043 system first increases and then decreases. In addition, the breakdown strength of the LR3043 matrix is not obviously higher than that of pure LR3043, and it shows a substantial negative correlation to temperature increases of ≥ 50°C.
This would indicate that the enhancement of breakdown strength as a consequence of elevated temperature is due to the enhanced thermal fluctuations of nanostructures, which leads to the enhanced diffusion of the nanoparticles’ scavenging capabilities [Citation13]. As the temperature increases, the nanostructures experience higher degrees of Brownian motion within the network [Citation45,Citation46]. This effect, combined with the increased instability of the charged entities, due to the lowering of network compactness and enhanced thermal diffusion, leads to more effective scavenging, resulting in the delayed merger of the breakdown in the network [Citation13]. This is illustrated in ). The presence of fillers with a high dielectric constant is responsible for changing the localized permittivity of the elastomer (), and this then leads to a decrease in positive streamer propagation velocity, thus delaying the breakdown event [Citation13]. Since the relative permittivity of TiO2 is much higher than that of the silicone matrix, the field lines converge onto the TiO2 particles, directing the streamers onto the TiO2 particles. These streamers are then efficiently scavenged by the TiO2 particles before they can travel to the opposite electrode, thereby enhancing breakdown strength [Citation13].
Figure 6. Illustration of the breakdown mechanism, which changes due to the addition of TiO2 particles into silicone films (indicated by blue-waved lines). White arrows indicate the traverse direction of free electrons (green dots) between the electrodes. Yellow arrows indicate how the TiO2 particles (orange dots) capture the charges, thereby enhancing breakdown strength. Green arrows indicate the liberated charges, which traverse through the voids among the particles, eventually leading to reduced breakdown strength.
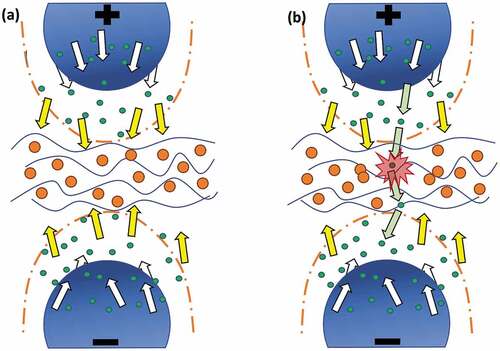
From the TGA curves shown in Figure S2 (SI), it is deduced that pure LR3043 has more silica particles embedded in the matrix compared to pure RT625. Hence, dispersing a high-filler loading of TiO2 into pure LR3043 leads to the formation of defects in the matrix because of the interfacial interactions between the TiO2 and silica particles and the subsequent voids. When there is an increase in the operating temperature, a looser network structure (as seen from the above modulus change with temperature in and Table S1) tends to form alongside the better dispersion of TiO2 particles, thus enhancing breakdown strength. Nonetheless, it seems that as the temperature increases further beyond 50°C, the possibility of particles touching each other results in aggregation and produces more electron shortcuts from one electrode to another. This phenomenon decreases breakdown strength and is illustrated in ), where some streamers manage to travel through the voids in TiO2 particles and reach the opposite electrode, thereby reducing breakdown strength. Likewise, this can also be seen in for the RT625 elastomer with the big R420 particles.
3.4. Weibull analysis of breakdown strength
One way of describing the statistical probability of the electrical breakdown of an elastomer involves employing the Weibull distribution [Citation47], which is given by three parameters, namely, η, which describes the characteristic life, β, which is the shape parameter, and γ, which is the location parameter [Citation47]. t is the variable, which in this case is the electrical field over the dielectric elastomer. The probability density function (pdf) is described by [Citation47]:
In most cases, due to the complexity of the above EquationEquation (2)(2)
(2) , the location parameter is set to γ = 0, which gives the more commonly known two-parameter Weibull distribution, given by:
The 0 value assigned to the location parameter indicates that electrical breakdown may occur instantaneously following the application of an electrical field, i.e. f(t) > 0 for t > 0. If the location parameter is given by γ = γ’, then f(t) > 0 for t > γ’. The approximation of γ = 0 seems reasonable for the electrical breakdown of dielectric elastomer films, since a conducting fiber or dust particle within the elastomer, for example, may lead to immediate breakdown upon the application of an electrical field [Citation47].
