ABSTRACT
The role of gut microbiota in host defense against nontuberculous mycobacterial lung disease (NTM-LD) was poorly understood. Here, we showed significant gut microbiota dysbiosis in patients with NTM-LD. Reduced abundance of Prevotella copri was significantly associated with NTM-LD and its disease severity. Compromised TLR2 activation activity in feces and plasma in the NTM-LD patients was highlighted. In the antibiotics-treated mice as a study model, gut microbiota dysbiosis with reduction of TLR2 activation activity in feces, sera, and lung tissue occurred. Transcriptomic analysis demonstrated immunocompromised in lung which were closely associated with increased NTM-LD susceptibility. Oral administration of P. copri or its capsular polysaccharides enhanced TLR2 signaling, restored immune response, and ameliorated NTM-LD susceptibility. Our data highlighted the association of gut microbiota dysbiosis, systematically compromised immunity and NTM-LD development. TLR2 activation by P. copri or its capsular polysaccharides might help prevent NTM-LD.
Introduction
The incidence and number of deaths from nontuberculous mycobacterial (NTM) lung disease (NTM-LD) have steadily increased globally.Citation1,Citation2 NTM exhibits heterogeneous pathogenicity in humans and is of concern as NTM-LD is difficult to treat, can be debilitating, and even cause death.Citation3 Potential human factors associated with NTM-LD susceptibility are diverse, including preexisting abnormal structural and chronic lung diseases such as chronic obstructive pulmonary disease (COPD)Citation4 and pulmonary clearance defects with poor clearance of secretions such as cystic fibrosis.Citation5 Nevertheless, it remains unclear why many patients with seemingly immune-competent phenotypes, including middle-aged nonsmokers without previously known lung disease, patients with a slender body, those with pectus excavatum, and postmenopausal women develop NTM-LD.Citation3,Citation6,Citation7 Decreased immunity and NTM-LD susceptibility in patients were frequently reported.Citation8,Citation9 These need to be addressed to clarify the mechanisms of NTM-LD.
The gut microbiota comprises a large population of microorganisms residing in the human gastrointestinal tract, constantly interacting with various host immune systems, aiding in host physiological homeostasis.Citation10-13 Regarding molecular immune modulation, the gut microbiota serves as an important source of microorganism-associated molecular patterns in the intestine. These molecules act as ligands and are recognized by pattern recognition receptors, which include toll-like receptors (TLRs) and nucleotide-binding receptors on the host’s innate immune cells.Citation14 Among the TLRs, TLR2 and TLR4 are mainly stimulated by bacterial lipoproteins and lipopolysaccharides (LPS), respectively, and involve in immune response regulation and treatment efficacy of many important diseases, including infections.Citation15,Citation16 Among these, the association between NTM-LD and reduced host TLR2 activation activity was mostly reported,Citation16-19 but the association with gut microbiota remains unclear.
Gut microbiota dysbiosis or loss of gut bacteria diversity has been linked to the development of several diseases,Citation20-22 although the underlying molecular mechanisms remain not completely defined.Citation23-25 Subsequently, aberrant effects derived from gut microbiota dysbiosis occurred in the local intestinal environment and systematically in the host.Citation26 Gut microbiota might closely interact with immune cells in the lung and maintain its immune homeostasis through vital crosstalk as the “gut – lung axis”.Citation27,Citation28 Specifically, gut microbiota dysbiosis and potentially compromised immunity seemed closely related to aberrant immunopathology in lung infections.Citation29-32 Complementation by gut microbiota and immunity enhancement restores the immune response and enhances lung immunity against infections.Citation33-35 Some important signaling molecules and pathways involved in gut – lung crosstalk, affecting lung susceptibility to pathogens, have been identified.Citation36-38 Therefore, enhancement of lung immune defenses against pathogens by gut microbiota may be among the useful strategies for ameliorating the compromised lung immune activity.Citation39
While the role of gut microbiota in TLR signaling modulation during viral infections is widely established; however, its role during mycobacterial pulmonary infection, especially in NTM-LD is poorly understood. In this study, we aimed to investigate the role of gut microbiota in host defense against NTM-LD.
Materials and methods
Human case enrollment
We enrolled NTM-LD patients fulfilled diagnostic critieria by American Thoracic Society,Citation40 their family members living in the same house, and healthy controls (HCs) from the National Taiwan University Hospital under the approval of the Research Ethics Committee (IRB number: 201803068RINA) (Table S1). The enrollment period was from May 2018 to April 2021. All participants provided written informed consent. In brief, NTM-LD was defined if all of the following criteria were met: 1) two or more sputum culture-positive specimens for the same NTM species; 2) chest images typical radiological findings (i.e., fibrocavitary lesions or nodular bronchiectasis); 3) presence of respiratory symptoms; and 4) no obvious alternative diagnosis at that time. We excluded participants using antibiotics in the last 3 months, with human immunodeficiency virus infection, recently received systemic steroid or other immunosuppressants, and with active malignancy. For validation of P. copri abundance, another cohort comprising of 19 NTM-LD patients and 25 HCs was enrolled from May 2021 to October 2022 per the same criteria and IRB approval.
Microbiota library construction and illumina MiSeq sequencing
Feces samples were stored at −80°C. DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). V3–V4 regions in the 16S rRNA gene were amplified by using primers listed in Table S3, purified, and sequenced using the MiSeq Illumina pyrosequencer platform following the manufacturer’s instructions.
16S rRNA gene-based metagenomics analysis pipeline
We used a protocol modified from our previous studiesCitation41-43 where V3–V4 regions of 16S rRNA amplicon sequencing were performed using 250-bp paired-end raw reads, and the entire paired-end reads were assembled using FLASH v.1.2.11. Low-quality reads (Q score < 20) were discarded in QIIME 1.9.1 pipeline. If three consecutive bases were <Q20, the read was truncated, and the resulting read was retained for data at least 75% of the original length (QIIME script split_libraries_fastq.py). Sequences were chimera-checked using UCHIME and filtered from the dataset before OTU (operational taxonomy units) picking of 97% sequence identity using the USEARCH v.7 pipeline (UPARSE function). For each representative sequence, the Silva Database v128 was used based on RDP classifier (v.2.2) algorithm to annotate taxonomy classification, which was performed with an 80% minimum confidence threshold to record an assignment. Any singleton-sequences were filtered out.
