?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the humid oral environment, 3Y-TZP ceramics always suffer from low-temperature degradation (LTD) for a long time, which results in the degradation of mechanical properties and catastrophic failure. The low-temperature degradation (LTD) and mechanical properties of low-cost tetravalent (Ge4+, Ti4+) element-doped 3Y-TZP were investigated by analysing grain boundary segregation in samples with deferent contents. The results show that GeO2 is superior to TiO2 in limiting LTD but results in lower flexural strength and fracture toughness when the content is ≥1.5 mol%. This dilemma can be improved by adding only 0.1%-0.5 wt% Al2O3, and the flexural strength and fracture toughness of 0.25 wt% Al2O3 zirconia are then increased to 898 MPa and 4.68 MPa·m1/2 compared with 1Ge-3Y, respectively. This work is expected to provide an effective reference for the development and application of budget dental materials.
GRAPHICAL ABSTRACT
1. Introduction
3Y-TZP is a 3% yttria-stabilized tetragonal polycrystalline material with a high fracture toughness and strength and is widely used for dental crowns, fixed bridges, and dental implants [Citation1]. However, their surface will spontaneously transform into the martensitic phase when subjected to a humid and complex oral environment for an extended period [Citation2]. The transformation of the tetragonal phase (t) to the monoclinic phase (m) will lead to surface microcracking, and water molecules will pass through these cracks and penetrate the material, increasing the roughness and decreasing the mechanical properties, which greatly shorted the clinical life of zirconia restorations. This phenomenon is also known as low-temperature degradation (LTD) [Citation3].
To solve this problem, researchers have introduced rare earth cations (including Ce4+, La3+, and Y3+) as dopants to ensure the aging resistance of 3Y-TZP ceramics. However, the price of rare earth cations is high, and there needs to be a systematic method for choosing a suitable dopant that simultaneously exhibits both aging resistance and excellent strength. According to the mechanism of electroneutrality, oxygen vacancies can be introduced by trivalent dopants [Citation4], which can occupy these oxygen vacancies and destabilize the tetragonal phase. Tetravalent dopants (Ce4+, Si4+, Ge4+, and Ti4+) do not create anion vacancies [Citation5] and can stabilize tetragonal zirconia against monoclinic distortion. Among these tetravalent dopants, Ce4+ (with an ionic radius of 0.097 nm) is a large cation that decreases oxygen overcrowding around Zr4+ (with an ionic radius of 0.084 nm) and stabilizes the tetragonal phase. CeO2-doped TZP (Ce-TZP) shows improved LTD resistance compared to 3Y-TZP without introducing oxygen vacancies [Citation6]. However, the flexural strength of Ce-TZP is much lower than that of standard 3Y-TZP due to its larger grain size. Ti4+ (with an ionic radius of 0.074 nm) and Ge4+ (0.053 nm) are smaller than Zr4+ and occupy a smaller space in the crystal lattice [Citation7,Citation8]. The smaller tetravalent cation radius will introduce tensile stresses, which can keep the tetragonal phase at room temperature [Citation9]. At the same time, these cations can also segregate to grain boundaries and block the diffusion pathways of water molecules; however, the detailed mechanism of their stability is not yet understood. Moreover, previous studies [Citation10] have estimated a decrease in flexural strength and fracture toughness with increasing tetravalent cation content. It is well known that [Citation11] doping 3Y-TZP with small amounts of Al2O3 can enhance its mechanical properties and suppress the t-m transformation. Previous studies [Citation12] have proposed that Al2O3 additions lead to Ce-TZP materials with a fine microstructure and significant transformability, as well as enhanced mechanical properties (including strength and fracture toughness). Recently, researchers [Citation13] have introduced small amounts of Al2O3 and 1 mol% CaO to 10Ce-TZP to retard LTD without compromising the mechanical properties. However, the solubility of Al2O3 in zirconia is extremely limited [Citation14,Citation15], so adding a suitable amount of Al2O3 is essential.
There needs to be more research on the LTD of tetravalent element (GeO2 or TiO2)-doped 3Y-TZP, the mechanism of the aging resistance still needs to be determined, and a suitable content remains to be studied. Therefore, this study investigated the effect of the ionic radius of the tetravalent dopant on the LTD behavior and mechanical properties in 3Y-TZP to further understand the diffusion stabilization process of GeO2 during the nonprecursor transformation of tetragonally stabilized zirconia. Further, we also focused on improving the mechanical properties and aging resistance of 3Y-TZP by codoping with Al2O3 and GeO2.
2. Materials and methods
2.1 Sample preparation
The 3 mol% yttria-stabilized zirconia powder (TZ-3Y, purity >99.99%, Tosoh, Japan) had an impurity concentration of approximately 0.1 wt%, with SiO2, Fe2O3, NaO2, and Al2O3 being the chief impurities. The specific surface area of 3Y-TZP was 16 ± 3 m2/g, and the mean particle diameter was 0.6 μm. The other starting powders were GeO2 (purity >99.999%, Aladdin), TiO2 (purity >99.8%, Aladdin), and Al2O3 (purity >99.99%, Aladdin) powders. The 3Y-TZP powders were mixed with 0–2 mol% GeO2, TiO2, or codoped GeO2 and Al2O3 powders by wet milling in a Teflon jar for 24 h using 5 mm and 1 mm zirconia balls as the milling media and ethanol as the mixing medium. TZ-3Y refers to 3Y-TZP, and the mol% is indicated in the nomenclature (e.g. 0.5Ge-3Y refers to 0.5 mol% GeO2 and 3 mol% Y2O3). Samples were dried in an oven at 80°C for 8 h to obtain xGe/Ti-3Y and 1Ge-xAl-3Y zirconia powders.