The above distribution can also be expressed as a cumulative density function (cdf) by the integration of the pdf, which results in:
where η is electrical breakdown strength and β is a measure of reliability. Usually, η agrees with the arithmetic mean within a few V/µm [Citation48].
For small-volume transducers, breakdown strength is most important for good product reliability, but for large-volume transducers, the β-parameter becomes the most important factor [Citation17]. shows the changes in β for the investigated elastomers at different temperatures. As operating temperatures increase, β decreases, thereby indicating the widening of the Weibull distribution law related to a higher dispersion of breakdown measurements for high temperatures, which indicates that there is a negative correlation between temperature and the homogeneity of the silicone material’s breakdown strength. In the previous section, it was discussed that for the formulations tested herein, breakdown strength increases up to a temperature of ≈ 50°C, and then it decreases with a further rise in temperature, which correlates to the above hypothesis of nonhomogeneous breakdown strength associated with increasing temperature.
Figure 7. Shape parameter β of the RT625 and LR3043 films, without and with 30phr TiO2 particles, as a function of the investigated temperature.
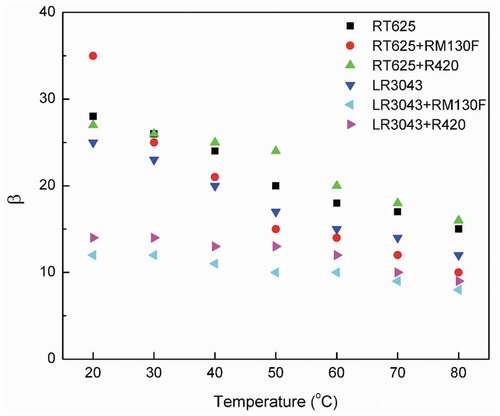
shows the Weibull distribution (pdf and cdf) in the investigated elastomers at different temperatures versus breakdown. For the RT625 system, it is observed that with an increase in temperature, the probability curves shift slightly toward higher breakdown fields, and the scale parameter η (which is determined from the Weibull plot as the value at which failure probability is F = 63.2% [Citation49],) also increases. On the contrary, the shape parameter β (i.e. the slope of the fitting straight line) decreases in line with increasing the temperature (). It can be hypothesized from these observations that as operating temperature increases, the breakdown strength of the silicone increases but the individual, local breakdowns becomes progressively more scattered.
Figure 8. Probability density function (pdf) and cumulative density function (cdf) of the RT625 and LR3043 films, without and with 30phr TiO2 particles, as a function of the investigated temperature.
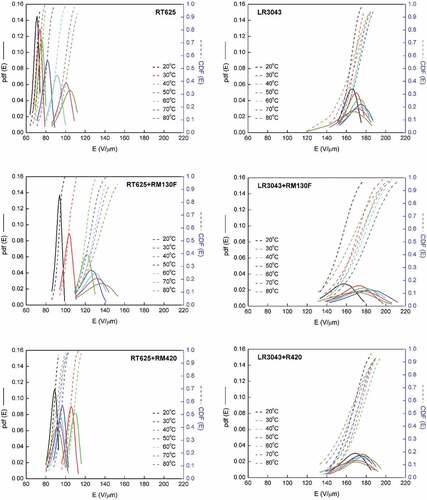
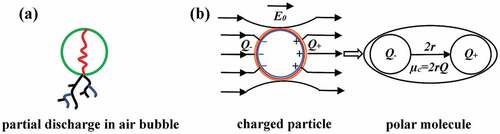
For the LR3043 system, it appears that the elastomer displays deviations in the distribution of the breakdown field values. For the study of polymers, a natural location parameter could be the dielectric breakdown field of air (breakdown strength of ~3 V/µm for dry air, and lower in the presence of contaminants or humidity), due to the existence of vacuoles in the film [Citation50]. In a solid dielectric, the partial discharges (PDs) in the bubble produce ionization capable of breaking the chemical bonds of the dielectric at the boundary of the bubble ()). Consequently, PD will progressively erode the internal surface of the bubble, either uniformly or by forming channels until a complete electrical short circuit is produced [Citation50].
