ABSTRACT
The world population is aging, which poses a significant burden to the economy and health care system. As people age, so do their gut microbiomes. Age-related changes in gut microbiome have been reported, including decreased microbial diversity and increased Proteobacteria. Recently, we characterized the gut microbiome of a group of long-living (≥ 90 years old) Chinese people. Interestingly, the diversity of their gut microbiome was greater than that of a young adult control group. We also identified several potentially beneficial bacteria enriched in the long-living Chinese group. These results were validated using data from an independent Italian cohort that included a group of long-living individuals. Other recent studies have found similar results. Here, we provide a summary of these discoveries and discuss their implications in healthy aging.
Introduction
The success of modern medicine, as well as better nutrition and medical care, has dramatically increased the human life span, leading to an increasing elderly population. By 2050, the global population of older people (> 65 years old) is projected to reach 1.6 billion.Citation1 The aging global population poses substantial challenges to the economy, society, and the health care system.Citation2 However, longer lifespan (i.e., longevity) does not necessarily equate to healthy aging. As people age, they have a higher chance of developing a variety of diseases such as heart disease, stroke, hypertension, cognitive impairment, and cancer.
The gastrointestinal tract houses trillions of microbial cells that are known as the gut microbiota. The microbiota has coevolved with the human body and is critical to human health. It plays an important role in human health including, but not limited to, education of the host immune system, vitamin biosynthesis, protection against pathogens, and regulation of intestinal endocrine functions.Citation3 Changes in the gut microbiome have been implicated in diseases such as inflammatory bowel disease (IBD), obesity, type 2 diabetes, and cancer.
Gut microbiota in the elderly
Interactions between the gut microbiota and humans occur at each stage of life, beginning in utero and continuing through old age. The temporal shifts in microbial diversity and composition are critical to human development and health.Citation3 Many factors affect the diversity and stability of the human microbiome (e.g., diet, genetics, environment, and antibiotics). During aging, changes in physiology, diet, medication, and lifestyles lead to changes in the gut microbiome.Citation4,Citation5 In general, gut microbiome diversity decreases during aging.Citation3,Citation6 Regarding gut microbiome composition, however, contradictory results have been reported at the genus level, with some studies showing an increase in a specific genus in the elderly and other studies showing a decrease in that same genus.Citation7 At the phylum level, however, an increase in Proteobacteria has been consistently demonstrated across most studies.
Centenarians as a model of healthy aging
The goal of healthy aging is to reduce and postpone age-related morbidities. Given the high morbidity rates among the elderly, it is difficult to find a cohort for healthy aging studies. Centenarians have consistently been used as a model for healthy aging studies because of their ability to delay, or even avoid, chronic diseases,Citation8,Citation9 and their genetics has been extensively examined.Citation10 However, much was unknown about their gut microbiome until recently. The latest research discoveries have been summarized in several recent review papers.Citation7,Citation11,Citation12
Identification of gut microbiome signatures for healthy aging
In China, there are five regions known as “longevous counties,” and Dujiangyan, Sichuan is one of these regions. To study the correlation between the gut microbiome and healthy aging, we enrolled a cohort of long-living (≥ 90 years old) people in Dujiangyan.Citation13
We characterized and compared the gut microbiota in this long-living cohort with that of a younger adult group. Surprisingly, the long-living group had a greater gut microbiome diversity than the younger adult group, which is different from the conventional wisdom (i.e., that gut microbial diversity decreases during aging). Additionally, we found several potentially beneficial bacterial taxa enriched in the long-living group.
To validate these discoveries, we re-analyzed an independent Italian dataset.Citation14 Consistent with our results, greater gut microbiome diversity was observed in the long-living Italian group. The gut microbiome composition of the long-living Chinese cohort was significantly different from that of the long-living Italian cohort. This was very likely due to differences in diet, genetics, geography, and environment. However, among the top 50 most indicative features, 11 were shared between the two long-living cohorts. In our previous report, we showed six of the 11 shared features,Citation13 including greater alpha diversity measures (e.g., Chao index and observed operational taxonomic units, OTUs) and an increase in potentially beneficial bacteria known as short-chain fatty acids producers (e.g. Akkermansia, Clostridium XIVa).