The powders were isostatically cold pressed at 250 MPa for 2 min and pressureless sintered in air at 1450°C for 2 h, with constant heating and cooling rates of 3°C/min and 10°C/min, respectively. The samples were then cut into 36 × 4 × 3 mm rods. The indicated sintered samples were ground in turn by 120, 240, 600, 800, 1200, 2000, 3000, 4000, and 5000 grade SiC papers and subsequently polished with 1 and 0.05 μm diamond pastes to obtain an optically reflective surface.
2.2 Microstructural analysis
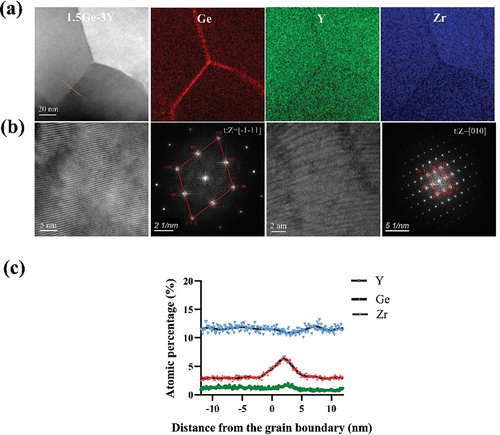
The densities of the samples were tested in deionized water according to Archimedes’ principle. The grain size distributions were obtained on polished surfaces after thermal etching at 1350°C for 20 min. The grain size was measured according to the linear intercept method, performed on pictures recorded by field-emission scanning electron microscopy (SEM, InLens, Germany). At least 1000 grains were counted by Nano Measurer 1.2.5 software, and the outcomes were reported as average results (± standard deviation). The distribution of dopant cations (Y3+, Ge4+, Ti4+, and Al3+) and Zr4+ near the grain boundaries in thin foils was analyzed by scanning transmission electron microscopy (STEM, FEI Talos F200×, U.S.A), energy-dispersive X-ray spectroscopy (EDS) and a high-angle annular dark-field (HAADF) detector.
2.3 Assessment of aging kinetics
Each group’s LTD of polished samples (n = 3) was tested in deionized water for 10, 15, 20, and 30 h at 134°C and a pressure of 2 bar using an autoclave. The amount of all specimens that transformed was measured by X-ray diffraction (XRD, Japan Smartlab) using Cu-Kα radiation at 40 kV and 40 mA, a scan range of 20° to 80° (2θ), and a step size of 0.02 s−1. Fractions of m-ZrO2 and t-ZrO2 were measured by the diffraction patterns in accordance with the method used by Garvie [Citation16] and modified by Toraya [Citation17]:
where Xm is the relative amount of the monoclinic phase, Im and It are the intensities of the monoclinic and tetragonal peaks, respectively.
2.4 Characterization of mechanical properties
According to ISO 6872, the three-point bending strength was measured on 36 × 4 × 3 mm3 test bars (5 bars were tested for each sample).
where F is the maximum load, L is the space of fixtures, b is the sample width, and d is the sample thickness.
The Vickers hardness and fracture toughness [Citation18,Citation19] were measured by the microindentation method. Indentations were made on the surfaces of samples with different compositions at 98.1 N, with expected cracks originating at the corners of the indentation after loading. SEM imaged indentations and the apparent indentation fracture toughness were analyzed by using the equation proposed by Niihara:
where is the Vickers hardness, E is the elastic modulus of ceramic materials (taken as 210 GPa [Citation20,Citation21]), a is half of the diagonal of the indentation, c is the separation between the indentation center and the tip of the crack, and l is the propagation crack length. EquationEquation (3)
(3)
(3) can be applied when 0.25≤(c-a)/a≤2.5 [Citation22] in the case of small indentation cracks.
2.5 Statistical analysis
One-way analysis of variance (ANOVA) with Tukey’s post hoc test and Tukey’s multiple comparisons test was employed in the statistical analysis of mechanical properties (n = 5). GraphPad Prism 8 software was employed in the analysis, where α was set to 0.05. Mean values plus standard deviations are presented in the results, and the different letters in the figures indicate statistical significance.
