ABSTRACT
Low molecular weight (6.5 kDa) Glycyrrhiza polysaccharide (GP) exhibits good immunomodulatory activity, however, the mechanism underlying GP-mediated regulation of immunity and gut microbiota remains unclear. In this study, we aimed to reveal the mechanisms underlying GP-mediated regulation of immunity and gut microbiota using cyclophosphamide (CTX)-induced immunosuppressed and intestinal mucosal injury models. GP reversed CTX-induced intestinal structural damage and increased the number of goblet cells, CD4+, CD8+ T lymphocytes, and mucin content, particularly by maintaining the balance of helper T lymphocyte 1/helper T lymphocyte 2 (Th1/Th2). Moreover, GP alleviated immunosuppression by down-regulating extracellular regulated protein kinases/p38/nuclear factor kappa-Bp50 pathways and increasing short-chain fatty acids level and secretion of cytokines, including interferon-γ, interleukin (IL)-4, IL-2, IL-10, IL-22, and transforming growth factor-β3 and immunoglobulin (Ig) M, IgG and secretory immunoglobulin A. GP treatment increased the total species and diversity of the gut microbiota. Microbiota analysis showed that GP promoted the proliferation of beneficial bacteria, including Muribaculaceae_unclassified, Alistipes, Lachnospiraceae_NK4A136_group, Ligilactobacillus, and Clostridia_vadinBB60_group, and reduced the abundance of Proteobacteria and CTX-derived bacteria (Clostridiales_unclassified, Candidatus_Arthromitus, Firmicutes_unclassified, and Clostridium). The studies of fecal microbiota transplantation and the pseudo-aseptic model conformed that the gut microbiota is crucial in GP-mediated immunity regulation. GP shows great potential as an immune enhancer and a natural medicine for treating intestinal inflammatory diseases.
1. Introduction
The intestine is the largest digestive and immunological organ in the body and is rich in microorganisms.Citation1 Long-term evolution has established a balanced and delicate symbiotic relationship between the gut microbiota and the host.Citation1,Citation2 Previous studies have shown that the gut microbiota is crucial in the development and maturation of the host immune system.Citation3,Citation4 The gut microbiota can regulate the differentiation and maturation of T and B lymphocytes by interacting with toll-like receptors (TLRs) on immune cell surfaces.Citation5,Citation6 TLRs can affect the activity of immune-related pathways and the secretion of cytokines by stimulating downstream signaling pathways, such as MAPK and NF-kB.Citation7,Citation8 Gut microbiota disorder will lead to decreased immunoglobulin secretion, weakened intestinal barrier function, and bacterial migration and cause immune-related diseases, including diarrhea, liver disease, cancer, and atherosclerosis.Citation1,Citation9 Additionally, metabolites produced by gut microbiota, including acetic acid and propionic acid, can directly affect immune cells and responses.Citation5,Citation10 Cyclophosphamide (CTX), an alkylating agent and immunosuppressant, can significantly damage the intestinal mucosal structure and reduce the immunological organ indexes, immune cell, and cytokine secretion.Citation11,Citation12 CTX results in increased intestinal permeability and bacterial translocation because of the increased abundance of harmful bacteria, inculuding Proteobacteria, Sutterella, and Pseudumonas.Citation13,Citation14
Polysaccharides can counteract the immunosuppression and hold promise as less toxic immunomodulatory agents.Citation3,Citation15,Citation16 Polysaccharides may modulate immune functions via three pathways. First, polysaccharides, such as those from Lycium barbarum and purple sweet potato, can stimulate macrophages and dendritic cells in the Peyer patch or mucosal lamina propria after transmembrane transport by intestinal epithelial cells and M cells.Citation17,Citation18 Second, by directly stimulating pattern recognition receptors (PRRs) in dendritic and intestinal epithelial cells to produce cytokines and chemokines, polysaccharides, such as those from Ganoderma atrum and Hericium erinaceus, can enhance intestinal epithelial barrier function.Citation19,Citation20 Finally, metabolites of polysaccharides, including short-chain fatty acids (SCFAs), can directly promote the proliferation of beneficial symbiotic bacteria and enhance intestinal and body immunity. Polysaccharides from Acaudina molpadioides and Holothuria leucospilota were confirmed to regulate immunity through SCFAs and probiotics.Citation21,Citation22
Glycyrrhiza polysaccharides (GPs), one of the main active ingredients in Glycyrrhiza, have immunomodulatory activities.Citation23,Citation24 In our previous research, a low-molecular-weight (6.5 kDa) GP with desirable immune activity was isolated and its structure was elucidated (Fig. S1; Table S1). This study aimed to reveal the pathways and relationships of GP in regulating immunity and gut microbiota using CTX-induced immunosuppression and intestinal mucosal injury models. We also performed the pseudo germ-free model and Fecal microbiota transplantation (FMT) methods to verify the causality between GP and gut microbiota.
2. Results
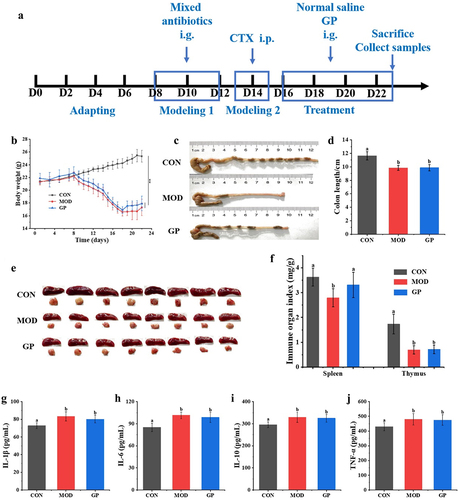
2.1. GP reversed CTX-induced intestinal structural damage
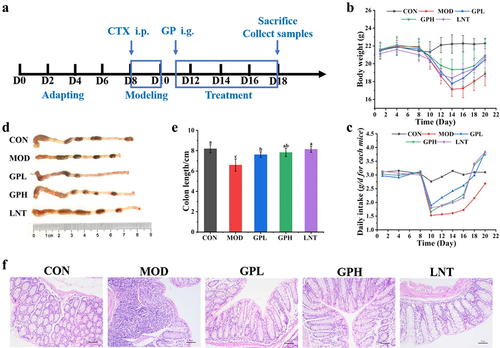
CTX is widely used in treating various malignant tumors and autoimmune diseases, however, it can cause toxic damage to the body.Citation25 Intraperitoneal injections of CTX can produce models of immunosuppression and intestinal mucosal damage. To study the effect of low-molecular-weight GP on CTX-induced immunosuppression and intestinal mucosal damage, an experimental schedule was designed (). After three days of consecutive intraperitoneal injections of CTX, the body weight and daily food intake of mice decreased by 15.23% and 50.54%, respectively ), and the length of colon reduced ). The intestinal tissues were infiltrated by inflammatory factors, and the villus structure and intestinal epithelial barrier function were damaged (). GP treatment significantly increased body weight and daily intake by 7.710–13.05% and 50.75–55.41%, respectively. The lengths of the colon and villi significantly increased, and the number of inflammatory cells decreased, implying improved functional integrity of the intestinal barrier. These results suggested that GP could effectively reverse the side effects of CTX, including weight loss, dietary reduction, shortened colon length, and intestinal structural damage.