Figure 9. Schematic representation of partial discharges in an air bubble and a charged particle as a polar molecule: Q+ is a positive charge, Q- is a negative charge, Q is total charge, µc is the dipole moment, 2 r is dipole length, and E0 is the electrical field.
Therefore, large volumes of air bubbles, produced by the evaporation of the high loading solvent in the LR3043 mixture, play an important role in diminishing the breakdown strength and homogeneity of the resulting LR3043 samples. The characteristic breakdown strength (η) and the shape parameter (β) of Weibull distribution for all test samples are summarized in Table S3. Data presented in Table S4 (SI) are statistical means of 20 different places for each sample.
In addition, for silicone elastomers filled with particles under an alternating current (AC) electric field, both polymer molecules and particles (such as initial SiO2 and added TiO2 in this work) will be polarized, as shown in ). Consequently, the ultimate dielectric properties of the elastomers are reliant on interactions between molecules, particles, and voids.
3.5. The influence of network structure on the dielectric properties of manufactured elastomers
An evident consequence of inhomogeneity in the network is that local strain is not uniformly distributed when operating temperatures rise. The imposed strain will be concentrated preferably in these inhomogeneous regions, facilitating the onset of yielding in these localized regions with extra free volume or higher-density fluctuations. The bigger defect zones in the network act as stress concentration points, leading to premature sample failure. Consequently, polymeric networks with small defect zones and smaller degrees of inhomogeneity become more resistant to dielectric breakdown [Citation51]. From the above results, it can be hypothesized that the breakdown strength and homogeneity of the two investigated silicone systems are dependent on the network structure, which is illustrated in . This observation helps illustrate that the shape parameter β is affected by temperature in all of the investigated elastomers. For the examined silicones, β decreases as a result of increasing temperature, because a rise in temperature affects network morphology and particle motion, which results in a decrease in overall homogeneity.
Figure 10. Illustration of the influence of network structure on the breakdown strength and shape parameter of the RT625 and LR3043 films, without and with 30phr TiO2 particles, at the investigated temperature. Red dots indicate the small RM130 F TiO2, and the orange dots indicate the large R420 TiO2, respectively. Black-waved lines indicate silicone networks.
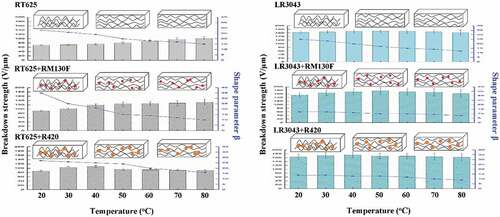
On the contrary, the influence of temperature on the breakdown strength of the elastomers varies somewhat, due to compositions with different silicone matrixes and TiO2 fillers. In both pure RT625 and RT625+ RM130 F elastomers, breakdown strength is augmented by increasing the temperature. As noted in the illustration, the network becomes looser, which in turn means that more free spaces are created amongst air bubbles and TiO2 fillers, hence boosting thermo diffusion in the elastomers and delaying breakdown accordingly. In the same way, the breakdown strength of the RT625+ R420 increases alongside an increase in temperature of ≤ 40°C. In contrast, an opposite trend appears at higher temperatures. As an illustration in , the looser network leads to the higher possibility of the presence of the larger air voids and more aggregations of the big-sized R420 fillers, resulting in a decrease in breakdown strength.
Furthermore, the competition between air voids and filler aggregations in the LR3043 elastomers with high filler loading leads to the durable compaction of the network structure and ultimately results in small variations in breakdown strength across multiple temperatures.