3. Results and discussion
3.1 Effect of the tetravalent GeO2/TiO2 content on the hydrothermal aging of 3Y-TZP
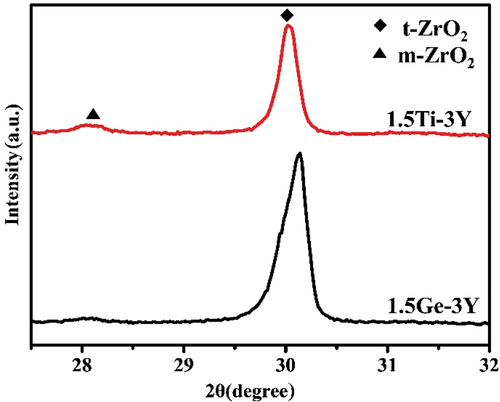
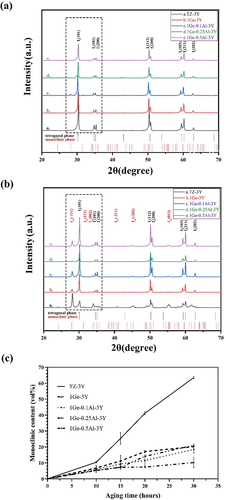
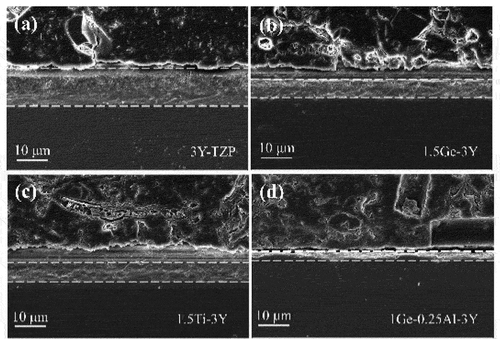
From the XRD analysis (, all of the samples before aging consisted of the tetragonal ZrO2 phase regardless of the amount of added GeO2/TiO2. After aging for 30 h (), the It (101) main peak at 30.2° (2θ) and the monoclinic Im (−111), Im (111), and Im (002) peaks positioned at 2θ values of 28.2°, 31.3°, and 34° start to emerge, with their intensities tending to decrease with increasing tetravalent dopant amount. The monoclinic phase content of 3Y-TZP containing different amounts of GeO2/TiO2 is plotted in against accelerated aging time. Note that compared with GeO2/TiO2-doped 3Y-TZP, undoped 3Y-TZP exhibited a higher monoclinic phase content. The lines indicate that increasing the stabilizer (Ge4+/Ti4+) will improve the resistance to hydrothermal degradation. Moreover, when the aging time exceeded 15 h, the 1.5Ge-3Y samples started to age slowly and stabilized at a monoclinic phase content of approximately 9%, less than the monoclinic phase content in 1.5Ti-3Y ceramics. This suggests that 1.5Ge-3Y displayed more excellent aging resistance than 1.5Ti-3Y after 30 h in steam, corresponding to 90 years in the human body [Citation23]. Since a transformation can only be detected at a depth of up to 8 μm by XRD, we used SEM to further study the degradation area of the cross-section (in ). After aging for 20 h, the thickness of the degradation layer in 1.5Ge-3Y (~6 μm) was smaller than that in 3Y-TZP (~16 μm) and 1.5Ti-3Y (~8 μm). These observations demonstrate that GeO2-doped 3Y-TZP shows better resistance to low-temperature aging than TiO2-doped 3Y-TZP.
Figure 1. X-ray diffraction pattern obtained from samples of GeO2-doped 3Y-TZP (a) before and (b) after 30 h aging at 134°C.
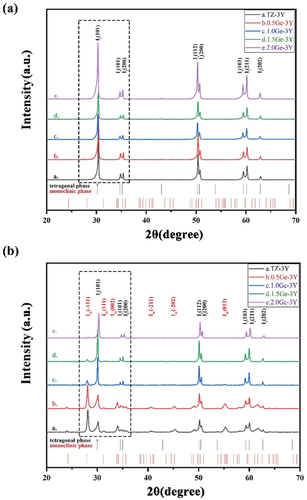
Figure 2. X-ray diffraction pattern obtained from samples of TiO2-doped 3Y-TZP (a) before and (b) after 30 h aging at 134°C.
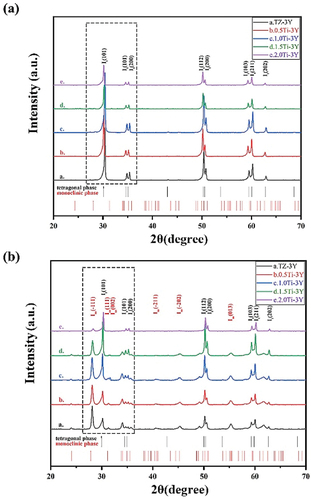
Figure 3. Monoclinic phase content from 3Y-TZP with different GeO2/TiO2 content against accelerated aging time.
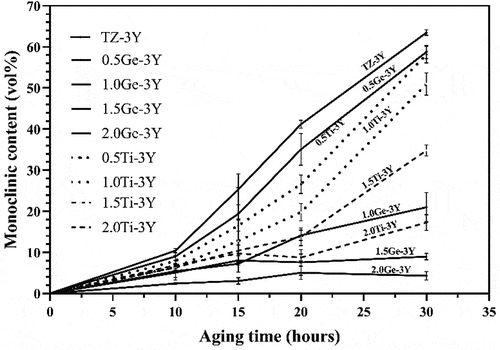
Figure 4. SEM images of the degraded zone from the cross-sections of (a) 3Y-TZP (b) 1.5ge-3Y (c) 1.5ti-3Y (d) 1ge-0.25al-3Y after 20 h aging at 134°C, the transformation zone is indicated by the area between the dotted lines, the upper zones are resin which was used to imbed the zirconia.