Sample-species complexity was evaluated by beta diversity analysis. Principal Coordinate Analysis (PCoA) evaluated and visualized variance based on OTU level of gut microbiota composition among the groups. Statistically significant biomarkers were identified using the linear discriminant analysis (LDA) effect size (LEfSe) analysis, an algorithm-based approach that performs the nonparametric Kruskal – Wallis and Wilcoxon rank-sum tests to identify bacterial taxa whose relative abundance is significantly different relative to the control.Citation44 LEfSe applies LDA to bacterial taxa identified as significantly different and assesses the effect size of each differentially abundant taxon. The relative abundance of microbial genera was also compared using Welch’s t-test.
Relative quantification of P. copri abundance
Determination of P. copri abundance in fecal DNA was performed by real-time qPCR using P. copri 16S rRNA gene specific primers listed in Table S3.Citation45 The 16S rRNA gene V3–V4 primers were used as the internal control for qPCR assay.
TLR2 ligand (TLR2L) and TLR4L activities
HEK-Blue-mTLR2 and HEK-Blue-mTLR4 cells (InvivoGen, USA) were used to determine TLR2L and TLR4L activities in stool supernatant, plasma, sera, and lung supernatant per manufacturer’s protocol, respectively. The NF-kB activation was determined by OD630 measurement.
Bacterial culture
Mycobacterium kansasii strain ATCC12478 was obtained from the American Type Culture Collection (ATCC, USA) and cultured on Middlebrook 7H11 agar plate (Difco, USA) containing 0.5% glycerol and 10% oleic acid-bovine albumin-dextrose-catalase (OADC) (Becton Dickinson, USA) at 35°C in the dark. P. copri (PC) strain DSM18205 was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Germany) and cultured on an anaerobic blood agar plate (Creative, Taiwan) and liquid thioglycollate medium (BD, USA) at 37°C in a Whitley DG250 anaerobic chamber (Don Whitley, Bingley, UK) with mixed anaerobic gas (5% carbon dioxide, 5% hydrogen, 90% nitrogen).
THP-1 cell culture and in vitro treatment
THP-1 monocytic leukemia cells were differentiated into macrophage-like phenotype by treatment with Phorbol 12-Myristate 13-Acetate (200 ng/ml PMA) for 48 hours. After differentiation by PMA, THP-1 derived macrophages were resting for 24 hours and then treated with heat-killed PC at the indicated multiplicity of infection (with and without a TLR2 (50-μM C29) or TLR4 (1-μM TAK-242) for inhibitor 24 hours. Reaction supernatants were collected, and TNF-α was measured using ELISA.
Animal experiments
All animal experiments were conducted following the approval of the Institutional Animal Care and Use Committee of Chang Gung University and performed according to the guidelines (Approval number: CGU106–216). C57BL/6 mice (7 weeks old) were purchased from the National Laboratory Animal Center (Taiwan). All mice were kept under a 12-h light/dark cycle. All mice were acclimatized for 1 week before the experiments.
For antibiotic cocktail treatment, mice were left untreated (CTL) or were orally treated with a four antibiotic cocktail (ABX: 1-g/l ampicillin, 1-g/l metronidazole, 1-g/l neomycin sulfate and 0.5-g/l vancomycin; Sigma, USA) in sterile drinking water for 3 weeks. Two days after the cessation of antibiotic drinking water, mice were infected with M. kansasii. As the demographic data of the enrolled patients (Table S1), M. kansasii accounted for 12% of NTM-LD infections. In addition, we had developed the M. kansasii lung infection model in our previous study.Citation46 Based on these, we selected M. kansasii as the NTM pathogen in this study. For M. kansasii infection, mice were anesthetized, followed by intranasal inoculation of M. kansasii [4 × 106 colony-forming units (CFUs)] in 25-μl sterile phosphate buffer saline solution. For in vivo treatment, mice were inoculated with 100-μg purified LTA-SA, a lipoteichoic acid from Staphylococcus aureus (Invivogen, USA), 1.5 × 109 CFUs of PC, or 100-μg purified CPS from PC via oral gavage once daily after antibiotic drinking water cessation. At 48 hours post-infection, mice were euthanized and sacrificed for sample collection and analysis. Mycobacterial proliferation in lung homogenates was determined by inoculating serial dilutions onto Middlebrook 7H11 agar plate containing 0.5% glycerol, 10% OADC, and 100-μg/ml ampicillin (Sigma). Colonies were enumerated, and results were expressed as CFUs/gram of organ.
For TLR2 inhibition in vivo, mice were treated twice with or without C29L (2 mg in 400 μl water) before administration of PC via oral gavage daily from the cessation of antibiotics to the end of experiment.Citation47,Citation48 In addition, TLR2 knockout mice (The Jackson Laboratory) were used to validate whether PC and PC CPS function on ameliorating NTM-LD susceptibility through TLR2 pathway.
Transcriptomic analysis
Total RNA was prepared from mice lung and colon tissues. The total RNA quality was assessed with a Qubit® RNA Assay Kit in a Qubit® 2.0 fluorometer (Life Technologies, CA, USA). RNA integrity was checked using an RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Then, the RNA was subjected for cDNA library construction and Illumina sequencing. RSEM quantified gene expression levels for each sample. First, clean data were mapped back onto the assembled transcriptome. Then, the read count for each gene was obtained from the mapping results. Differential expression analysis to identify differentially expressed genes (DEGs) was performed using the DESeq R package, and the resulting p values were adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate. The genes with an adjusted p < 0.05 and |log2 (fold change) |>1 were designated as significant DEGs. The Gene-Ontology (GO) enrichment of the DEGs was performed by using STRING databases (https://string-db.org/).
ELISA
For TLR2 and some DEGs (TLR2, Reg3b, CCR6, and Gbp2) noted from transcriptome analysis, we used ELISA kits (MyBioSource company) to measure the protein levels for confirmation.
Gene expression levels
Lung and colon tissues were harvested from the mice, and total RNAs were extracted using the Genezol TriRNA pure kit (Geneaid, New Taipei City, Taiwan). RNA was reversely transcribed using the Quant II fast reverse transcriptase kit (BioTools, New Taipei City, Taiwan). The resulting cDNA was used as a template for quantitative PCR (qPCR) using primers listed in Table S3. The PCR conditions include an initial pre-incubation-step at 95°C for 3 min, 50 PCR cycles at 95°C for 10 sec, 60°C for 20 s, 72°C for 5 s, and one melting curve cycle. The GAPDH primers were used as the internal control for qPCR assay. The 2−(ΔΔCT) method calculated relative gene expression levels.