Figure 1. Effect of GP on CTX-induced immunosuppressed in mice. (a) diagram of experimental schedule; (b) the change of body weight; (c) the change of diet; (d) Representative physical image of colons; (e) mean colon length in all groups; (f) Representative image of H&E-stained pathological section of colon tissue (200х). Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GPL: low does GP group; GPH: high does GP group; LNT: lentinan.
2.2. GP increased CD4+ T lymphocytes, goblet cells, and mucin content
Goblet cells are secretory cells distributed in the columnar epithelium of the mucosa. By synthesizing and secreting mucin to form a mucosal barrier, goblet cells protect epithelial cells from physical damage and adhesion of harmful bacteria to epithelial cells. The changes in goblet cells and mucin in the intestinal tissues weaken epithelial barrier function and shorten villi.Citation26,Citation27 Intraperitoneal injection of CTX caused a 51.06% and 41.67% decrease in goblet cells and mucin in the intestinal epithelium, respectively, and 10.37% decrease of CD4+ T lymphocytes in intestinal lamina propria ). After GP intervention, goblet cell and mucin contents in the mice intestinal epithelium significantly increased in three regions (). Compared with that in the MOD group, the number of goblet cells and mucin content increased by approximately 2.28 and 1.61-fold (p < .05), respectively ).
Figure 2. Effects of GP on CD4+ and CD8+ T lymphocytes, goblet cell number and mucous volume in colon tissue. (a) Representative image of AB-PAS-stained section of colon tissue (40X and 200X); (b) number of goblet cells; (c) grayscale of the mucus; (d) Representative image of immunohistochemical-stained section of colon tissue (200X); (e) number of CD4+ and CD8+ T lymphocytes. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GPL: low does GP group; GPH: high does GP group; LNT: lentinan.
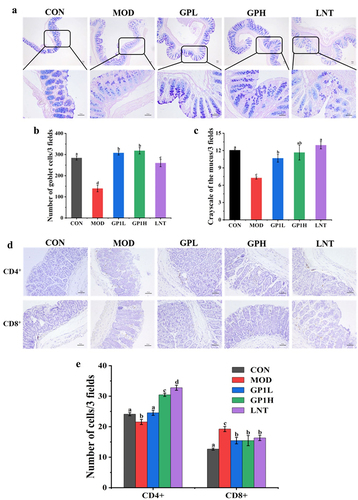
In addition, intraperitoneal CTX injection reduced CD4+ T lymphocytes levels in intestinal tissues approximately 11.86% compared with those in the CON group (p < .05, ). CD4+ and CD8+ T lymphocytes are important immune cells in the intestinal lamina propria that maintain intestinal immune homeostasis. CD4+ T lymphocyte depletion leads to immune dysfunction and diseases, including cold, viral infection, and tuberculosis. After oral administration of GP, CD4+ T lymphocyte level in intestinal tissues in the GPH group increased to 30.45 ± 0.51 (p < .05), which was approximately 20.82% higher than that in the CON group (24.11 ± 0.52), similar to the LNT group (32.78 ± 0.84). Similarly, GP increased the number of CD8+ T lymphocytes in the intestinal tissues by 17.94% compared to that in the CON group (). However, CD8+ T lymphocyte level increased by 33.85% after CTX treatment compared to those in the CON group, which may be related to inflammation in the body. Because CD8+ T lymphocytes usually refer to cytotoxic T lymphocytes, otherwise called viral T cells, high levels of CD8+ T lymphocytes usually represent inflammation or viral infection in the body. AB-PAS staining and immunohistochemical results showed that GP improved CTX-induced intestinal mucosal damage and immunosuppression by increasing the number of CD4+ T lymphocytes and goblet cells and mucin content.
2.3. GP maintained Th1/Th2 balance in CD4+ T lymphocyte subsets
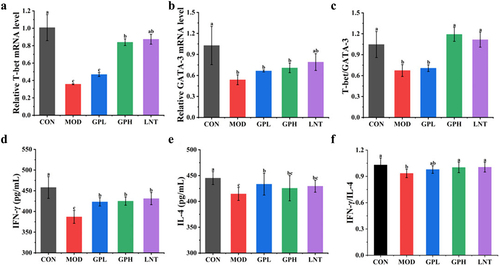
Immunohistochemical results showed that GP increased the number of CD4+ T lymphocytes. CD4+ cells, otherwise called helper T cells (Ths), can be classified into Th1 and Th2 subgroups, which are the most prominent T cell groups involved in intestinal adaptive immunity. T-bet and GATA-3 are nuclear transcription factors of Th1 and Th2, respectively. The mRNA expression ratio of T-bet/GATA-3 represents the dynamic equilibrium state of Th1/Th2. Furthermore, the cytokine IFN-γ/IL-4 ratio represents the Th1/Th2 balance because IFN-γ (a Th1 cytokine) and IL-4 (a Th2 cytokine) are secreted by CD4+ Th1 and Th2 lymphocytes to promote cellular immunity and humoral immunity, respectively.Citation28,Citation29 Intraperitoneal injection of CTX decreased the mRNA expression of T-bet and GATA-3 by 2.82 and 1.91 times ), respectively, while the T-bet/GATA-3 ratio decreased from 1.04 to 0.67 (). The levels of cytokines IFN-γ and IL-4 decreased by 1.12 and 1.15 times, respectively (), while the IFN-γ/IL-4 ratio reduced from 1.03 to 0.87 (). The decreased T-bet/GATA-3 and IFN-γ/IL-4 ratios indicated a weakened degree of differentiation induced by Th0 cells to Th1 and Th2 cells, thereby weakening the body’s immune response. Moreover, a decrease in T-bet/GATA-3 and IFN-γ/IL-4 ratios lead to homeostasis breakdown between Th1 and Th2 cells, further inducing various immune diseases.Citation29 After oral administration of GP, the mRNA levels of T-bet and GATA-3 in the GPH group increased by 1.82 and 1.32 times, respectively, while the T-bet/GATA-3 ratio recovered from 0.67 to 1.19 (). IFN-γ and IL-4 levels in the GP group increased by 1.04 times and 1.15 times, respectively, while IFN-γ/IL-4 ratio recovered from 0.87 to 1.01 (). The recovered ratios in the GPH group reached levels similar to those in the LNT group, indicating that GP enhanced intestinal immunity by maintaining the Th1/Th2 balance in CD4+ T lymphocyte subsets.
Figure 3. Effects of GP on Th1 and Th2 balance of CD4+ T lymphocyte subsets. (a) T-bet; (b) GATA-3; (c) T-bet/GATA-3; (d) IFN-γ; (e) IL-4; (f) IFN-γ/IL-4. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GPL: low does GP group; GPH: high does GP group; LNT: lentinan.
2.4. GP down-regulated the ERK/p38/NF-κBp50 pathway and increased cytokines secretion
Real-time polymerase chain reaction (RT-PCR) and ELISA were used to further explore the effects of GP on signaling pathways and cytokine secretion in immune cells to detect the expression of immune-related pathways and secretion of immune cytokines, respectively.Citation30 The MAPK signaling pathway regulates various cellular physiological and pathological processes, including cell growth, differentiation and inflammatory response. NF-κB signaling pathway is crucial in regulating the immune response to infection.Citation31 Intraperitoneal CTX injection up-regulated the expression of p38, ERK, and p50 by approximately 2.66, 1.97, and 1.28-fold, respectively (p < .05, )). Activation of the ERK/p38/NF-κBp50 pathway may signify increased apoptosis of immune cells and the activation of inflammatory cells, leading to tumor occurrence and an inflammatory response in the body.Citation32 Oral GP administration reduced the mRNA levels of p38, ERK, and p50 in the GPH group by 3.14, 2.72, and 3.86-fold, respectively ).