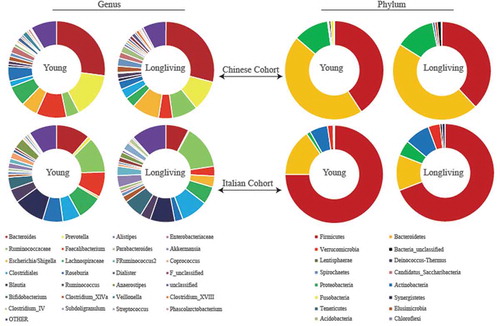
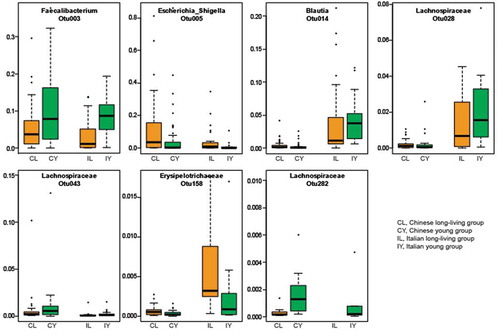
shows the relative abundance of the other five shared features. Of note, OTU5, which is associated with Escherichia/Shigella was increased in both the Chinese and the Italian long-living cohorts. Aggregated pie charts at the genus () and phylum () levels show the greater portion of Escherichia/Shigella and Proteobacteria, consistent with other recent reports showing increased Proteobacteria in the elderly. Therefore, increases in Proteobacteria during aging might be a common change in gut microbiome, regardless of typical elderly or long-living subjects. In addition, the relative abundance of Faecalibacterium in the long-living people decreased compared to the younger group in both the Chinese and Italian cohorts. These data suggest that although the discoveries in our previous study are promising, further studies are warranted to determine whether or not the gut microbiome plays critical roles in healthy aging.
Figure 1. Box plots of five shared features between the Italian and Chinese cohorts. We identified the top 50 features that differentiated the long-living and younger cohorts and in both our Chinese and in the independent Italian cohort. Among the 50 features, 11 were shared between the two cohorts. We previously showed 6 of these 11 features.Citation13 Here, we show the remaining five shared features.
Gut microbiome diversity in the elderly
Microbial diversity has been used as a measure of a healthy microbiome.Citation15 Decreased microbial diversity has been correlated with increased frailty and diseases such as obesity, IBD, cancer, and type 2 diabetes.Citation16–Citation21 It has been conventionally accepted that microbial diversity decreases during aging,Citation3,Citation6 indicating an unbalanced gut microbiome in the elderly. Elderly people almost always have some gut-associated comorbidities.Citation22 Claesson, et al investigated correlations between gut microbiota, diet and health in the elderly. The microbial diversity of the long-stay group, elderly people in long-term residential care, was significantly lower than that of the community-dwelling group. The decreased diversity in the long-stay group was associated with increased inflammation (e.g., increased serum C-reactive protein), decreased healthy food diversity, and decreased overall health status (e.g., functional independence measure, FIM).Citation5 These results suggest it is important to stratify the elderly by their health status for studies focusing on the gut microbiome and aging.
The Chinese long-living subjects in our study were very independent and healthy at enrollment, as demonstrated by their FIM values. FIM is a well-accepted metric to measure physical and cognitive disability. None of the people in this cohort were sick, hospitalized, or receiving any antibiotic treatments. These long-living people lived with family members, had a high quality of life, and enjoyed regular daily activities and games. Therefore, this cohort was not only long living but also a healthy aging cohort. The greater gut microbiome diversity observed in this healthy long-living cohort indicates a healthier gut microbiome in this group.
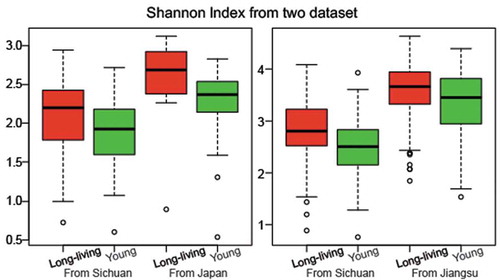
Two recent studies support our discoveries. Odamaki et al. performed a cross-sectional study that characterized 367 healthy Japanese subjects ranging from 0 to 104 years old.Citation23 Re-analysis of a subset of their data from subjects who had similar age range as the Chinese cohort in our study showed that the long-living Japanese cohort had greater gut microbiome diversity than the younger group (). In another recent study, Bian et al. examined the gut microbiome of a cross-sectional cohort of > 1,000 healthy Chinese subjects (mainly from Jiangsu Province, China) aged 3–100 years old and found that the gut microbiota composition of the healthy elderly group was similar to that of the younger people.Citation24 After re-analyzing their age-matched data, we found that the gut microbiome of the long-living Chinese in the Jiangsu province was also more diverse than that of the younger controls, consistent with the data from our Sichuan cohort (). It is important to note that, although microbial diversity has been used as a parameter for a healthy microbiome, it must be interpreted in context.Citation25 For example, due to differences in sample collection, DNA extraction, and sequencing errors, diversity should be only compared between groups within a study; it is risky to compare the diversity between studies without considering the context.