Increasing the content of GeO2/TiO2 is expected to improve the susceptibility of 3Y-TZP to LTD, and the chemistry of the dopant and its concentration are closely related to grain boundary segregation, which can enhance the stability of local zirconia grain boundaries and thus decrease the susceptibility to LTD [Citation5].
3.2 Grain boundary segregation in MO2-3Y-TZP: M=Ge/Ti
Grain boundary segregation in tetravalent cation-doped 3Y-TZP is common when sintering at high temperatures. Tetravalent cations such as Ce4+, Mn4+, Si4+, Ge4+, and Ti4+ [Citation24–28] can stabilize the tetragonal phase by inducing grain boundary segregation at room temperature, with the detailed mechanisms depending on the cation type and ionic radius. In the current study, GeO2 and TiO2 were specifically chosen, as their cations have a lower tetravalent ionic radius than the host cation. The cation radius evolution was as follows: Y3+ (0.104 nm) [Citation7] >Zr4+ (0.084 nm) > Ti4+ (0.074 nm) > Ge4+ (0.053 nm) [Citation5,Citation29]. We aimed to research the influence of cation radius on low-temperature degradation.
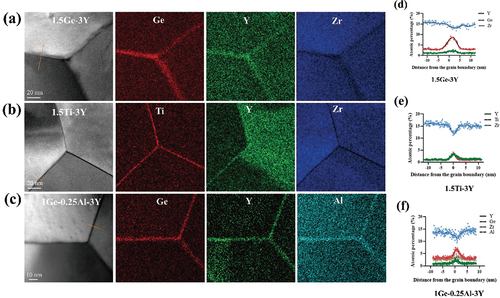
The EDS elemental maps reveal the segregation of Ge4+, Ti4+, Y3+, and Zr4+ to the grain boundaries (). The distribution of Ge4+, Ti4+, Y3+, and Zr4+ across the grain boundary is plotted in line scans (). Ge4+ tends to segregate to a wide area within up to 10 nm from the grain boundary, while Y3+ is apt to segregate to a narrow area within ~5 nm from the grain boundary. At the grain boundaries, the content of GeO2 reached 8%, while the content of Y2O3 was 2%, and the concentration of GeO2 was greatly enhanced at the grain boundaries. In 1.5Ti-3Y, Y3+ ions and Ti4+ ions both tend to segregate to a wide area approximately 5 nm from the grain boundary, with concentrations of ~3–4%.
Figure 5. HAADF-STEM images, EDS elemental mappings and corresponding chemical compositions of Ge4+, Ti4+, Y3+, Zr4+ and Al3+ across the grain boundaries of (a,d) 1.5ge-3Y, (b,e) 1.5ti-3Y and (c,f) 1ge-0.25al-3Y. The dots represent the experimental data, and the black lines represent the fitting curves.
The discrepancies between the radii of dopant cations and the host cation were mainly responsible for the different grain boundary segregation behaviors of various cations. The differences among the cation sizes of Y3+, Ti4+, and Zr4+ are similar, resulting in similar grain boundary segregation behavior. The Ge-O bond is much smaller than the Ti-O and Zr-O bonds. The smaller substitutional Ge4+ cation could decrease the bond length and increase the binding strength, allowing for greater mobility of the Ge4+ atoms and therefore strengthening grain boundary segregation [Citation7,Citation29,Citation30]. Grain boundary segregation is more substantial when the difference between the dopant cation radius and the host cation radius is more significant. Increased tetravalent element grain boundary segregation can strengthen grain boundaries and inhibit water molecules’ diffusion, leading to excellent aging resistance in GeO2-doped 3Y-TZP.
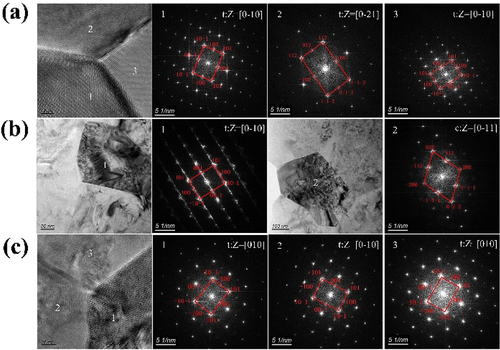
The diffraction analysis indicates that both GeO2 and TiO2 can stabilize the tetragonal phase ; the GeO2-doped grains have a tetragonal crystal structure, whereas the TiO2-doped grains have a tetragonal or cubic crystal structure. According to the grain boundary segregation-induced phase transformation mechanism (GBSIPT), a large amount of Y3+ starts to segregate to grain boundaries when temperatures exceed 1300°C, decreasing its concentration in the tetragonal phase grain regions. Consequently, the regions in 1.5Ti-3Y with high Y3+ ion concentrations transform into cubic grains. A possible explanation for this behavior was proposed by Chevalier, who suggested that large cubic grains will act as new nucleation sites for tetragonal-to-monoclinic (t-m) martensite phase transformation, and monoclinic phase grains are more susceptible to degradation than tetragonal grains [Citation30]. This mechanism may also explain why 1.5Ge-3Y is superior to 1.5Ti-3Y in resisting LTD.