Capsular polysaccharide extraction
Capsular polysaccharides (CPS) were isolated by using hot phenol-water extractionCitation49. The bacterial pellet of 1200-ml overnight culture was suspended in 30-ml warm water, and an equal volume of phenol was added and then stirred at 65°C for 30 min. After centrifugation, the aqueous layers were collected, and the organic layers were added to an equal volume of warm water to perform the extraction twice. The crude extract obtained after dialysis and lyophilization was treated with DNase, RNase, and Proteinase K. Further dialysis, lyophilization, and LPS removal using EntoxinOUT™ resin (G-Bioscience, USA) were performed to obtain the purified CPS, which was used in the animal model.
Statistical analysis
Data were shown as means ± standard deviation (SD). Differences between the two groups were assessed using an unpaired two-tailed Student’s t-test or Mann–Whitney test. Enrolled case number was estimated by difference of the abdundence of Prevotella_9 between NTM-LD and the control using alpha error of 0.05 and power of 0.8. Datasets involving more than two groups were assessed by one-way ANOVA with post hoc Dunnett’s multiple comparisons (see Figure legends).
Results
Gut microbiota dysbiosis in NTM-LD patients
We enrolled 25 NTM-LD patients and 21 HCs (Table S1). The NTM-LD group did not show major underlying co-morbidity diseases, but they were older, more likely to be female, and had lower body mass index (BMI) than the controls. The percentage of current smokers, percentage of those working night shift works, body weight loss, and medical history were not significantly different between the two groups.
The gut microbiota compositions were determined by using the 16S microbiota ribosomal DNA sequencing approach (). Regarding the alpha diversity in the gut microbiota, Shannon and Chao1 index were decreased significantly in the NTM-LD group, compared with HCs (). Beta diversity analyzed by unweighted and weighted UniFrac and PCoA was also significantly different between the two groups (). Whereas age, sex, and BMI were different between the two groups, these were not significant influencing factors on gut microbiota composition in the Adonis analysis (Table S2). Briefly, these results revealed significant gut microbiota dysbiosis occurring in NTM-LD patients.
Figure 1. Gut microbiota dysbiosis in NTM-LD patients.
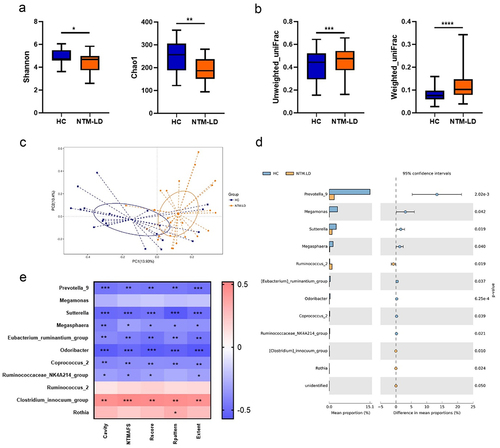
Reduced abundance of a commensal P. copri is closely associated with disease development and severity
The compositions of gut microbiota between the two groups were further compared. LEfSe analysis indicated that relative abundance of Prevotellaceae family, Prevotella_9 genus, Selenomonadales order, Negativicutes class, Veillonellanceae family, and Megmonas genus was more significantly reduced (LDA score < 4.0), while that of Bifidobacterium longum subspecies longum, Clostridium species, Eubacterium hallii, and Ruminococcaceae UCG_014 genus was more significantly increased (LDA score > 3.0) in the feces of NTM-LD patients than in HCs (Figure S1). Altogether, 11 bacterial genera were significantly altered in NTM-LD patients than in HCs, according to the Welch’s t-test (). Correlations between the 11 identified bacterial genera and disease severity, including the presence of a cavity, the grading of the sputum specimen’s acid-fast smear, and the radiographic score, were further analyzed (). The Clostridium innocuum group was significantly positively correlated with NTM-LD severity, while a negative correlation between disease severity and Prevotella_9 genus which was dominant in HCs was observed (). The enrolled case number of 46 could reach a power of 0.8 and alpha error of 0.05 for the abundance difference of Prevotella_9. In order to identify the gut bacteria that might contribute to decrease the NTM-LD susceptibility, we focused on the bacterial genera that showed the most significant negative association with the disease severity. By measuring the relative abundance, we found that Prevotella_9 genus ranked as the top one in this aspect, in contrast to the other bacterial genera (). In addition, Prevotella have been also identified to be the predominant genera in the human colonic microbiota in previous reports.Citation50 Therefore, together with all the data obtained, Prevotella_9 genus was selected for subsequent studies.
Figure 2. P. copri predominantly induce TLR2 pathway and NTM-LD patients had compromised TLR2 activation activity.
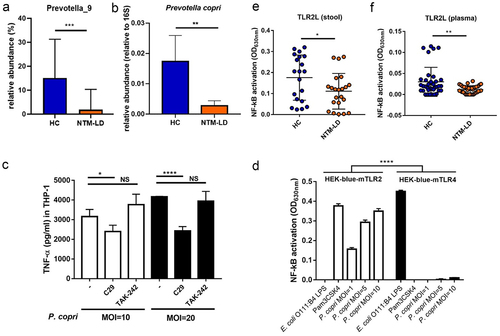
The sequence of V3–V4 region in the 16S rRNA gene of the Prevotella_9 genus showed 99.57% identity to that of Prevotella copri (PC) by NCBI database matching (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Additionally, in the real-time qPCR for quantifying the abundance of PC in a second cohort comprising of 19 NTM-LD patients and 25 HCs, decreased abundance was also observed in the feces samples in NTM-LD patients (). Thus, a reduced abundance of PC was closely associated with disease development and severity.
P. copri predominantly induces TLR2 pathway and NTM-LD patients had compromised TLR2 activation activity
We used heat-killed PC to stimulate THP-1 derived macrophages and found that TNF-α production was significantly inhibited by the addition of TLR2 inhibitor (C29), but not by TLR4 inhibitor (TAK-242) (). Additionally, PC could dose-dependently induce NF-kB activation in HEK-blue-mTLR2 reporter cells, but it only elicited a weak response in HEK-blue-mTLR4 reporter cells (). These results indicated that PC is a potent TLR2 agonist. Speculation that reduced abundance of PC in NTM-LD patients might lead to compromised TLR2 activation activity in feces and plasma, which were further examined. In stool supernatants and plasma, TLR2L, measured by NF-kB activation in HEK-blue-mTLR2 reporter cells, was significantly lower in NTM-LD patients than in HCs (), although reduced TLR4L in stool supernatants but not in plasma were detected in NTM-LD patients (Figure S2a and S2b).