Conclusions
Our study shows that the gut microbiome of Chinese long-living people is more diverse than that of a younger group. This finding is supported by two additional recent studies.Citation23,Citation24 We observed enrichment of several potentially beneficial bacterial taxa in the long-living cohort, members of which are short-chain fatty acids producers. However, we also noticed the decrease of certain OTUs associated with beneficial bacteria (e.g. Faecalibacterium) in this cohort. In addition, we found the increase of some OTUs related to potential bacterial pathogens (e.g. Escherichia_Shigella) in these long-living people. While it is still early to draw any causal relationships between gut microbiota and healthy aging, several recent studies have used animal models to show the causality of gut microbiome and age-related phenotypes. Smith et al showed that recolonizing the gut of middle-age fishes with bacteria from young donors led to life span extension and delayed behavioral decline.Citation26 In another mouse model study, Thevaranjan and colleagues showed that co-housing germ-free mice with old conventionally raised mice increased pro-inflammatory cytokines in the blood, such phenotype was not observed in these germ-free mice when co-housed with young mice.Citation27 Further studies using multi-omics approaches and animal models by fecal microbiota transplantation from young, elderly and long-living people will provide insights into the functions of the gut microbiome in healthy aging. Nevertheless, our study suggests that maintaining a diverse and balanced gut microbiome might contribute to healthy aging and provide a modulation target to promote healthy aging.
Acknowledgments
F.K. and F.D. are supported by the China Scholarship Council (CSC) Scholarship. This research was supported by the State Key laboratory of Genetic Resources and Evolution, Kuming Institute of Zoology, CAS (GREKF14-03); the Innovative Research Team at the University of Sichuan Bureau of Education; and an NSFC (31471997) grant to Y.L.
Additional information
Funding
References
- He W, Goodkind D, Kowal P. An aging world: 2015. Washington (DC): United States Census Bureau; 2016.
- Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591.
- Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379.
- Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4586–4591.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184.
- Kumar M, Babaei P, Ji B, Nielsen J. Human gut microbiota and healthy aging: recent developments and future prospective. Nutr Healthy Aging. 2016;4:3–16.
- Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M, Franceschi C. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018;75:129–148.
- Engberg H, Oksuzyan A, Jeune B, Vaupel JW, Christensen K. Centenarians–a useful model for healthy aging? A 29-year follow-up of hospitalizations among 40,000 Danes born in 1905. Aging Cell. 2009;8:270–276.
- Cevenini E, Invidia L, Lescai F, Salvioli S, Tieri P, Castellani G, Franceschi C. Human models of aging and longevity. Expert Opin Biol Ther. 2008;8:1393–1405.
- Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet. 2013;132:1323–1338.
- Biagi E, Rampelli S, Turroni S, Quercia S, Candela M, Brigidi P. The gut microbiota of centenarians: signatures of longevity in the gut microbiota profile. Mech Ageing Dev. 2017;165:180–184.
- O’Toole PW, Jeffery IB. Microbiome-health interactions in older people. Cell Mol Life Sci. 2018;75:119–128.
- Kong F, Hua Y, Zeng B, Ning R, Li Y, Zhao J. Gut microbiota signatures of longevity. Curr Biol. 2016;26:R832–833.
- Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, et al. Gut Microbiota and Extreme Longevity. Curr Biol. 2016;26:1480–1485.
- Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484.
- Hansen R, Russell RK, Reiff C, Louis P, McIntosh F, Berry SH, Mukhopadhya I, Bisset WM, Barclay AR, Bishop J, et al. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn’s but not in ulcerative colitis. Am J Gastroenterol. 2012;107:1913–1922.
- Gopalakrishnan V, Spencer C, Nezi L, Reuben A, Andrews M, Karpinets T, Prieto P, Vicente D, Hoffman K, Wei S, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103.
- Menni C, Jackson MA, Pallister T, Steves CJ, Spector TD, Valdes AM. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int J Obes. 2017;41:1099–1105.
- Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O’Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8.
- O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350:1214–1215.
- Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5:80.
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90.
- Bian G, Gloor GB, Gong A, Jia C, Zhang W, Hu J, Zhang H, Zhang Y, Zhou Z, Zhang J, et al. The gut microbiota of healthy aged chinese is similar to that of the healthy young. mSphere. 2017;2. doi:10.1128/mSphere.00327-17.
- Shade A. Diversity is the question, not the answer. ISME J. 2017;11:1–6.
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife. 2017;6:e27014. doi:10.7554/eLife.27014.
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–466 E4.