Figure 6. Diffraction analysis of the area where the Roman numerals located form (a) 1.5ge-3Y, (b)1.5ti-3Y, and (c) 1ge-0.25al-3Y.
To further understand the grain boundary segregation behavior of GeO2, we studied the diffusion mechanism of GeO2 in 1.5Ge-3Y at lower temperatures. At 1250°C (), the EDS elemental mappings also reveal the segregation of Ge4+, Ti4+, and Y3+ to the grain boundaries, whereas at 1450°C, Ge4+ tends to segregate to a wide area within up to 10 nm from the grain boundary, and the GeO2 content at the grain boundaries reaches 6.7%; however, the grain boundary segregation behavior of Y3+ is slightly weaker than that of 1.5Ge-3Y sintered at 1450°C.
3.3 Mechanical properties of MO2-3Y-TZP: M=Ge/Ti
Grain boundary segregation can affect not only the aging resistance of 3Y-TZP but also the mechanical properties of the material. First, increasing the doping amount of GeO2 or TiO2 slightly lowers the hardness. Second, as shown in , the flexural strength of 3Y-TZP decreases with GeO2/TiO2 doping and decreases after a significant increase in TiO2 doping amount (and has almost no effect on that doped with 1.5 mol%). The flexural strength will drop greatly with increasing grain size for samples containing the same dopant. The grains of xTi-3Y are smaller than those of xGe-3Y, so the flexural strength of xTi-3Y is higher than that of xGe-3Y because doping with tetravalent elements will promote grain growth and coarsening and lead to a decrease in density and flexural strength. Another possible explanation for the difference between the strengths of xGe-3Y and xTi-3Y is that a large amount of GeO2 grain boundary segregation leads to an increase in the number of impurities at the grain boundaries, which affects bridging between grains and decreases the density, thus influencing mechanical properties [Citation30] (in ). In , the flexural surface was investigated by SEM, and the 1.5Ge-3Y materials exhibited predominantly intergranular fracture modes. The segregation of many impurities results in much lower cohesion between grains, leading to intergranular fracture and lower flexural strength [Citation3].
Figure 8. SEM images of polished and thermally etched surfaces of (a) 1.5ge-3Y and (b) 1ge-0.25al-3Y zirconia ceramics sintered for 2 h at 1450°C and fractured surfaces of (c) 1.5ge-3Y and (d) 1ge-0.25al-3Y after a flexural strength test. TEM microstructure images of (e) 1.5ge-3Y and (f) 1ge-0.25al-3Y.
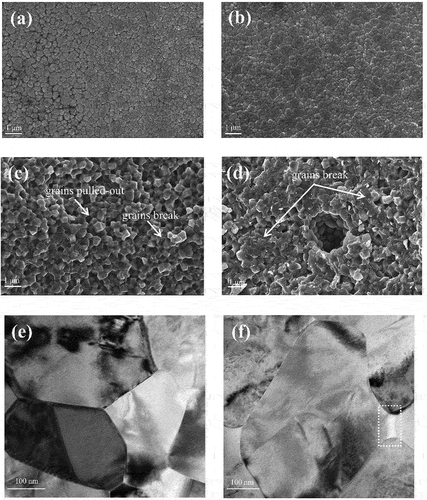
Table 1. Group, density, grain size, and mechanical properties of GeO2/TiO2-doped 3Y-TZP.
Moreover, fracture toughness is likely related to the segregation behavior of many solute atoms at grain boundaries; however, the detailed mechanism underlying this behavior is unclear. Generally, the fracture toughness of xTi-3Y is typically higher than that of xGe-3Y. On the one hand, based on the line scan in , more Y2O3 is located at grain boundaries in 1.5Ti-3Y than in 1.5Ge-3Y, which leads to less Y3+ within grains. The composition of internal grains is responsible for the higher fracture toughness of 1.5Ti-3Y due to the t-m transformation toughening mechanism [Citation31–34]. The monoclinic phase content on the fracture surface represents the stress-induced phase transformation rate, and the phase transformation rate of 1.5Ti-3Y is 12.7%, which is higher than the 3.1% phase transformation rate of 1.5Ge-3Y in .
On the other hand, we can observe stacking faults in 1.5Ti-3Y (areas 1 and 2 in . The scattered regions of a raised surface may result from the volume increase associated with martensitic transformation near grain boundaries [Citation35]. Thus, local tensile stress will be present at the grain boundaries and lead to the formation of metastable tetragonal zirconia. Cubic grains will act as nucleation sites for the tetragonal-to-monoclinic transformation [Citation36], which increases the fracture toughness of xTi-3Y; however, this phenomenon is not common [Citation37].
The grain boundary structure, and the presence of stacking faults near the grain boundary can affect the distribution of solute elements and influence the strength and toughness of the materials [Citation35,Citation38]. In short, the degree of segregation depends on the impurity concentration at the grain boundaries and can explain the differences between the mechanical properties of GeO2- and TiO2-doped 3Y-TZP.