4. Conclusions
From analyzing dielectric breakdown behavior, and the electrical and mechanical properties of the 30phr rutile TiO2 (20 nm and 300 nm)-filled silicones at commercially relevant operating temperatures of 20°C to 80°C, it is concluded that the dielectric properties of the filled silicone elastomers ultimately depend on the network structure. It can be hypothesized that the network structure is influenced by interactions among the molecules, particles, and voids, and these interactions change significantly under various operating temperatures. The storage modulus decreases in line with increasing temperature, indicating that more free volume in the network is created as temperature increases. Furthermore, the small RM130 F (20 nm) TiO2 enhances the more interfacial polarization in the filled elastomers, resulting in higher permittivity compared to the big R420 (300 nm) TiO2-filled elastomers. The relative permittivity of pure silicone and big TiO2-filled elastomers decreases as the temperature increases, thus obeying the Debye model. On the contrary, the permittivity of the small TiO2-filled elastomers increases as a result of an increase in temperature, which is attributable to the accumulation of unbounded charges at the interface between the silicone matrix and small fillers, causing an increase in polarization and dielectric permittivity. Additionally, from the Weibull analysis of breakdown strength, it is notable that the shape parameter (β) decreases as temperature increases, and as such the inhomogeneity of the investigated elastomers is apparent. This is due to the increase in the probability of finding impurities in network morphology leading to failure. Moreover, the small TiO2 particles with larger specific surface area values form excellent electron traps, thereby causing the filled elastomers to exhibit further enhanced breakdown strength compared to the big TiO2-filled elastomers. As the temperature increases, the higher degrees of Brownian motion experienced by the nanoparticles enhances thermal diffusion, due to the lowering of network compactness, resulting in the delayed merger of the breakdown in the network and thus increasing breakdown strength. In contrast, in the silicone elastomers with high filler loading, the incremental possibility of particles touching each other results in the aggregation and appearance of more big voids. This is due to lowering of the network compactness as the temperature increases, thereby limiting the enhancement of breakdown strength or even weakening it. This work provides a formulation approach for the temperature-dependent behavior of TiO2-silicone elastomers, which will help subsequently in the layout of more reliable dielectrics in high-power devices.
Supplemental Material
Download MS Word (870.4 KB)Acknowledgments
The authors gratefully acknowledge the financial support of the Independent Research Fund Denmark.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Jahns TM, Blasko V. Recent advances in power electronics technology for industrial and traction machine drives. Proc IEEE. 2001;89:963.
- Kim IC, Lee SJ. Characterization of a miniature thermal shear-stress sensor with backside connections. Sens Actuators A. 2006;128:305.
- Ortiz C, Skorek AW, Lavoie M, et al. Parallel CFD analysis of conjugate heat transfer in dry-type transformer. IEEE Trans Ind Appl. 2009;45:1530.
- Sim LC, Ramanan SR, Ismail H, et al. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim Acta. 2005;430:155.
- Hikita M, Ieda M, Sawa G. Numerical analysis of steady-state thermal breakdown. J Appl Phys. 1983;54:2025.
- Nagao M, Kimura T, Mizuno Y, et al. Detection of Joule heating before dielectric breakdown in polyethylene films. IEEE Trans Electr Insul. 1990;25:715.
- Madsen FB, Daugaard AE, Hvilsted S, et al. The current state of silicone-based dielectric elastomer transducers. Macromol Rapid Commun. 2016;37:378.
- Zhou W, Qi S, Zhao H, et al. Thermally conductive silicone rubber reinforced with boron nitride particle. Polym Composites. 2007;28:23.
- Shankar R, Ghosh TK, Spontak RJ. Dielectric elastomers as next-generation polymeric actuators. Soft Matter. 2007;3:1116.
- Shit SC, Shah P. A review on silicone rubber. Nat Acad Sci Lett. 2013;36:355.
- Plante JS, Dubowsky S. Large-scale failure modes of dielectric elastomer actuators. Int J Solids Struct. 2006;43:7727.