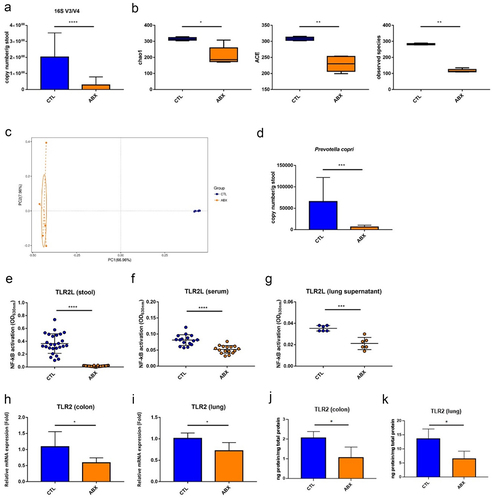
Gut microbiota dysbiosis and compromised TLR2 activation activity in mice receiving four antibiotics
The mice treated with four antibiotics (ABX mice) for 3 weeks were used as the study model. Gut microbiota dysbiosis was successfully induced, including a reduced abundance of gut microbiota, decreased alpha diversity, and altered beta diversity (). The robustly changed compositions of gut microbiota with increased Proteobacteria phylum and decreased Bacteroidetes phylum were observed in mice (Figure S3), while the abundance of PC was also observed to be significantly reduced (). TLR2Ls but not TLR4Ls were significantly lower in stool supernatants, serum, and lung supernatants of ABX mice than in the controls ( and Figure S2). Meanwhile, mRNA and protein expressions of TLR2 receptor were also significantly reduced in the colon and lung of ABX mice (). Altogether, gut microbiota dysbiosis, specifically decreased abundance of PC and reduced TLR2 activation activity in the intestine, blood, and lung tissue in mice receiving four antibiotics, was consistent with the findings observed in NTM-LD patients.
Figure 3. Gut microbiota dysbiosis and compromised TLR2 activation activity in mice receiving four antibiotics.
Compromised immunity in both lung and colon of mice receiving four antibiotics
Subsequently, whether reduced TLR2 activation resulted in compromised immunity was examined. The lung and colon tissues of mice treated with drinking water (CTL) and drinking water with antibiotics (ABX) for 3 weeks were collected at 2 days after antibiotic treatment cessation and then subjected to transcriptomic analysis ( for lung tissue and Figure S4 for colon tissue). In the colons of ABX mice, while the genes responsible for T lymphocyte cell proliferation and activation were significantly upregulated, those responsible for cell division were significantly downregulated (Figure S4a and S4b). Additionally, expressions of genes related to cellular response to oxidative stress and acute-phase response, including TNF-α, Reg3b, and Reg3g (regenerating islet-derived proteins with bactericidal activities), were significantly reduced (Figure S4c-e).
Figure 4. Transcriptomic analysis of lung tissues of ABX mice.
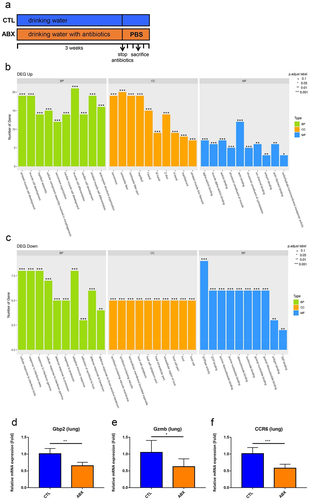
Although the lung histology was not obviously altered in ABX mice compared with the CTL mice (Figure S5), genes responsible for myofilament and actin filament development and binding were significantly upregulated. Contrarily, those responsible for responses to bacteria and protozoan were significantly downregulated (). The emapplot of GO enrichment indicated that the two networks related to the defense of bacteria and adaptive immune response were downregulated in the lung of ABX mice (Figure S6a). The linkages of downregulated genes and response to interferon-beta (IFN-β) and interferon-gamma (IFN-γ) also formed a network under cnetplot analysis (Figure S6b). Further RT-qPCR assay validated the downregulated relative expression levels of guanylate binding protein 2, granzyme B, and C-C motif chemokine receptor 6 (). We confirmed the results of some significantly differential expressed gene including TNF-α, Reg3b, Gbp2 and CCR6 by using ELISA (Figure S7). In addition to analysis of differential expression genes, the transcriptome of lung tissues was also analyzed by using GSEA (Gene Set Enrichment Analysis). As shown in Figure S8, the significantly activated (ex. gene sets responsible for actin binding, cytoskeleton protein binding and muscle organ development) and suppressed (ex. gene sets responsible for cellular response to IFN-β as well as IFN-γ and adaptive immunity) gene sets were in concordance with the results shown in .
Therefore, transcriptomic study demonstrated that genes associated with immunocompromised activity occurred in both intestine and lung tissues of ABX mice. Notably, RNAseq only shows indirect evidence of immune regulation, and the pathway of gut-NTM lung infection axis might be more complex and is still not totally clarified.
Administration of TLR2 agonist or treatment of P. copri leads to immunity restoration and NTM-LD amelioration
We further examined whether NTM pulmonary infection susceptibility was affected in ABX mice. The CTL and ABX mice were infected with M. kansasii at 2 days after antibiotic treatment cessation and then were sacrificed after 48 hours (). Compared with controls, the bacterial burdens in lung tissues increased significantly in ABX mice (), and the pathological changes such as immune cell infiltration were also apparent in ABX mice (). The more infiltrations of immune cells in ABX mice might be a compensatory result from high burden of NTM pulmonary infection. Next, whether activation of TLR2 pathway in the colon of immune-compromised ABX mice might restore the defense to NTM pulmonary infection in the lung was determined. The susceptibility to NTM pulmonary infection was examined in mice with oral inoculation of a TLR2 agonist (TLR2L, 100-μg purified LTA-SA) or 1.5 × 109 CFUs of PC once daily from cessation of antibiotics to end of experiment (). ABX mice with orally administration of TLR2 agonist or PC had significantly reduced bacterial burdens and decreased lung inflammation by pathology in lung tissues (). The lung tissues were collected and subjected to transcriptomic analysis. The heatmap revealed that supplementation of TLR2L or PC significantly altered the gene expressions in ABX mice and reversed to an extent similar to that of the control mice (). Functional associations of supplementation of TLR2L or PC-altered genes with TLR2 receptor were observed in the prediction of the protein – protein interaction network (Figure S9). The genes highly expressed in ABX mice (left panel, in yellow) comprised those responsible for the immune system process and activated T cell proliferation, including integrin and interferon-activated genes (). Contrarily, genes induced by administration of TLR2L or PC (right panel, in blue) were associated with responses to other organisms and bacteria, including mucin 5B, secretoglobin 3A1, Bpifa1 and Bpifb1 (BPI fold containing family), and chemokine ligand 8 genes (). Thus, the administration of TLR2 agonist or PC restored the responses to mycobacterial infection and ameliorated NTM-LD.