3.4 Co-doping 3Y-TZP with Al2O3 and GeO2 to improve aging resistance and mechanical properties
GeO2 doping can significantly improve the aging performance of 3Y-TZP, but it has a detrimental influence on mechanical properties. This conclusion is consistent with the research of Mastui et al. [Citation39]. Therefore, how to improve the mechanical properties while maintaining the excellent aging resistance of GeO2-doped 3Y-TZP ceramics is the focus of current research.
As shown in , the flexural strength and fracture toughness of 1Ge-3Y were enhanced by adding 0.1 wt% to 0.5 wt% Al2O3. This enhancement was not observed for the hardness. From the micrographs provided in , all samples were densified without obvious porosity and adding Al2O3 slightly enhanced the grain growth of 1Ge-3Y. In addition to the role of Al2O3 on the flexural strength of the materials, 0.25 wt% Al2O3-doped 1Ge-3Y exhibited more transgranular fracture than 1.5Ge-3Y , which may be related to the segregated Al3+ increasing grain boundary cohesion [Citation40–42]. In our study, addingAl2O3 can slightly enhance the fracture toughness of 1Ge-3Y; the material doped with 0.25 wt% Al2O3 had a 12.32% higher Vickers indentation hardness than 1Ge-3Y.
Table 2. Group, density, grain size, and mechanical properties of GeO2 and Al2O3 co-doped 3Y-TZP.
Three effects may explain the influence of alumina on toughness. One possible explanation may be that increased Y3+ segregation can slightly improve the indentation toughness [Citation43]. A second explanation is that the various laws of grain size and fracture toughness are similar (); a larger grain size corresponds to a greater tendency for phase transformation, contributing the stress-induced phase transformation toughening mechanisms. To further determine the toughening mechanism of ceramic materials, Raman spectra were collected from the indentation center, crack root and crack tip in . For any component, the monoclinic phase content at the crack root is the largest, followed by that at the indentation center, with that at the crack tip being the smallest. In addition, 1Ge-0.25Al-3Y induces greater t-m transformability around the crack than 1Ge-3Y, thus resulting in higher toughness.
Figure 10. Raman spectra near the indentation zone of zirconia ceramics: (a)1.0ge-3Y, (b) 1ge-0.25al-3Y. (c) SEM images of indentation-induced cracking in 1ge-0.25al-3Y ceramics.
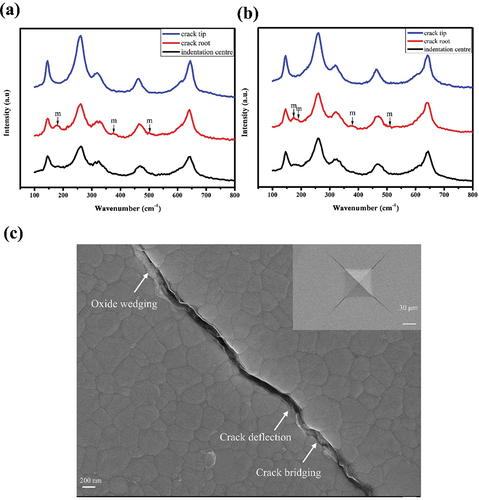
Moreover, other traditional toughening mechanisms, including fracture bridging, oxide wedging, and crack deflection, are also presented in .
Third, an amorphous phase is located at the triple junctions, which may reduce residual strains at the grain boundaries of rounded grains compared with faceted and sharp grains in 1.5Ge-3Y . The amorphous phase mainly consists of Ge4+ and Al3+ (). According to a previous study [Citation11], the formation of a grain boundary amorphous phase caused by the addition of alumina may contribute to the improved mechanical properties and resistance to t-m transformation; however, the detailed mechanism of LTD resistance has not been determined.
Table 3. The elements compositions of the amorphous phase.
According to ISO standard 13,356:2008, a monoclinic phase content of less than 25% should be expected for dental ceramics after heating for 5 h at 134°C in an autoclave [Citation44]. In the present study, XRD analysis () indicates that the aging resistance of GeO2- and Al2O3-codoped 3Y-TZP is superior to that of GeO2 singly doped 3Y-TZP. Moreover, shows the average thickness of the degradation layer of 1Ge-0.25Al-3Y (varying from 1 to 3 μm, which was in accordance with the XRD results) after 20 h of hydrothermal treatment, which is much lower than that of 1Ge-3Y. We can conclude that GeO2- and Al2O3-codoped 3Y-TZP materials have sufficient aging resistance compared with currently known bioceramics and meet clinical requirements. From the TEM and diffraction analyses of 1Ge-0.25Al-3Y in , Ge4+ is more segregated to the grain boundaries than Al3+ and Y3+ [Citation45] and stabilizes the tetragonal phase at room temperature. Previous studies [Citation3] have reported that segregated Al2O3 may also play a significant role in influencing the nucleation of aging and the concentration of free oxygen vacancies, thus reducing the sensitivity to low-temperature aging. In the present study, adding 0.25 wt% Al2O3 improved the aging resistance of 1Ge-3Y; however, the aging resistance was not further improved with the addition of 0.1 wt% or 0.5 wt% Al2O3, which may be related to the solubility of Al2O3 in 3Y-TZP. It is likely that Ge4+ and Al3+ segregated at grain boundaries and played major and minor roles, respectively, in retarding the LTD of 1Ge-xAl-3Y.