Figure 4. Effects of GP on signaling pathways and cytokines in serum and intestinal tissues. (a) p50; (B) p38; (c) ERK; (d) IL-2; (e) IL-10; (f) IL-22; (g) TGF-β3; (h) IgM; (i) IgG; (j) SIgA; (k) MIP-1α; (L) MCP-1. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GPL: low dose GP group; GPH: high dose GP group; LNT: lentinan.
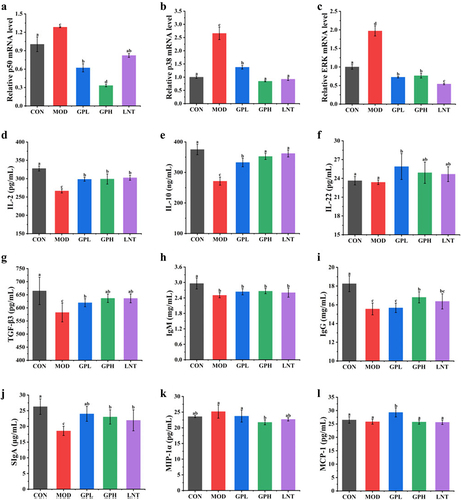
Additionally, intraperitoneal CTX injection decreased the levels of immune cytokines IL-2, IL-10, TGF-β3, IgM, and IgG by approximately 1.23, 1.39, 1.14, 1.18 and 1.17-fold, respectively (p < .05, ); the levels of MIP-1α increased by approximately 1.11-fold, whereas those of MCP-1 did not change significantly ). Cytokines secretion is another indicator used to evaluate the immune response. The cytokines IL-2, IFN-γ (Th1 cell cytokines), IL-10 (Th2 cell cytokine), IL-22 (Th17 cell cytokine) and TGF-β3 (regulatory T (Treg) cell cytokine) are related to innate and adaptive immunity.Citation1 Moreover, the cytokines IgM and IgG secreted by B cells and SIgA secreted by intestinal mucosal epithelial cells play important roles in maintaining intestinal epithelial barrier function. Reduced secretion of these cytokines increases the risk of inflammatory diseases, including vasculitis and arthritis.Citation1 After GP intervention, IL-2, IL-10, and TGF-β3 levels were 1.12–1.3 times higher than those in the MOD group (p < .05), similar to those in the LNT group. MIP-1α decreased approximately 1.16 times, which was higher than that in the LNT group. IL-22 levels in the GPL group, and IgG and SIgA levels in the GPH group increased by 1.21, 1.11 and 1.31 times, respectively, relative to those in the MOD group. These results suggested that GP can alleviate CTX-induced immunosuppression and inflammatory responses by regulating ERK/p38/NF-κBp50 signals and increasing immune cytokine secretion.
2.5. GP increased the diversity of the gut microbiota
To explore the effect of GP on the gut microbiota, the diversity of the gut microbiota was detected by high-throughput sequencing.Citation33 Eight fecal samples were randomly selected from each group, and 2,791,872 effective sequences were obtained from 40 samples, averaging 69,797 sequences per sample (Table S3). A sparse curve was used to compare species diversity and explain the reliability of the sequenced data. A stable dilution curve was observed when the extracted sequence was ≥ 20,000, indicating that the sequencing quantity was sufficient to cover all the taxa in the sample (Fig. S2A). A rank-abundance curve was used to explain species abundance and evenness. The species in each group are evenly distributed with reasonable data (Fig. S2B). Intraperitoneal CTX injection reduced the α-diversity of the gut microbiota. The Chao 1 index, indicating the total number of species, decreased by 6.8%, whereas the Simpson and Shannon indexes, representing the microbial diversity, had no significant changes ). GP intervention increased the α-diversity of the gut microbiota, Chao1 and Shannon indexes increased by 19.41% and 6.82%, respectively. After intraperitoneal CTX injection, the gut microbiota samples from the CON and MOD groups were significantly different (p ≤0.05) (). The results showed that GP reversed the decrease in the total number of CTX-induced species by increasing the total number and diversity of the gut microbiota in mice.
Figure 5. Effects of GP on gut microbiota structure in fecal. (a) α-diversity – Chao 1 (p = .016); (b) α-diversity – Simpson (p = .36); (c) α-diversity – Shannon (p = .11); (d) β-diversity – PCA; (E) microbiota composition – phylum level;(f) dominant bacteria in phylum level: (a) Bacteroidota; (b) Firmicutes; (c) Proteobacteria; (d) Actinobacteria; (g) microbiota composition – genus level; (H) dominant bacteria in genus level: (a) Muribaculaceae; (b) Alistipes; (c) Lachnospiraceae_ NK4A136_group; (d) Clostridiales_unclassified; (e) Clostridia_UCG-014_unclassified; (f) Lachnospiraceae_unclassified; (g) Ligilactobacillus; (h) Lactobacillus; (I) Venn diagram; (J-K) LEfSe difference analysis. The values among groups were compared by Kruskal-Wallis rank sum test, and p < .05 was considered significant (n = 8). CON: control group; MOD: model group; GPL: low dose GP group; GPH: high dose GP group; LNT: lentinan.
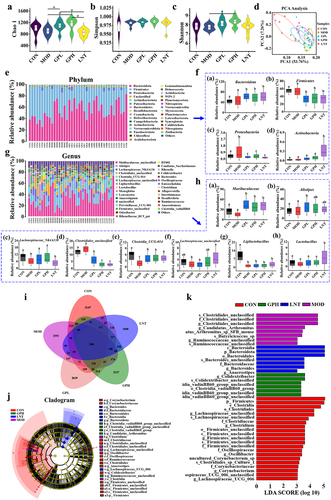
2.6. Effects of GP on the gut microbiota composition
Intraperitoneal CTX injection resulted in a 5.85% decrease in the abundance of Bacteroides and a 3.59% and 34.92% increase in that of Firmicutes and Proteobacteria, respectively ). An increase in the abundance of Firmicutes may cause obesity, hyperlipidemia, hypertension, diabetes, and other metabolic syndromes, while that of Proteobacteria may lead to metabolic disorders, inflammation, and cancer. The abundance of harmful Firmicutes, including Clostridiales_unclassified, Candidatus_Arthromitus, Firmicutes_unclassified, and Clostridium, increased by 15.26%, 29.93%, 37.17% and 6.25%, respectively ( and Fig. S4A-D), which may trigger digestive disorders and production of inflammatory factors. After oral GP administration, 11 bacteria had an average abundance of more than 1% in the GP groups, namely Muribaculaceae_unclassified, Alistipes, Lachnospiraceae_NK4A136_group, Clostridiales_unclassified, Clostridia_UCG-014_unclassified, and Lachnospiraceae_unclassified, Ligilactobacillus, Lactobacillus, Monoglobus, Leuconostoc, and Anaerotignum ). Specifically, the abundance of Firmicutes and Proteobacteria decreased by 45.00% and 63.49%, respectively, in the GPH group. The abundance of CTX-derived bacteria (Clostridiales_unclassified, Candidatus_Arthromitus, Firmicutes_unclassified and Clostridium) decreased by 78.04%, 51.09%, 71.73%, and 60.42%, respectively (Fig. S4A-D). In addition, the ratio of B/F (Bacteroidetes/Firmicutes) increased by 36.99% from 0.92 to 1.46 after oral GP administration (Fig S3), favoring the reduced incidence of cardiovascular disease, inflammation, and obesity.