- Zakaria SB, Morshuis PHF, Benslimane MY, et al. The electrical breakdown of thin dielectric elastomers: thermal effects. Proc SPIE. 2014;9056:90562V.
- Katiyar A, Dhar P, Nandi T, et al. Superior dielectric breakdown strength of graphene and carbon nanotube infused nano-oils. IEEE Trans Dielectr Electr Insul. 2016;23:943.
- González N, Custal MÀ, Tomara GN, et al. Dielectric response of vulcanized natural rubber containing BaTiO3 filler: the role of particle functionalization. Eur Polym J. 2017;97:57.
- Jung HM, Kang JH, Yang SY, et al. Barium titanate nanoparticles with diblock copolymer shielding layers for high-energy density nanocomposites. Chem Mater. 2010;22:450.
- Zhang X, Chen H, Ma Y, et al. Preparation and dielectric properties of core-shell structural composites of poly(1H,1H,2H,2H-perfluorooctyl methacrylate)@BaTiO3 nanoparticles. Appl Surf Sci. 2013;277:121.
- Skov AL, Yu L. Optimization techniques for improving the performance of silicone-based dielectric elastomers. Adv Eng Mater. 2018;20:1700762.
- Ishmael SA, Slomski M, Luo H, et al. Thermal conductivity and dielectric properties of a TiO2-based electrical insulator for use with high temperature superconductor-based magnets. Supercond Sci Technol. 2014;27:095018.
- Xue Q, Liu W, Zhang Z. Friction and wear properties of a surface-modified TiO2 nanoparticle as an additive in liquid paraffin. Wear. 1997;213:29.
- Balasubramanian B, Kraemer KL, Reding NA, et al. Synthesis of monodisperse TiO2-paraffin core-shell nanoparticles for improved dielectric properties. ACS Nano. 2010;4:1893.
- Reyes-Coronado D, Rodríguez-Gattorno G, Espinosa-Pesqueira ME, et al. Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology. 2008;19:145605.
- Yu L, Skov AL. Silicone rubbers for dielectric elastomers with improved dielectric and mechanical properties as a result of substituting silica with titanium dioxide. Int J Smart Nano Mater. 2015;6:268.
- Carpi F, Rossi DD. Improvement of electromechanical actuating performances of a silicone dielectric elastomer by dispersion of titanium dioxide powder. IEEE Trans Dielectr Electr Insul. 2005;12:835.
- González N, Custal MÀ, Rodríguez D, et al. Influence of ZnO and TiO2 particle sizes in the mechanical and dielectric properties of vulcanized rubber. Mater Res. 2017;20:1082.
- Vudayagiri S, Zakaria SB, Yu L, et al. High breakdown-strength composites from liquid silicone rubbers. Smart Mater Struct. 2014;23:105017.
- Silva VP, Paschoalino MP, Goncalves MC, et al. Silicone rubbers filled with TiO2: characterization and photocatalytic activity. Mater Chem Phys. 2009;113:395.
- Choi C, Yoo HS, Oh JM. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr Appl Phys. 2008;8:710.
- Diaham S, Zelmat S, Locatelli ML, et al. Dielectric breakdown of polyimide films: area, thickness and temperature dependence. IEEE Trans Dielectr Electr Insul. 2010;17:18.
- Baudot A, Mazuer J, Odin J. Thermal conductivity of a RTV silicone elastomer between 1.2 and 300 K. Cryogenics. 1998;38:227.
- Humphrey D, Condra L, Pendse H, et al. An avionics guide to uprating of electronic parts. IEEE Trans Compon Packag Technol. 2000;23:595.
- Rahim HRBA, Lokman MQB, Harun SW, et al. Temperature sensing by side coupling of light through zinc oxide nanorods on optical fibers. Sens Actuators A. 2017;257:15.
- Madsen FB, Yu L, Mazurek P, et al. A simple method for reducing inevitable dielectric loss in high-permittivity dielectric elastomers. Smart Mater Struct. 2016;25:075018.