4. Conclusion
The present investigation has shown that tetravalent oxides (GeO2 or TiO2) with different cation radii resulted in different trends in the aging behavior and mechanical properties as a function of dopant concentration in the range of 0.5–2 mol%. On the one hand, the present work showed that adding more significant amounts of GeO2 or TiO2 resulted in substantially higher hydrothermal aging resistance than undoped 3Y-TZP. More importantly, GeO2 is superior to TiO2 in limiting low-temperature degradation, which could be directly related to the large discrepancy between the radii of the tetravalent cation and Zr4+; Ge4+ exhibited strong segregation at the grain boundaries with increasing temperature, which was preferred. On the other hand, TiO2 is better than GeO2 in terms of mechanical properties, which may also be relevant to the grain boundary structure. Various parameters must be considered, including the mechanical properties, in selecting the most suitable zirconia ceramic for biomedical applications. Therefore, we codoped GeO2 and Al2O3 in 3Y-TZP and found that 1Ge-0.25Al-3Y exhibited excellent mechanical properties while maintaining the hydrothermal aging resistance. However, within the scope of this work, additional efforts will be made further to explore the aging resistance and mechanical properties of Al2O3- and GeO2-codoped 3Y-TZP sintered at different temperatures. It is necessary to study the stable zirconia obtained from the transformation process of nonprecursors and the diffusion stabilization process of the subsequent addition of rare earth elements to further guide grain boundary engineering.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Tovar-Vargas D, Ferrari B, Sanchez-Herencia AJ, et al. Low temperature degradation and mechanical properties of alumina reinforced ceria-zirconia by colloidal processing. J Eur Ceram Soc. 2021;41:1459–1470.
- Camposilvan E, Flamant Q, Anglada M. Surface roughened zirconia: towards hydrothermal stability. J Mech Behav Biomed. 2015;47:95–106.
- Samodurova A, Kocjan A, Swain MV, et al. The combined effect of alumina and silica co-doping on the ageing resistance of 3Y-TZP bioceramics. Acta Biomaterialia. 2015;11:477–487.
- Fabris S, Paxton AT, Finnis MW. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002;50(20):5171–5178.
- Zhang F, Batuk M, Hadermann J, et al. Effect of cation dopant radius on the hydrothermal stability of tetragonal zirconia: grain boundary segregation and oxygen vacancy annihilation. Acta Mater. 2016;106:48–58.
- Chevalier J, Gremillard L, Virkar AV, et al. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J Am Ceram Soc. 2009;92(9):1901–1920.
- Shannon RD. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A. 1976;32(5):751–767.
- Kuwabara A, Yokota S, Ikuhara Y, et al. Grain boundary energy and tensile ductility in superplastic cation-doped TZP. Mater Trans. 2004;45(7):2144–2149.
- Schaedler TA, Leckie RM, Kramer S, et al. Toughening of nontransformable t ’-YSZ by addition of titania. J Am Ceram Soc. 2007;90:3896–3901.
- Matsui K, Yoshida H, Ikuhara Y. Review: microstructure-development mechanism during sintering in polycrystalline zirconia. Int Mater Rev. 2018;63(6):375–406.
- Tsubakino H, Fujiwara T, Satani K, et al. Composition of grain boundary phase formed in zirconia-3 mol% yttria containing alumina. J Mater Sci Lett. 1997;16(17):1472–1475.
- Fornabaio M, Palmero P, Traverso R, et al. Zirconia-based composites for biomedical applications: role of second phases on composition, microstructure and zirconia transformability. J Eur Ceram Soc. 2015;35:4039–4049.
- Tovar-Vargas D, Turon-Vinas M, Anglada M, et al. Enhancement of mechanical properties of ceria-calcia stabilized zirconia by alumina reinforcement. J Eur Ceram Soc. 2020;40:3714–3722.
- Lakiza SM, Lopato LM. Stable and metastable phase relations in the system alumina-zirconia-yttria. J Am Ceram Soc. 1997;80(4):893–902.
- Wu ZK, Li N, Jian C, et al. Low temperature degradation of Al2O3-doped 3Y-TZP sintered at various temperatures. Ceram Int. 2013;39(6):7199–7204.
- Garvie RC, Nicholson PS. Phase analysis in zirconia systems. J Am Ceram Soc. 1972;55(6):303–&.
- Toraya H, Yoshimura M, Somiya S. Calibration curve for quantitative analysis of the monoclinic-tetragonal zro 2 system by x-ray diffraction. J Am Ceram Soc. 1984;67(6):C119–121.
- Quinn GD, Bradt RC. On the Vickers indentation fracture toughness test. J Am Ceram Soc. 2007;90(3):673–680.
- Nastic A, Merati A, Bielawski M, et al. Instrumented and vickers indentation for the characterization of stiffness, hardness and toughness of zirconia toughened al2o3 and sic armor. J Mater Sci Technol. 2015;31(8):773–783.
- Nawa M, Nakamoto S, Sekino T, et al. Tough and strong Ce-TZP/alumina nanocomposites doped with titania. Ceram Int. 1998;24:497–506.