Venn diagrams were used to present common and unique numbers to each group. shows that 846 overlapping Operational Taxonomic Units (OTUs) in each group. The unique OTUs were 3147, 2392, 2619, 3227, and 1880. The number of specific bacteria in the gut microbiota of the GP1H group was higher than that of the other groups. A linear discriminant analysis (LDA) effect size (LEfSe) was performed to further analyze the differences in the gut microbiota among each group (). The results showed that Firmicutes, Clostridiales, Oscillospiraceae, Ruminococcaceae, and Lachnospiraceae_unclassified were dominant in the gut microbiota of the CON group, whereas Candidatus_Arthromitus, Clostridiales_unclassified, and Ruminococcaceae_unclassified played principal roles in the MOD group. Candidatus_Arthromitus, a member of the Proteobacteria, can cause multiple infections, intestinal diseases, and inflammation. Clostridiales_unclassified, and Ruminococcaceae_unclassified, members of Firmicutes, are opportunistic pathogens that cause obesity, cancer, and digestive tract diseases. After GP intervention, Candidatus_Arthromitus, Clostridiales_unclassified, and Ruminococcaceae_unclassified decreased; Clostridia_vadinBB60_group and Colidextribacter were the specific bacteria in GP1H, and Bacteroidota and Anaerostipes were the specific bacteria in the LNT group. Clostridia_vadinBB60_group and Colidextribacter belong to Firmicutes, and their increased abundance is associated with weight control, protection against damage, and reduced inflammatory responses. Similar results were observed in the LDA (LDA>3) (), with 21 dominant taxa in the CON group, 6 in the GP1H group, and 7 in the LNT group.
2.7. GP increased SCFA production
SCFAs, metabolites produced by gut microbiota, were determined using gas chromatography.Citation34 Intraperitoneal CTX injection significantly reduced the production of total SCFAs by 36.9%, among which acetic acid and propionic acid were the most significantly reduced by 41.45% and 42.51%, respectively (p < .05, ). However, after administering GP orally, the total SCFAs increased by 41.31% relative to those in the MOD group; acetic acid and propionic acid increased by 47.29% and 41.23% in the GP1H group, respectively (p < .05). The increase in SCFAs provides energy to intestinal epithelial cells, repairs the intestinal barrier, enhances immune function, and prevents ulcers and enteritis.Citation34 According to the results of microbial composition and specific bacterial analysis in the gut, the increase in the abundance of SCFA producing bacteria (Colidextribacter, Bacteroides and Clostridia_vadinBB60_group) after GP treatment may be related to the increase in SCFA production.
Figure 6. (a) concentration of SCFAs in fecal; (b) the correlation between gut microbiota in genus level; (c) the correlation between gut microbiota and relative index. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GPL: low does GP group; GPH: high does GP group; LNT: lentinan.
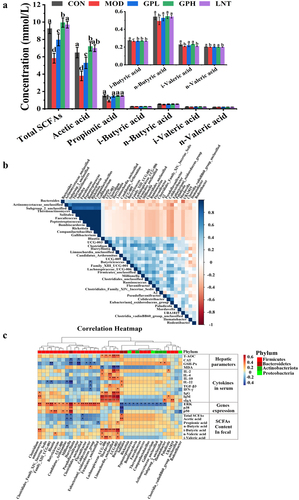
2.8. Relationship between characteristic bacteria and immunoassay index
The correlation between GP-derived bacteria and immune detection indexes was explored using Spearman correlation analysis to analyze the relationship between gut microbiota and immune function.Citation33 Kruskal-Wallis rank sum test was used to detect all the characteristic species, and 37 microbiotas with significant differences were obtained (). The results showed that Bacteroides, Actinomycetace-unclassified, Subgroup-2-unclassified, Thermoactinomyces, Solitalea, Faecalicoccus, Peptostreptococcus, Bombiscardovia, Rickttsia, Companilactobadillus and Gallibacterium were all positively correlated. The correlation between the GP-derived bacteria and immune detection indices is shown as a heat map (). Lachnospiraceae_UCG_006, A2, UCG_003, Mordavella, Limnochordia_unclassified, and Clostridium were positively correlated with hepatic parameters, cytokines, and SCFAs, whereas some microbiota (including Millionella etc.) were negatively correlated. Clostridium etc. were positively correlated with the mRNA level of the correlation pathway in the colon tissue, while Bacteroides etc. showed a negative correlation.
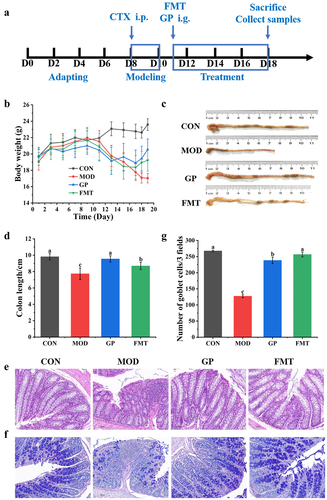
2.9 Gut microbiota enhanced the immune regulation function of GP
To explore a causal relationship between GP-derived bacteria and enhanced immune function, we performed a daily gavage of fecal microbiota from the GP group to FMT group of immunosuppressed mice (). After eight days of FMT, the FMT group showed a significantly increase in body weight by 11.38% compared with the MOD group (); the mean colon length significantly increased by 10.97% ). Histological results showed that the colon was structurally-intact, with longer villi and reduced numbers of inflammatory cells (), whereas the number of goblet cells significantly increased by 50.39% ). These results showed that gavage of the fecal microbiota from GP group enhanced intestinal barrier function, reduced intestinal inflammation, and increased goblet cell production in immunosuppressed mice.
Figure 7. Effect of GP-derived bacteria on intestinal structure of immunosuppressed mice. (a) diagram of experimental schedule; (b) the change of body weight; (c) Representative physical image of colons;(d) mean colon in all groups; (e) Representative image of H&E-stained pathological section of colon tissue (400х); (f) (a) Representative image of AB-PAS-stained section of colon tissue (400X); (G number of goblet cells. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GP: GP group; FMT: fecal microbiota transplantation group.