- Brook MA, Saier HU, Schnabel J, et al. Pretreatment of liquid silicone rubbers to remove volatile siloxanes. Ind Eng Chem Res. 2007;46:8796.
- Zakaria SB, Morshuis PHF, Benslimane MY, et al. The electrical breakdown strength of pre-stretched elastomers, with and without sample volume conservation. Smart Mater Struct. 2015;24:055009.
- Barber P, Balasubramanian S, Anguchamy Y, et al. Polymer composite and nanocomposite dielectric materials for pulse power energy storage. Materials. 2009;2:1697.
- Wang J, Hu J, Sun Q, et al. Dielectric and energy storage performances of PVDF-based composites with colossal permittivitied Nd-doped BaTiO3 nanoparticles as the filler. AIP Adv. 2017;7:125104.
- Hao YN, Wang XH, O’Brien S, et al. Flexible BaTiO3/PVDF gradated multilayer nanocomposite film with enhanced dielectric strength and high energy density. J Mater Chem C. 2015;3:9740.
- Mazurek P, Yu L, Gerhard R, et al. Glycerol as high-permittivity liquid filler in dielectric silicone elastomers. J Appl Polym Sci. 2016;133:44153.
- Madsen FB, Daugaard AE, Hvilsted S, et al. Dipolar cross-linkers for PDMS networks with enhanced dielectric permittivity and low dielectric loss. Smart Mater Struct. 2013;22:104002.
- Gun’ko VM, Borysenko MV, Pissis P, et al. Polydimethylsiloxane at the interfaces of fumed silica and zirconia/fumed silica. Appl Surf Sci. 2007;253:7143.
- Vu-Cong T, Jean-Mistral C, Sylvestre A. Impact of the nature of the compliant electrodes on the dielectric constant of acrylic and silicone electroactive polymers. Smart Mater Struct. 2012;21:105036.
- Papageorgiou AC, Beglitis NS, Pang CL, et al. Electron traps and their effect on the surface chemistry of TiO2. PNAS. 2010;107:2391.
- Du YF, Lv YZ, Zhou JQ, et al. Effect of TiO2 nanoparticles on the breakdown strength of transformer oil. Annual Report Conference on Electrical Insulation and Dielectric Phenomena, IEEE; 2010, 1–4, West Lafayette, Indiana, USA.
- Christensen LR, Hassager O, Skov AL. Electro-thermal model of thermal breakdown in multilayered dielectric elastomers. AIChe J. 2019;65:859.
- Yamaguchi M, Doan VA, Nobukawa S. Structure and properties of rubbers with silica nanoparticles as petroleum-free fillers. Advanced Structured Materials. 2015;74:563.
- Minoura I, Katayama E, Sekimoto K, et al. One-dimensional Brownian motion of charged nanoparticles along microtubules: a model system for weak binding interactions. Biophys J. 2010;98:1589.
- Silau H, Stabell NB, Petersen FR, et al. Weibull analysis of electrical breakdown strength as an effective means of evaluating elastomer thin film quality. Adv Eng Mater. 2018;20:1800241.
- Zakaria SB, Madsen FB, Skov AL. Post curing as an effective means of ensuring the long-term reliability of PDMS thin films for dielectric elastomer applications. Polym Plast Technol Eng. 2017;56:83.
- Razak A, Yu L, Skov AL. Voltage-stabilized elastomers with increased relative permittivity and high electrical breakdown strength by means of phase separating binary copolymer blends of silicone elastomers. RSC Adv. 2017;7:17848.
- Tommasini D, Proceedings of CAS Courses on Magnets, Bruges, Belgium, Dielectric insulation and high-voltage issues, 16-25 June 2009, pp. 335–355.
- Sangram KR, Sharma SK, Sudarshan K, et al. Subnanoscopic inhomogeneities in model end-linked PDMS networks probed by positron annihilation lifetime spectroscopy and their effects on thermomechanical properties. Polymer. 2016;101:358.