Figure 5. Administration of TLR2 agonist or treatment of P. copri restored immune response and ameliorated NTM-LD.
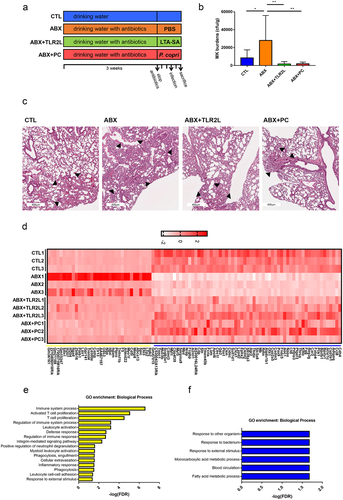
Administration of capsular polysaccharide of PC enhances TLR2 activation activity and ameliorates NTM-LD
CPSs were reported as potent TLR2 activators.Citation51–53 Like PC administration, purified CPS of PC also dose-dependently elicited NF-kB activation in HEK-blue-mTLR2 cells (). PC CPS was subsequently investigated to determine its potential in ameliorating NTM-LD susceptibility. The burden of NTM pulmonary infection was examined in mice with oral inoculation of 100-μg purified PC CPS once daily from cessation of antibiotics to end of experiment (). The bacterial burdens and pathology in lung tissues were significantly reduced by inoculation of PC CPS (). Therefore, the administration of PC CPS ameliorated NTM-LD susceptibility.
Figure 6. Administration of capsular polysaccharide of P. copri ameliorated NTM-LD.
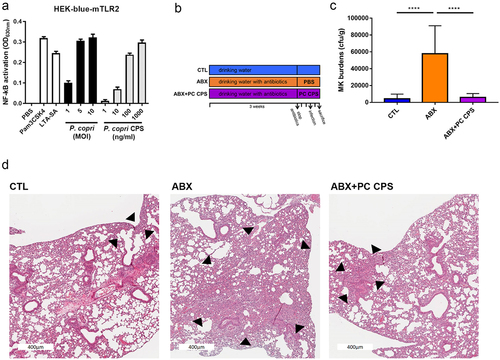
Administration of capsular polysaccharide of PC cannot ameliorate NTM-LD under TLR2 inhibitor
We further examined if treatment of TLR2 inhibitor (C29L) could inhibit the ameliorative effect of PC or PC CPS administration in vivo. After the cessation of antibiotic drinking water, mice were treated twice with or without C29L before administration of PC or purified CPS from PC daily (Figure S10a). In concordance with the above results, the administration of PC or PC CPS significantly ameliorated NTM-LD (Figure S10a, right panel). Upon treatment with C29L, the NTM burdens of ABX mice with administration of PC CPS were not significantly different with those of ABX mice, whereas administration of PC still revealed the ameliorative effect (Figure S10a, right panel). The results could be repeated in experiments using TLR2 knockout mice (Figure S10b). These results suggested that PC CPS but not PC ameliorated NTM-LD mainly or solely through TLR2 activation.
Discussion
Although recent advances in diagnostic and imaging techniques provided a much better understanding of the clinical manifestation caused by NTM-LD, the parameters causing NTM-LD development are still not completely clarified. Even so, currently known risk factor analysis strongly indicated compromised respiratory tract immunity and functions seemed to play important causal effects. Although NTM-LD was widely identified in immunocompromised subjects, such infection was also frequently reported in patients without previously known risk factors (Table S1). Subsequent laboratory and clinical studies on host immunity in NTM-LD, especially on measuring cytokine levels in serum or cell culture supernatants derived from cell preparations stimulated with antigens or mitogens stimulating cellular immunity, have shed light on some potential factors resulting in compromised immunity and opportunistic infections. A comparison of MAC-LD patients without significant risk factors and HCs showed that peripheral blood mononuclear cells (PBMCs) from infected patients stimulated with mycobacterial antigens produced decreased IFN-γ, IL-12, IL-17, and TNF-α levels.Citation54,Citation55 Contrarily, the serum concentrations of type 1 cytokine-associated molecules, including CD40L, IFN-γ, IL-8 and IL-23, were significantly reduced in MAC-LD patients versus HCs at the time of diagnosis.Citation56 Our previous study also indicated that patients with MAC-LD had lower TNF-α and IFN-γ responses compared to HCs in PBMC stimulation assays with MAC bacilli.Citation57 These results indicated that patients with unknown risk factors may have been immunocompromised at the time of NTM-LD diagnosis.
For the NTM-LD patients with unknown risk factors in the present study, compromised immunity was detected in the intestines and systemically in the blood (). Subsequent studies indicated gut microbiota dysbiosis was significantly observed in these patients, indicating that the change in gut microbiota may be related to development of compromised immunity and might even cause underlying compromised immunity leading to NTM-LD development (). Using mice treated with the antibiotic mixture, which led to gut microbiota dysbiosis, as the study model, a similar decrease in immune responses detected in the intestines, blood, and lung tissues also occurred (). These were closely related to the development of M. kansasii lung infection (). Subsequent studies indicated decreased TLR2 activation activity in the intestines and blood was consistently identified in samples harvested from animals and patients, with TLR2 activation activity also being decreased in lung tissues of mice (). Briefly, the systematic immune-compromised phenomena observed might be related to the change in gut microbiota structure, which is closely associated with decreased TLR2 immune response. These observations, therefore, suggested that the restoration of immunity by activating TLR2 signaling may be among the strategies for NTM-LD amelioration. Accordingly, restoration of immunocompromised activity by TLR2 agonist administration reversed the severity of M. kansasii pulmonary infection (), validating the important role of TLR2 signaling through the gut – lung axis against M. kansasii lung disease.