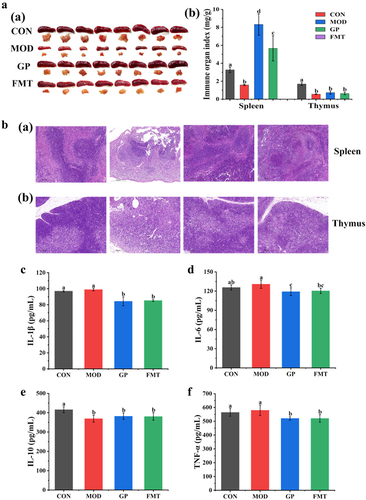
Meanwhile, the spleen and thymus indices of the FMT group increased by 72.09% and 11.65%, respectively, compared with those of the MOD group (). And FMT of fecal microbiota significantly reversed the spleen and thymus structures damage induced by CTX, including white pulp atrophy, cell necrosis, cortical and medullary structures blurred, thymic corpuscle destruction, and the number of immune cells decreased (). Moreover, fecal microbiota significantly reduced the secretion of proinflammatory cytokines IL-1β, IL-6, and TNF-α by 13.75%, 8.01%, and 10.22%, respectively, and reduced the inflammatory response ). These results showed that gavage of fecal microbiota enhanced immune function by increasing the immune organ index, repairing spleen and thymus lesions, and reducing proinflammatory factors level.
Figure 8. Effect of GP-derived bacteria on immune function of immunosuppressed mice. (a) (a) physical image of spleen and thymus; (b) the indices of spleen and thymus; (b) (a) Representative image of H&E-stained pathological section of spleen tissue (200х), (b) thymus tissue; (c) IL-1β; (d) IL-6; (e) IL-10; (f) TNF-α. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GP: GP group; FMT: fecal microbiota transplantation group.
In order to explore the effect of GP on immune response in the absence of microorganisms, we used combined antibiotics to eliminate microorganisms in mice. The results showed that intraperitoneal CTX injection significantly reduced in body weight, spleen and thymus indices, and colon length by 31.70%, 22.98%, 59.98%, and 15.36%, respectively, ). Moreover, CTX significantly increased the secretion of cytokines IL-1β, IL-6, IL-10, and TNF-α by 12.72%, 16.20%, 10.30%, and 7.66%, respectively, ). However, after eight days of GP, the GP group showed no significantly change in body weight, spleen and thymus indices, and colon length. These results showed that the ability of GP to regulate immune response significantly reduced in the pseudo germ-free model.
Figure 9. Effect of GP on immune function in pseudo germ-free mice. (a) diagram of experimental schedule; (b) the change of body weight; (c) Representative physical image of colons;(d) mean colon in all groups; (e) physical image of spleen and thymus; (f) the indices of spleen and thymus; (g) IL-1β; (h) IL-6; (i) IL-10; (j) TNF-α. Multiple comparisons were performed by using one-way ANOVA using SPSS 26.0 software. Means with different letters are significantly different (p < .05) (n = 8). CON: control group; MOD: model group; GP: GP group.
3. Discussion
The intestinal tract, the largest digestive and immunological in the body, can digest food and resist the invasion of harmful substances and pathogens.Citation1 The structural integrity of the intestinal epithelium is important for human health and intestinal immunity.Citation35 Polysaccharides, as an approved immunomodulator, regulate immunosuppression and intestinal mucosal damage caused by multiple factors.Citation3 Here, we showed that GP could protect the intestinal structure, improve antioxidant capacity (Fig. S5), regulate immune cells and cytokines, activate immune-related pathways, and improve the gut microbiota structure and metabolites.
The intestinal tissue contains a large number of T lymphocytes as the first line of defense. CD4+ and CD8+ T lymphocytes are the two main subtypes involved in cellular and adaptive immunity.Citation5 T lymphocytes specifically bind to target cells of infection antigens and excrete them; moreover, they expand and enhance immune effects by releasing cytokines.Citation3 GP increased CD4+ T lymphocytes in colon tissue by 29.30%, more than that by Cordyceps sinensis polysaccharide (NCSP) (28.57%),Citation36 because GP has a smaller molecular weight and a 1, 6-linked Glcp structure, which is related to immune activity.Citation37,Citation38 Furthermore, GP enhanced the immune activity by promoting the secretion of cytokines, including IFN-γ, IL-2, IL-4, IL-10, IL-22, and TGF-β3. The immunological activity of GP was similar to that of polysaccharides from Hericium erinaceus (HEP) and ginger (UGP1).Citation11,Citation33 However, GP increased IL-2, IL-4, and IL-10 levels by 12.33%, 13.36%, and 23.01%, respectively, relative to HEP and UGP1. GP affects the secretion of immunoglobulin (IgG, IgM, and SIgA) and chemokines (MIP-1α and MCP-1) and maintains the homeostasis of immune cells.
Another mechanism by which GP regulates immunity may be related to the ERK/p38/NF-κBp50 signaling pathway. After entering the digestive tract, GP is degraded by the gut microbiota and produces metabolites in the colon. Microbiota and metabolites can be recognized by TLRs on the cell surface, thereby activating intracellular signal transduction pathways, including MAPK and NF-κB; this leads to changes in gene expression level and the synthesis of various molecules, including cytokines, chemokines, and immunoglobulins.Citation1,Citation5 Polysaccharides from Ganoderma lucidum and Chinese wolfberry regulate immune function in the body through TLR4/p65/MyD88 and TLR4/MyD88/NF-κB signaling, respectively, and secrete cytokines, including IgA, IL-1β, TNF-α, TGF-β1, and IL-10.Citation37,Citation38 GP down regulated the downstream pathways of TLRs, including MAPK and NF-κB, suggesting that GP could inhibit the body’s inflammatory response by changing the levels of immune-related genes.Citation39 The regulatory mechanism was consistent with the results of ELISA and intestinal histopathological analysis.
Gut microbiota and metabolites are related to immune regulation. The study demonstrated that GP improved the structure of the gut microbiota by promoting the proliferation of specific bacteria and reducing the abundance of harmful bacteria. GP intervention produced several unique microbiotas, including Ligilactobacillus, Clostridia_UCG-014_unclassified, Lachnospiraceae_unclassified, Monoglobus, Leuconostoc, and Anaerotignum, which may have promoted carbohydrate metabolism. Similar to polysaccharides from Hericium erinaceus and Auricularia cornea var. Li.,Citation33,Citation38 GP enhanced the abundance of the probiotics Muribaculaceae, Alistipes, Lachnospiraceae_NK4A136_group, Lachnospiraceae, and Lactobacillus. Spearman correlation analysis suggested that GP induced bacteria Lachnospiraceae_UCG_006, A2, UCG_003, Mordavella, Limnochordia_unclassified, and Bacteroides interacted to produce SCFAs, immune cytokines, and immunoglobulins. Bacteroides, Solitalea, Rickettsia, Gallibacterium, and Rodentibacter may be related to the down regulation of the p38/ERK/NF-κBp50 pathway. Similar to polysaccharides from Fuzhuan brick tea and Auricularia cornea var. Li.,Citation40,Citation41 This study explored the interaction among GP-induced bacteria, chemokines, and the p38/ERK/NF-κBp50 pathways, indicating that GP regulates immunity in multiple ways.
Polysaccharide intervention can significantly alter the structure of gut microbes.Citation3 The FMT experiment confirmed that fecal microbiota from the GP group significantly improved CTX-induced immunosuppression, which was almost consistent to the GP immunological regulation. The pseudo germ-free experiment showed the reduced regulatory effect of GP on immune response, indicating the crucial role of gut microbiota in GP-mediated immunity regulation.Citation42 The immune enhancement mechanism of GP-derived bacteria maybe include the following: (1) improve intestinal epithelial barrier function and reduce the penetration of pathogens into the body; (2) promote the proliferation of immune cells in intestinal tissue and strengthen intestinal immune function; and (3) optimize the immunological capacity of immune organs, regulate cytokine secretion, and improve the immune defense. Notably, the importance of gut microbiota in regulating the immune response to GP was clarified, the future research should focus on identifying the pathways involved in GP-mediated regulation of gut microbiota and the immune system.