- Zhang F, Vanmeensel K, Inokoshi M, et al. Critical influence of alumina content on the low temperature degradation of 2–3mol% yttria-stabilized TZP for dental restorations. J Eur Ceram Soc. 2015;35:741–750.
- Niihara K. A Fracture-mechanics analysis of indentation-induced palmqvist crack in ceramics. J Mater Sci Lett. 1983;2(5):221–223.
- Deville S, Gremillard L, Chevalier J, et al. A critical comparison of methods for the determination of the aging sensitivity in biomedical grade yttria-stabilized zirconia. J Biomed Mater Res B. 2005;72(2):239–245.
- Ramesh S, Meenaloshini S, Tan CY, et al. Effect of manganese oxide on the sintered properties and low temperature degradation of Y-TZP ceramics. Ceram Int. 2008;34:1603–1608.
- Camposilvan E, Marro FG, Mestra A, et al. Enhanced reliability of yttria-stabilized zirconia for dental applications. Acta Biomaterialia. 2015;17:36–46.
- Takigawa Y, Shibano T, Kanzawa Y, et al. Effect of small amount of insoluble dopant on tetragonal to monoclinic phase transformation in tetragonal zirconia polycrystal. Mater Trans. 2009;50(5):1091–1095.
- Yoshida H. Doping dependence of high temperature plastic flow behavior in TiO2 and GeO2-Doped Tetragonal ZrO2 Polycrystals. J Ceram Soc Jpn. 2006;114(1326):155–160.
- Yoshida H, Morita K, Kim BN, et al. Doping amount and temperature dependence of superplastic flow in tetragonal ZrO2 polycrystal doped with TiO2 and/or GeO2. Acta Mater. 2009;57(10):3029–3038.
- Behtash M, Wong J, Jiang SC, et al. First-principles study of impurity segregation in zirconia, hafnia, and yttria-stabilized-zirconia grain boundaries. J Eur Ceram Soc. 2019;39:3812–3820.
- Chen IW. Mobility control of ceramic grain-boundaries and interfaces. Mat Sci Eng A Struct. 1993;166(1–2):51–58.
- Luo P, Zhang J, You ZY, et al. Effect of TiO2 content on the microstructure and mechanical and wear properties of yttria-stabilized zirconia ceramics prepared by pressureless sintering. Mater Res Express. 2019;6(12):125211.
- Vleugels J, Yuan ZX, Van Der Biest O. Mechanical properties of Y2O3/Al2O3-coated Y-TZP ceramics. J Eur Ceram Soc. 2002;22:873–881.
- Yuan ZX, Vleugels J, Van Der Biest O. Preparation of Y2O3-coated ZrO2 powder by suspension drying. J Mater Sci Lett. 2000;19(5):359–361.
- Basu B, Vleugels J, Van Der Biest O. Y-TZP ceramics with tailored toughness. Key Eng Mat. 2002;206-2:1185–1188.
- Zhao M, Ren XR, Pan W. Effect of lattice distortion and disordering on the mechanical properties of titania-doped yttria-stabilized zirconia. J Am Ceram Soc. 2014;97(5):1566–1571.
- Chevalier J. Critical effect of cubic phase on aging in 3mol% yttria-stabilized zirconia ceramics for hip replacement prosthesis. Biomaterials. 2004;25(24):5539–5545.
- Munoz-Tabares JA, Jimenez-Pique E, Anglada M. Subsurface evaluation of hydrothermal degradation of zirconia. Acta Mater. 2011;59(2):473–484.
- Kibsey M, Romualdez J, Huang X, et al. Mechanical properties of titania-doped yttria stabilized zirconia (TiYSZ) for use as thermal barrier coating (TBC. J Eng Gas Turbines Power. 2011;133(12). DOI:10.1115/1.4004125
- Matsui K, Yoshida H, Ikuhara Nanocrystalline Y. Ultra-degradation-resistant zirconia: its grain boundary nanostructure and nanochemistry. Sci Rep. 2014;4(1). DOI:10.1038/srep04758
- Zhang F, Vanmeensel K, Inokoshi M, et al. 3Y-TZP ceramics with improved hydrothermal degradation resistance and fracture toughness. J Eur Ceram Soc. 2014;34:2453–2463.
- Nogiwa-Valdez AA, Rainforth WM, Zeng P, et al. Deceleration of hydrothermal degradation of 3Y-TZP by alumina and lanthana co-doping. Acta Biomaterialia. 2013;9:6226–6235.
- Zhang F, Li LF, Wang EZ. Effect of micro-alumina content on mechanical properties of Al2O3/3Y-TZP composites. Ceram Int. 2015;41(9):12417–12425.
- Bucevac D, Kosmac T, Kocjan A. The influence of yttrium-segregation-dependent phase partitioning and residual stresses on the aging and fracture behaviour of 3Y-TZP ceramics. Acta Biomaterialia. 2017;62:306–316.
- Cattani-Lorente M, Scherrer SS, Ammann P, et al. Low temperature degradation of a Y-TZP dental ceramic. Acta Biomaterialia. 2011;7:858–865.
- Ross IM, Rainforth WM, McComb DW, et al. The role of trace additions of alumina to yttria–tetragonal zirconia polycrystals (Y–TZP). Scripta Mater. 2001;45(6):653–660.