Although M. kansasii is not the most prevalent species for NTM-LD,Citation58 M. kansasii is one of representatives for slowly growing mycobacteria after MAC and has been well set up in an animal infection model.Citation46 In addition, the major pathogenesis for all NTM species is similar though there should be some different immune response between different NTM species,Citation5,Citation59 requiring further studies before generalizing our results to other NTM species. In addition, because we would like to address the role of gut microbiota against NTM-LD in host defense efficacy, especially at the early infection stage, we therefore examined the early pulmonary infection burden, instead of the sequelae or treatment effect after NTM pulmonary infection. In this study, we used 2 days for short-term observation. Previous report of mice model has shown that the NTM pulmonary infection in mice can be made in post-infection Day 1.Citation60 In addition, to prevent the decreased effect of gut dysbiosis after we discontinued antibiotics feeding, we thus used short-term observation.Citation61
Regarding the change in the composition of gut commensals in immunocompromised hosts, including human patients and animals, the reduced abundance of PC was significantly highlighted compared to controls (). Considering the practical applicability of establishing an immune-compromised animal model, in association with gut microbiota dysbiosis, therefore, ABX mice were used as the study model for immune-compromise in animals, examining the effect of gut dysbiosis, and the effect of P. copri supplements on NTM-LD susceptibility in this study. Altough the ABX might affect many genera of gut microbiota and gut dysbiosis by ABX could not be the same for NTM-LD status. Gut dysbiosis induced by ABX could lead lung susceptibility of high NTM infection burden (), possibly indicating that some of the ABX affecting gut microbiota is associated with NTM development. In addition, after we gave OG supplement of Prevotella copri, the NTM infection burden of ABX mice decreased to similar level with control mice. Therefore, Prevotella copri might involve in the NTM-LD susceptibility although there are many factors warranted clarifying and investigating.
Prevotella was previously found to elevate serum IL-6 and IL-1β levels and BMDCs of mice treated with a combination of broad-spectrum antibiotics, which was mainly mediated through the interaction with TLR2.Citation62 Furthermore, Prevotella predominantly activates TLR2, producing cytokines, including IL-1, IL-6, and IL-23.Citation63,Citation64 In concordance, our study also indicated PC treatment enhanced TLR2-associated immune responses both in vitro and in vivo and restored the immune responses caused by decreased TLR2 signaling (). By contrast, the ameliorative effect of PC CPS but not PC was significantly inhibited by treatment of TLR2 inhibitor in vivo or by an TLR2 knockout mice model, suggesting that PC CPS but not PC ameliorated NTM-LD mainly or solely through TLR2 activation. As shown in , the TNF-α activation by heat-killed PC was partially, not completely, blocked by TLR2 inhibitor, suggesting signals other than TLR2 might exist. Therefore, PC might use multiple mechanisms of action through other possible pathways, which can compensate TLR2,Citation65,Citation66 to prevent NTM-LD although the mechanism required further study. These results might suggest that PC or PC CPS may be further developed as a probiotic for ameliorating NTM-LD in immunocompromised patients. However, some PC strains are closely related to the development of chronic inflammation-associated diseases such as arthritis, increased susceptibility to Listeria monocytogenes infection, and inflammatory bowel disease.Citation67 Therefore, care should be taken when developing PC as a therapeutic agent for NTM-LD.
The PC CPS significantly enhanced TLR2 activation activity and restored the immune compromise phenomenon and its associated M. kansasii pathology of the ABX mice (), suggesting that PC CPS may be an important component involved in restoring the compromised immunity. The CPS purified from other bacteria were also reported to activate TLR2-related signaling. For example, the cell surface of a human intestinal commensal Bacteroides fragilis is composed of CPS, within which capsular polysaccharide A (PSA), a zwitterionic polysaccharide symbiosis factor, induces regulatory T cells in the intestinal tract and inhibit enteritis in a TLR2 dependent manner.Citation51 Besides, PSA protects against central nervous system demyelination and inflammation during experimental autoimmune encephalomyelitis, an animal model for multiple sclerosis. Activation of TLR2 by PSA resulted in a tissue-specific expansion of a critical regulatory CD39+ CD4 T cell subset. These results also emphasized the therapeutic potential of PSA in ameliorating CNS-targeted demyelination.Citation68 Whether CPS derived from P. copri showed similar immune modulation activities in contrast to those from B. fragilis needs clarification.
In the present study, the lung microbiota were not examined in this study and their role in the NTM-LD might be neglected and needs future investigation. In fact, some studies had shown the lung microbiota changed under antibiotics use.Citation69,Citation70 However, the comparison of NTM-LD mice model with or without PC orally supplement were both received pre-treatment of oral antibiotics for 3 weeks. We assumped that the lung microbiota changes were not different between the mice subgroups.
In conclusion, our results established an important linkage of the gut – lung axis, in that gut microbiota dysbiosis with reduced P. copri abundance might be associated with compromised immunity of low TLR2 activation, resulting in NTM-LD susceptibility. Furthermore, specific P. copri strain-related probiotics might be developed as a preventive or therapeutic agent for NTM-LD, although this requires future validation.
Author contributions
TLL, YLK, CCL, HCL, JCS and CCS designed this work. TLL, JHL, and CYH performed the experimental study. TLL, JHL, CTY, CYH, BSY, LSHW, TSW, JYW, CJY, CCS, JCS and HCL performed case enrollment, data analysis and interpretation. TLL, YLK, CCL, HCL, JCS and CCS wrote the manuscript. All authors discussed, reviewed, and approved the manuscript.
Availability of data and materials
All relevant data is contained within the article. The original contributions presented in the study are included in the article/supplementary information, further inquiries can be directed to the corresponding authors.
The 16S sequence reads have been deposited under NCBI BioProject numbers PRJNA997120 and PRJNA997600. The sequence reads of transcriptome have been deposited under NCBI BioProject numbers PRJNA998172, PRJNA998187 and PRJNA998205.
Ethics approval and consent to participate
All experiments were conducted under the approval of the institutional review board described in the Methods section.