In conclusion, GP regulates the immune response through three mechanisms: including increasing the number of immune cells and cytokine secretion, regulating the mRNA expression levels of immune-related pathways, and adjusting the structure and metabolites of the gut microbiota. Thus, GP has great potential as an immune enhancer and a natural medicine for treating intestinal inflammatory diseases.
4. Methods
4.1. Animal and experimental design
All the experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council. This study was approved by the Ethics Committee on Laboratory Animals of the First Affiliated Hospital of Shihezi University (no. SCXK (Yu) 2020–0005). Forty male specific pathogen-free BALB/c mice, aged 6 weeks (weight 20 ± 2.0 g), were purchased from Henan Skobes Biotechnology Co., Ltd (Henan, China). After 1 week of adaptation under temperature (23 ± 2°C), 55 ± 5% humidity, and an ordinary diet, the mice were randomly divided into five groups (eight per group): control (CON), model (MOD), low-dose GP (100 mg/kg bw, GPL), high-dose GP (200 mg/kg bw, GPH) and lentinan (Solarbio, China)-treated positive (20 mg/kg bw, LNT) groups. Low-molecular-weight GP (6.5 kDa) was obtained by hot water extraction and purification on a diethylethanolamine cellulose-52 column and Sephadex G-100 chromatogram. The immunosuppression model was established by CTX intraperitoneal injection (100 mg/kg body weight; Macklin, China) once daily for three days. For the next 8 days, the CON and MOD groups were intragastrically administered normal saline once daily, whereas the GPL, GPH, and LNT groups were administered different doses of GP or lentinan. Changes in the diet and weight of mice were observed and recorded.
On the 11th day, feces and orbital blood samples were collected. Serum was collected at 4◦C by centrifuging at 12,000 g for 15 min. The colon tissue was isolated. A part of the intestinal tissue was removed and placed in 10% formalin for pathological analysis. The remaining samples were stored in a − 80°C refrigerator.
4.2. FMT
The FMT experiment was performed using previously described method with slight modifications.Citation43 Fresh fecal samples (0.1 g) were collected from mice in the GPH group under sterile conditions and resuspended in sterile saline (0.5 mL) in an anaerobic chamber. The solution was thoroughly mixed and centrifuged at 500 g for 5 min (4°C). The supernatant was collected and centrifuged at 12,000 r/min for 5 min. Sterile saline was added to the sediment at a ratio of 1:3, and the sediment was resuspended, centrifuged, and collected. Part of the precipitate was used to verify the flora extraction rate, and the other part was used for FMT. All fresh bacterial solutions were prepared 30 min before FMT to prevent significant changes in the flora in vitro. The other three groups of mice (eight per group) were prepared using immunosuppressive models (described as “4.1” section), except for the CON group. Four groups of mice were intragastrical administered of normal saline (CON and MOD groups), GP (GP group, 200 mg/kg), or freshly transplanted flora from the GP group (FMT group, 0.2 mL) for eight days.
4.3 Antibiotics treatment experiment
The operation of antibiotic treatment experiment was similar to “FMT” section. The difference was that on days 8, Balb/c mice were administrated with vancomycin (2.5 g/L), ampicillin (5 g/L), neomycin (5 g/L), metronidazole (5 g/L) for consecutive five days to induce the pseudo germ-free model. Samples were collected and measured at days twenty-three.
4.4. Histopathological and immunohistochemical analysis
The collected intestinal tissues were fixed with 10% formalin, embedded in paraffin, sliced, and stained using the standard hematoxylin and eosin (H&E) method to observe the histopathological changes.Citation44 The changes in goblet cells and mucin content were obtained using the alcian blue and periodic acid Schiff (AB-PAS) method and a light microscope (Leica, Germany). The number of immune cells (CD4+ and CD8+) was measured by immunohistochemistry.Citation34 The tissue sections were dewaxed and antigen-repaired. Endogenous peroxidase was blocked with a 3% hydrogen peroxide solution. After incubation of tissue sections with primary (anti-CD4+ and CD8+) and secondary antibodies, changes in CD4+ and CD8+ T lymphocytes were observed using optical microscopy. The numbers of goblet cells and CD4+, and CD8+ T lymphocytes and mucins content were assessed using Image-J analysis software.
4.5. Determination of cytokines, chemokines, and immunoglobulins
The contents of immune cytokines (IL-2, IL-10, IL-4, IL-22, IFN-γ and TGF-β3), chemokines (MCP-1 and MIP-1α), and immunoglobulins (IgG and IgM) in serum and sIgA in colon tissue were measured using ELISA as per the manufacturer’s instructions.
4.6. Determination mRNA levels of p38, ERK, p50, T-bet and GATA3
RT-qPCR was used to detect the mRNA levels of p38, ERK, p50, T-bet and GATA3, with β-actin as the reference gene.Citation45 Total RNA was extracted from intestinal tissues using Trizol reagent and reverse transcribed to cDNA using an M5 Super Plus qPCR RT kit (Juomei, China). The reaction mixture (10 μL) included 5 μL 2× M5HiPer SYBR Premix Es Taq (Juomei, China), 0.3 μL forward primer, 0.3 μL reverse primer, 1 μL cDNA, and 3.4 μL ddH2O. The PCR cycle conditions were 95°C for 2 min, followed by 45 cycles at 95°C for 5 s, and 60°C for 34 s. the solution curve conditions were 95°C for 5 s, 60°C for 1 min. The primer sequences are listed in Table S2.
4.7. DNA extraction and high-throughput sequencing
Total bacterial DNA was extracted from fresh mice feces using cetyltrimethylammonium bromide and evaluated using 2% agarose gel electrophoresis.Citation33 PCR products were purified using AMPure XT beads (Beckman, USA), assessed using Agilent 2100 Biochromateter (Agilent, USA) and Illumina (Kapa Biosciences, Woburn, MA, USA) library quantitative kits, and then sequenced using a NovaSeq 6000 sequencer. The α-diversity, β-diversity, Venn diagram, species composition, heatmap, and linear discriminant analysis effect size (LEfSe) were used to analyze the structure of the gut microbiota.
4.8. Determination of SCFAs
The SCFA concentration was determined using a GC.Citation46 Fresh mice feces (100 mg) were resuspended in 700 μL ultra-pure water, ultrasonicated for 10 min, and stored in an ice bath for 20 min. After fully vortexing, the mixture was centrifuged at 5000 g and 4°C for 15 min. Three repeat extracts were combined and passed through a 0.22 μm filter for GC analysis. The filtrates were analyzed using the Shimadzu GC-2014 system (Shimadzu Co. Ltd, Kyoto, Japan) equipped with an SH-Rtx-Wax capillary column (30 m × 0.25 mm × 0.25 μm, Shimadzu Co. Ltd, Kyoto, Japan) and a hydrogen flame ion detector (FID). The injection volume was 1 μL. The temperature of the FID detector and injection port was 240°C. The initial column temperature was 100°C for 4 min, which was elevated at a speed of 10°C/min to 170°C and maintained for 1 min and then elevated at a speed of 50°C/min to 220°C and maintained for 5 min.
4.9. Statistical analysis
The experiments were repeated three times, and the data are shown as mean ± standard deviation unless otherwise stated. Graphs were created using Origin 64 software (Origin Lab Corporation, Northampton, Mass. USA). The number of goblet cells and CD4+ and CD8+ T lymphocytes and mucin content were assessed using ImageJ software. The 16S rDNA sequencing data were analyzed using the Kruskal-Wallis and Wilcoxon rank sum tests. Multiple comparisons were performed using a one-way analysis of variance, followed by the least significant difference post hoc test using SPSS software. p < 0.05 was considered statistically significant.
Supplemental Material
Download MS Word (539.9 KB)Acknowledgments
This work was supported by Science and Technology Program, grant number 2020AB026, the National Natural Science Foundation of China, grant number 51863018, and Young Doctor Fund of Higher Education in Gansu Province, grant number 2021QB-105.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Immune index detection and gut microbiome data were deposited at Mendeley Data (https://data.mendeley.com/) with Reserved DOI: 10.17632/xgp4p6ndmr.2. Source data are provided with this paper.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2276814
Additional information
Funding
References
- Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut microbiota and immune System interactions. Microorganisms. 2020;8:12. doi:10.3390/microorganisms8101587. PMID: 33076307.
- Sandoval DA, Seeley RJ. The microbes made me eat it. Sci. 2010;328:179–20. doi:10.1126/science.1188876. PMID: 20378804.
- Tang C, Ding R, Sun J, Liu J, Kan J, Jin C. The impacts of natural polysaccharides on intestinal microbiota and immune responses - a review. Food Funct. 2019;10:2290–2312. doi:10.1039/c8fo01946k. PMID: 31032827.
- Tang M, Cheng L, Liu Y, Wu Z, Zhang X, Luo S. Plant polysaccharides modulate immune function via the gut microbiome and May have potential in COVID-19 therapy. Molecules. 2022;27:27. doi:10.3390/molecules27092773. PMID: 35566123.
- Wang G, Huang S, Wang YM, Cai S, Yu HT, Liu HB, Zeng X, Zhang G, Qiao S. Bridging intestinal immunity and gut microbiota by metabolites. Cell Mol Life Sci. 2019;76:3917–3937. doi:10.1007/s00018-019-03190-6. PMID: 31250035.
- Brown DG, Soto R, Yandamuri S, Stone C, Dickey L, Gomes-Neto JC, Pastuzyn ED, Bell R, Petersen C, Buhrke K, et al. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. Elife. 2019;8. doi:10.7554/eLife.47117. PMID: 31309928.
- Sun Y, Zhang ZP, Cheng L, Zhang X, Liu YN, Zhang RL, Weng PF, Wu ZF. Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota. Food Res Int. 2021;149:110675. doi:10.1016/j.foodres.2021.110675. PMID: 34600677.
- Grao-Cruces E, Lopez-Enriquez S, Martin ME, Paz M. High-density lipoproteins and immune response: A review. Int J Biol Macromol. 2022;195:117–123. doi:10.1016/j.ijbiomac.2021.12.009. PMID: 34896462.
- Liu ZQ, Hu YY, Tao XY, Li JJ, Guo XM, Liu G, Song S, Zhu BW. Metabolites of sea cucumber sulfated polysaccharides fermented by parabacteroides distasonis and their effects on cross-feeding. Food Res Int. 2023;167. doi:10.1016/j.foodres.2023.112633. PMID: 37087229.
- Zhang J, Zhu S, Ma N, Johnston LJ, Wu C, Ma X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: a therapeutic target to control intestinal inflammation. Med Res Rev. 2020;41:1061–1088. doi:10.1002/med.21752. PMID: 33174230.
- Liu JP, Wang J, Zhou SX, Huang DC, Qi GH, Chen GT. Ginger polysaccharides enhance intestinal immunity by modulating gut microbiota in cyclophosphamide-induced immunosuppressed mice. Int J Biol Macromol. 2022;223:1308–1319. doi:10.1016/j.ijbiomac.2022.11.104. PMID: 36395935.
- Li C, Duan S, Li Y, Pan X, Han L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: a review. Carbohyd Polym. 2021;261:117876. doi:10.1016/j.carbpol.2021.117876. PMID: 33766363.
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune Effects of cyclophosphamide. Sci. 2013;342:971–976. doi:10.1126/science.1240537. PMID: 24264990.
- Wu XC, Huang XJ, Ma WN, Li MZ, Wen JJ, Chen CH, Liu LD, Nie SP. Bioactive polysaccharides promote gut immunity via different ways. Food Funct. 2023;14:1387–1400. doi:10.1039/d2fo03181g. PMID: 36633119.
- Eswar K, Mukherjee S, Ganesan PAR, Rengan AK. Immunomodulatory natural polysaccharides: an overview of the mechanisms involved. Eur Polym J. 2023;188:111935. doi:10.1016/j.eurpolymj.2023.111935.
- Hu YY, He Y, Niu ZQ, Shen T, Zhang J, Wang XF, Hu WC, Cho JY. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J Ginseng Res. 2022;46:23–32. doi:10.1016/j.jgr.2021.06.003. PMID: 35058724.
- Cao C, Gong GP, Huang WQ, Huang LJ, Song S, Zhu BW, Zhu B. Effects of Lycium barbarum polysaccharides on immunity and metabolic syndrome associated with the modulation of gut microbiota: a review. Foods. 2022;11:3177. doi:10.3390/foods11203177. PMID: 37430929.
- Sun J, Chen H, Kan J, Gou Y, Jin C, Zhang X, Wu X, Tang S, Sun R, Qian C. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int J Biol Macromol. 2020;153:708–722. doi:10.1016/j.ijbiomac.2020.03.053. PMID: 32169445.
- Yang Y, Ye HQ, Zhao CH, Ren L, Wang CN, Georgiev MI, Xiao JB, Zhang TH. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohyd Polym. 2021;262:117668. doi:10.1016/j.carbpol.2021.117668. PMID: 33838836.
- Ying MX, Zheng B, Yu Q, Hou KY, Wang H, Zhao MM, Chen Y, Xie JH, Nie SP, Xie MY. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food Chem Toxicol. 2020;138:111244. doi:10.1016/j.fct.2020.111244. PMID: 32151603.
- Zhao FQ, Ma TT, Zhang X, Zhao QC, Zhu KX, Cao J, Liu ZY, Shen XR, Li C. Holothuria leucospilota Polysaccharides Improve Immunity and the Gut Microbiota in Cyclophosphamide-Treated Immunosuppressed Mice. Mol Nutr Food Res. 2022;67:e2200317. doi:10.1002/mnfr.202200317. PMID: 36401832.
- Shi HJ, Chang YG, Gao Y, Wang X, Chen X, Wang YM, Xue CH, Tang QJ. Dietary fucoidan of acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017;8(9):3383–3393. doi: 10.1039/c7fo00932a. PMID: 28861559.