Supplemental Material
Download Zip (1.1 MB)Acknowledgments
We thank Dr Yu-Lun Kuo (Biotools) for the kind assistance with microbiota sequencing and analysis, National Laboratory Animal Center (NLAC), NARLabs, Taiwan, for technical support in service of isolator, and Second, Seventh and Eighth Core as well as Department of Medical Research of National Taiwan University Hospital for technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2361490
Additional information
Funding
References
- Ratnatunga CN, Lutzky VP, Kupz A, Doolan DL, Reid DW, Field M, Bell SC, Thomson RM, Miles JJ. The rise of non-tuberculosis mycobacterial lung disease. Front Immunol. 2020;11:303. doi:10.3389/fimmu.2020.00303.
- Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. Managed care health plan, 2008–2015. Ann Am Thorac Soc. 2020;17(2):178–18. doi:10.1513/AnnalsATS.201804-236OC.
- Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;54(1):54. doi:10.1183/13993003.00250-2019.
- Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008;31(6):1322–33. doi:10.1183/09031936.00140007.
- Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36(1):1–11. doi:10.1016/j.ccm.2014.10.001.
- Marras TK, Vinnard C, Zhang Q, Hamilton K, Adjemian J, Eagle G, Zhang R, Chou E, Olivier KN. Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med. 2018;145:80–88. doi:10.1016/j.rmed.2018.10.022.
- Shu CC, Wei YF, Chen KH, Chuang S, Wang YH, Wang CY, Wang H-C. Inhaled corticosteroids increase risk of nontuberculous mycobacterial lung disease: a nested case-control study and meta-analysis. J Infect Dis. 2021;225(4):627–36. doi:10.1093/infdis/jiab428.
- Lake MA, Ambrose LR, Lipman MC, Lowe DM. ”Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016;14:54.
- Mortaz E, Moloudizargari M, Varahram M, Movassaghi M, Garssen J, Kazempour Dizagie M, Mirsaeidi M, Adcock IM. What immunological defects predispose to non-tuberculosis mycobacterial infections? Iran J Allergy Asthma Immunol. 2018;17(2):100–9.
- Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4–14. doi:10.4161/gmic.19320.
- Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. doi:10.1038/nri.2016.42.
- Kho ZY, Lal SK. The human gut microbiome – a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. doi:10.3389/fmicb.2018.01835.
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi:10.1038/s41579-020-0433-9.
- Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12(4):496–508. doi:10.1016/j.chom.2012.09.009.
- Mukherjee S, Karmakar S, Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016;20(2):193–204. doi:10.1016/j.bjid.2015.10.011.
- Skevaki C, Pararas M, Kostelidou K, Tsakris A, Routsias JG. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseases. Clin Exp Immunol. 2015;180(2):165–177. doi:10.1111/cei.12578.
- Ryu YJ, Kim EJ, Lee SH, Kim SY, Suh GY, Chung MP, Kim H, Kwon OJ, Koh W-J. Impaired expression of Toll-like receptor 2 in nontuberculous mycobacterial lung disease. Eur Respir J. 2007;30(4):736–742. doi:10.1183/09031936.00039507.
- Urban-Wojciuk Z, Khan MM, Oyler BL, Fahraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR, Goodlett DR. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front Immunol. 2019;10:2388. doi:10.3389/fimmu.2019.02388.
- Wu MF, Shu CC, Wang JY, Yan BS, Lai HC, Chiang BL, Wu LSH, Yu C-J. NLRP3 inflammasome is attenuated in patients with Mycobacterium avium complex lung disease and correlated with decreased interleukin-1β response and host susceptibility. Sci Rep. 2019;9(1):12534. doi:10.1038/s41598-019-47609-3.
- Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7:455. doi:10.3389/fmicb.2016.00455.
- Tang TWH, Chen HC, Chen CY, Yen CYT, Lin CJ, Prajnamitra RP, Chen L-L, Ruan S-C, Lin J-H, Lin P-J, et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139(5):647–659. doi:10.1161/CIRCULATIONAHA.118.035235.
- Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59–72. doi:10.1016/S2213-2600(15)00427-0.
- Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):2. doi:10.1186/s12865-016-0187-3.
- Ruff WE, Greiling TM, Kriegel MA. Host–microbiota interactions in immune-mediated diseases. Nat Rev Microbiol. 2020;18(9):521–538. doi:10.1038/s41579-020-0367-2.
- Ahlawat S, Asha, Sharma KK. Gut–organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2021;72(6):636–668. doi:10.1111/lam.13333.
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi:10.1038/s41422-020-0332-7.
- Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5(1):7–18. doi:10.1038/mi.2011.55.
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi:10.1038/nrmicro.2016.142.
- He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J. Gut–lung axis: The microbial contributions and clinical implications. Crit Rev Microbiol. 2017;43(1):81–95. doi:10.1080/1040841X.2016.1176988.
- Openshaw PJ. Crossing barriers: infections of the lung and the gut. Mucosal Immunol. 2009;2(2):100–2. doi:10.1038/mi.2008.79.
- Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi:10.3389/fimmu.2018.01830.
- Rosenow EC 3rd, Wilson WR, Cockerill FR 3rd. Pulmonary disease in the immunocompromised host. 1. Mayo Clin Proc. 1985;60(7):473–487. doi:10.1016/S0025-6196(12)60872-6.
- Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol. 2012;188(3):1411–1420. doi:10.4049/jimmunol.1101682.
- Felix KM, Jaimez IA, Nguyen TV, Ma H, Raslan WA, Klinger CN, Doyle KP, Wu HJJ. Gut microbiota contributes to resistance against pneumococcal pneumonia in immunodeficient rag−/− mice. Front Cell Infect Microbiol. 2018;8:118. doi:10.3389/fcimb.2018.00118.
- Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS, Bomberger JM. Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio. 2020;11(1):11. doi:10.1128/mBio.03236-19.
- Negi S, Pahari S, Bashir H, Agrewala JN. Gut microbiota regulates mincle mediated activation of lung dendritic cells to protect against mycobacterium tuberculosis. Front Immunol. 2019;10:1142. doi:10.3389/fimmu.2019.01142.
- Ito T, Carson W, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–9. doi:10.1016/j.yexcr.2010.12.018.
- Luxameechanporn T, Kirtsreesakul V, Klemens J, Khoury P, Thompson K, Naclerio RM. Evaluation of importance of Toll-like receptor 4 in acute Streptococcus pneumoniae sinusitis in mice. Arch Otolaryngol Head Neck Surg. 2005;131(11):1001–06. doi:10.1001/archotol.131.11.1001.
- Deng JC, Moore TA, Newstead MW, Zeng X, Krieg AM, Standiford TJ. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J Immunol. 2004;173(8):5148–55. doi:10.4049/jimmunol.173.8.5148.
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF. et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST.
- Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu C-C, Young JD, Lai H-C. et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68(2):248–262. doi:10.1136/gutjnl-2017-315458.
- Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng S-F, Wu T-R, Chen YYM, Young JD. et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6(1):7489. doi:10.1038/ncomms8489.
- Chang CJ, Lu CC, Lin CS, Martel J, Ko YF, Ojcius DM, Wu T-R, Tsai Y-H, Yeh T-S, Lu J-J. et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int J Obes (Lond). 2018;42(2):231–243. doi:10.1038/ijo.2017.149.
- Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10(12):1200–02. doi:10.1038/nmeth.2658.
- Gray L, Hasebe K, O’Hely M, Ponsonby AL, Vuillermin P, Collier F, BIS Investigator Group. Rapid PCR identification of Prevotella copri in an Australian cohort of pregnant women. J Dev Orig Health Dis. 2020;11(3):228–234. doi:10.1017/S2040174419000849.
- Lai HC, Chang CJ, Lin CS, Wu TR, Hsu YJ, Wu TS, Lu J-J, Martel J, Ojcius DM, Ku C-L. et al. NK cell–derived IFN-γ protects against nontuberculous mycobacterial lung infection. J Immunol. 2018;201(5):1478–90. doi:10.4049/jimmunol.1800123.
- Mistry P, Laird MH, Schwarz RS, Greene S, Dyson T, Snyder GA, Xiao TS, Chauhan J, Fletcher S, Toshchakov VY. et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci USA. 2015;112(17):5455–60. doi:10.1073/pnas.1422576112.
- Deng Y, Liu K, Pan Y, Ren J, Shang J, Chen L, Liu H. TLR2 antagonism attenuates the hippocampal neuronal damage in a murine model of sleep apnea via inhibiting neuroinflammation and oxidative stress. Sleep Breath. 2020;24(4):1613–21. doi:10.1007/s11325-020-02030-3.
- De Castro C, Parrilli M, Holst O, Molinaro A. Microbe-associated molecular patterns in innate immunity: Extraction and chemical analysis of gram-negative bacterial lipopolysaccharides. Methods Enzymol. 2010;480:89–115.
- Prasoodanan PKV, Sharma AK, Mahajan S, Dhakan DB, Maji A, Scaria J, Sharma VK. Western and non-western gut microbiomes reveal new roles of Prevotella in carbohydrate metabolism and mouth–gut axis. Npj Biofilms Microbiomes. 2021;7(1):77. doi:10.1038/s41522-021-00248-x.
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203(13):2853–63. doi:10.1084/jem.20062008.
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–977. doi:10.1126/science.1206095.
- Kayama H, Takeda K. Polysaccharide a of Bacteroides fragilis: actions on dendritic cells and T cells. Mol Cell. 2014;54(2):206–7. doi:10.1016/j.molcel.2014.04.002.
- Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, Fordham von Reyn C, Girard WJ, Wallace R Jr., Barnes P. et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis. 2001;183(3):478–484. doi:10.1086/318087.
- Lim A, Allison C, Price P, Waterer G. Susceptibility to pulmonary disease due to Mycobacterium avium–intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol. 2010;137(2):296–302. doi:10.1016/j.clim.2010.07.011.
- Kim SY, Koh WJ, Park HY, Jeon K, Kwon OJ, Cho SN, Shin SJ. Changes in serum immunomolecules during antibiotic therapy for Mycobacterium avium complex lung disease. Clin Exp Immunol. 2014;176(1):93–101. doi:10.1111/cei.12253.
- Shu CC, Wang JY, Wu MF, Wu CT, Lai HC, Lee LN, Chiang B-L, Yu C-J. Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci Rep. 2017;7(1):42004. doi:10.1038/srep42004.
- Lee MR, Chang LY, Ko JC, Wang HC, Huang YW. Nontuberculous mycobacterial lung disease epidemiology in Taiwan: A systematic review. J Formos Med Assoc. 2020;119 Suppl 1:S4–S12. doi:10.1016/j.jfma.2020.05.019.
- Lore NI, Yamasaki S, Simmonds RE, Jo EK. Editorial: Host-pathogen interactions in nontuberculous mycobacterial infections. Front Immunol. 2023;14:1201159. doi:10.3389/fimmu.2023.1201159.
- Andrejak C, Almeida DV, Tyagi S, Converse PJ, Ammerman NC, Grosset JH. Characterization of mouse models of Mycobacterium avium complex infection and evaluation of drug combinations. Antimicrob Agents Chemother. 2015;59(4):2129–35. doi:10.1128/AAC.04841-14.
- Schuijt TJ, Lankelma JM, Scicluna BP, de Sousae Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi:10.1136/gutjnl-2015-309728.
- Huang Y, Tang J, Cai Z, Zhou K, Chang L, Bai Y, Ma Y. Prevotella induces the production of Th17 cells in the colon of mice. J Immunol Res. 2020;2020:1–14. doi:10.1155/2020/9607328.
- Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–374. doi:10.1111/imm.12760.
- Leite AZ, Rodrigues NC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, Omori WP, Pinheiro DG, Brisotti JL, Matheucci Junior E. et al. Detection of increased plasma Interleukin-6 levels and prevalence of prevotella copri and bacteroides vulgatus in the Feces of type 2 diabetes patients. Front Immunol. 2017;8:1107. doi:10.3389/fimmu.2017.01107.
- Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34(5):629–636. doi:10.1016/j.immuni.2011.05.009.
- Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol Rev. 2007;219(1):75–87. doi:10.1111/j.1600-065X.2007.00548.x.
- Iljazovic A, Amend L, Galvez EJC, de Oliveira R, Strowig T. Modulation of inflammatory responses by gastrointestinal Prevotella spp. – from associations to functional studies. Int J Med Microbiol. 2021;311(2):151472. doi:10.1016/j.ijmm.2021.151472.
- Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL. et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun. 2014;5(1):4432. doi:10.1038/ncomms5432.
- Pfeiffer S, Jatzlauk G, Lund JV, Boateng E, Kovacevic D, Nh M, Bartel S, Schloter M, Krauss‐Etschmann S. Oral application of vancomycin alters murine lung microbiome and pulmonary immune responses. Immun Inflam Dis. 2022;10(8):e675. doi:10.1002/iid3.675.
- Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, Storti C, Triulzi T, Castelli C, Balsari A. et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018;24(13):3528–38. doi:10.1016/j.celrep.2018.08.090.