- Aipire A, Yuan PF, Aimaier A, Cai SS, Mahabati M, Lu J, Ying TL, Zhang BH, Li JY. Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis. Biomolecules. 2020;10:10. doi:10.3390/biom10010159. PMID: 31963790.
- Simayi Z, Rozi P, Yang XJ, Ababaikeri G, Maimaitituoheti W, Bao XW, Ma SJ, Askar G, Yadikar N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: systematic review. Int J Biol Macromol. 2021;183:387–398. doi:10.1016/j.ijbiomac.2021.04.099. PMID: 33887291.
- Xie H, Fang J, Farag MA, Li Z, Sun P, Shao P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. 2022;13:100235. doi:10.1016/j.fochx.2022.100235. PMID: 35499019.
- Zhou RR, He D, Xie J, Zhou QYJ, Zeng HL, Li HM, Huang LQ. The synergistic effects of polysaccharides and ginsenosides from American ginseng (Panax quinquefolius L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol. 2021;12:665901. doi:10.3389/fimmu.2021.665901. PMID: 33968068.
- Bilate AM, London M, Castro T, Mesin L, Mucida D, Kongthong S, Harnagel A, Victora GD, Mucida D. T cell receptor is required for differentiation, but not maintenance, of intestinal CD4+ intraepithelial lymphocytes. Immunity. 2020;53:1001–1014.e20. doi:10.1016/j.immuni.2020.09.003. PMID: 33022229.
- Chen S, Wang J, Fang Q, Dong N, Nie S. Polysaccharide from natural Cordyceps sinensis ameliorated intestinal injury and enhanced antioxidant activity in immunosuppressed mice. Food Hydrocoll. 2019;89:661–667. doi:10.1016/j.foodhyd.2018.11.018.
- Belghith M, Maghrebi O, Cherif A, Bahrini K, Saied Z, Belal S, Sassi SB, Barbouche MR, Kchaou M. Increased T-bet/GATA-3 and ROR-γt /Foxp3 Ratios in Cerebrospinal Fluid as Potential Criteria for Definite Neuro-Behçet’s Disease. J Clin Med. 2022;11:11. doi:10.3390/jcm11154415. PMID: 35956031.
- Fan ST, Nie SP, Huang XJ, Wang SN, Hu JL, Xie JH, Nie QX, Xie MY. Protective properties of combined fungal polysaccharides from cordyceps sinensis and Ganoderma atrum on colon immune dysfunction. Int J Biol Macromol. 2018;114:1049–1055. doi:10.1016/j.ijbiomac.2018.04.004. PMID: 29626602.
- Dong N, Li XR, Xue CY, Zhang L, Wang CS, Xu XY, Shan A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J Cell Physiol. 2020;235:5525–5540. doi:10.1002/jcp.29452. PMID: 32037545.
- Xu H, Liu L, Ding M, Fan W, Song H, Hui S. Effect of Ganoderma applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells. Int J Biol Macromol. 2020;146:353–362. doi:10.1016/j.ijbiomac.2020.01.010. PMID: 31911173.
- Tian BM, Liu RJ, Xu TR, Cai M, Mao RL, Huang LS, Yang K, Zeng XX, Sun PL. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J Sci Food Agric. 2023;103:3050–3064. doi:10.1002/jsfa.12404. PMID: 36546454.
- Wang X, Qu YH, Wang Y, Wang X, Xu JL, Zhao HL, Zheng DL, Sun L, Tai GH, Zhou YF, et al. β-1,6-Glucan from Pleurotus eryngii Modulates the Immunity and Gut Microbiota. Front Immunol. 2022;13:859923. doi:10.3389/fimmu.2022.859923. PMID: 35585984.
- Song QQ, Wang YK, Huang LX, Shen MY, Yu Y, Yu Q, Chen Y, Xie JH. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res Int. 2021;140:109858. doi:10.1016/j.foodres.2020.109858. PMID: 33648176.
- Chen SP, Wang JQ, Fang QY, Dong N, Fang QY, Cui SW, Nie SP. A polysaccharide from natural Cordyceps sinensis regulates the intestinal immunity and gut microbiota in mice with cyclophosphamide-induced intestinal injury. Food Funct. 2021;12:6271–6282. doi:10.1039/d1fo00596k. PMID: 34105571.
- Guo CL, Guo DD, Fang L, Sang TT, Wu JJ, Guo CJ, Wang YJ, Wang Y, Chen CJ, Chen JJ, et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr Polym. 2021;267:118231. doi:10.1016/j.carbpol.2021.118231. PMID: 34119183.
- Zhao Y, Yan Y, Zhou W, Chen D, Huang K, Yu S, Mi J, Lu L, Zeng X, Cao Y. Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice. J Funct Foods. 2020;72:104057. doi:10.1016/j.jff.2020.104057.
- Liu H, Guo H, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Copper induces hepatic inflammatory responses by activation of MAPKs and NF-κB signalling pathways in the mouse. Ecotox Environ Safe. 2020;201:110806. doi:10.1016/j.ecoenv.2020.110806. PMID: 32512418.
- Wang N, Ren D, Zhang L, Han N, Zhao Y, Yang X. Effects of sheep whey protein combined with fu brick tea polysaccharides and stachyose on immune function and intestinal metabolites of cyclophosphamide-treated mice. J Sci Food Agric. 2023;103:3402–3413. doi:10.1002/jsfa.12477. PMID: 36722467.
- Zhao M, Shi W, Chen X, Liu Y, Yang Y, Kong X. Regulatory effects of Auricularia cornea var. Li. polysaccharides on immune system and gut microbiota in cyclophosphamide-induced mice. Front Microbiol. 2022;13:1056410. doi:10.3389/fmicb.2022.1056410. PMID: 36406390.
- Sun WL, Hua S, Li XY, Shen L, Wu H, Ji HF. Microbially produced vitamin B12 contributes to the lipid-lowering effect of silymarin. Nat Commun. 2023;14:1. doi:10.1038/s41467-023-36079-x. PMID: 36717576.
- Wang L, Xian YF, Loo SKF, Ip SP, Yang W, Chan WY, Lin ZX, JCY W. Baicalin ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice through modulating skin barrier function, gut microbiota and JAK/STAT pathway. Bioorg Chem. 2022;119:119. doi:10.1016/j.bioorg.2021.105538. PMID: 34929516.
- Chen SY, Shen YC, Lin JA, Yen GC. Rhinacanthus nasutus and okara polysaccharides attenuate colitis via inhibiting inflammation and modulating the gut microbiota. Phytother Res. 2022;36:4631–4645. doi:10.1002/ptr.7582. PMID: 35918881.
- Cai GF, Wu Y, Wusiman A, Gu PF, Mao NN, Xu SW, Zhu TY, Feng Z, Liu ZG, Wang DY. Alhagi honey polysaccharides attenuate intestinal injury and immune suppression in cyclophosphamide-induced mice. Food Funct. 2021;12:6863–6877. doi:10.1039/d1fo01008e. PMID: 34128029.
- Li Y, Qin J, Cheng YH, Ai YQ, Han ZY, Li M, Qi YX, Zhao QC, Li ZB. Polysaccharide from Patinopecten yessoensis skirt boosts immune response via modulation of gut microbiota and short-chain fatty acids metabolism in mice. Foods. 2021;10:2478. doi:10.3390/foods10102478. PMID: 34681527.
