Abstract
Aging is universally associated with organism-wide dysfunction and a decline in cellular fitness. From early development onwards, the efficiency of self-repair, energy production, and homeostasis all decrease. Due to the multiplicity of systems that undergo agingrelated decline, the mechanistic basis of organismal aging has been difficult to pinpoint. At the cellular level, however, recent work has provided important insight. Cellular aging is associated with the accumulation of several types of damage, in particular damage to the proteome and organelles. Groundbreaking studies have shown that replicative aging is the result of a rejuvenation mechanism that prevents the inheritance of damaged components during division, thereby confining the effects of aging to specific cells, while removing damage from others. Asymmetric inheritance of misfolded and aggregated proteins, as well as reduced mitochondria, has been shown in yeast. Until recently, however, it was not clear whether a similar mechanism operates in mammalian cells, which were thought to mostly divide symmetrically. Our group has recently shown that vimentin establishes mitotic polarity in immortalized mammalian cells, and mediates asymmetric partitioning of multiple factors through direct interaction. These findings prompt a provocative hypothesis: that intermediate filaments serve as asymmetric partitioning modules or “sponges” that, when expressed prior to mitosis, can “clean” emerging cells of the damage they have accumulated.
Replicative Rejuvenation
Two important discoveries point to essential clues in the search for the mechanistic basis of aging: replicative rejuvenation and induced pluripotency, or reprogramming of induced pluripotent stem cells.Citation1-3 Studies of replicative aging have shown that a robust mechanism for aging avoidance promotes the “replicative rejuvenation” of individual cells, from prokaryotes, to budding yeast, mammalian cell lines, and even differentiating stem cells.Citation4-7 These cells are thought to mitigate the causes and consequences of cellular aging by asymmetrically partitioning aging determinants during mitosis. Although several of these factors have been identified (oxidatively damaged proteins, old or reduced mitochondria, circular DNA, and misfolded proteins, among others),Citation6,Citation9-12 most factors, as well as the mechanism governing asymmetric inheritance, remain a mystery. Understanding the mechanism of replicative rejuvenation will offer definitive insight into the determinants of aging and the interplay between these determinants and disease.
iPSC reprogramming technology offers another conceptual window into the mechanism of aging, since reprogramming can effectively “undo” the aging process: it has been suggested that old or even senescent cells can be reprogrammed into “youthful” pluripotent cells.Citation1,2 This phenomenon demonstrates that aging and associated damage accumulation can be reversed through a reprogramming process that is not yet understood at the molecular level.
Despite these important advances, 2 key questions remain unanswered: How do cells recognize some materials as being old or unfit versus new and youthful? And how are unfit components retained in specific cells?
Asymmetric inheritance of damaged factors in eukaryotes
Asymmetric mitosis, yielding 2 daughter cells that are different in their components or their fates, is an essential feature of organismal development, stem cell renewal and differentiation, the creation of a germ line, and the establishment of fitness asymmetry through rejuvenation.Citation13-16 Replicative rejuvenation is the process of partitioning damaged cellular factors during mitosis away from a cell that has been dedicated to staying young (the renewing daughter), and into a daughter cell that ages ().Citation17-19 It was only relatively recently that groundbreaking work in budding yeast Saccaromyces cerevisiae led to the discovery of this process.Citation8,Citation20-22 Budding yeast is a single cell organism in which every division is polar in 2 respects: 1. the mother and daughter cells are physically distinguishable from one another; and 2. with each division the mother cell becomes older while the daughter cell is rejuvenated. After a finite number of daughters, the mother cell shows signs of “aging decline,” stops dividing and eventually dies.Citation23 Replicative rejuvenation ensures that each of the daughters turns into a new mother that enjoys a full replicative potential.Citation24 Because of this polarity, yeast presents an exquisitely tractable system for probing the mechanism of asymmetry-based rejuvenation, which we have exploited in previous work to elucidate one of the mechanisms for asymmetric inheritance of aggregate inclusions.Citation25,26 Other work by the Nystrom group and colleagues has demonstrated that genes which participate in the regulation of asymmetric partitioning of aggregates (including Sir2, Actin, Hsp104) directly influence the yeast replicative lifespan, indicating that misfolded proteins and aggregates are bona fide determinants of aging.Citation9,11,Citation27-29 These studies have shown that replicative rejuvenation relies on the coordinated function of overlapping mechanisms that identify aging factors and carefully partition them away from the daughter cell and into the mother cell during mitosis. The aging markers identified so far in yeast include aged organelles such as mitochondria, oxidatively damaged proteins, and extra-chromosomal DNA circles.Citation11,19,Citation30-34
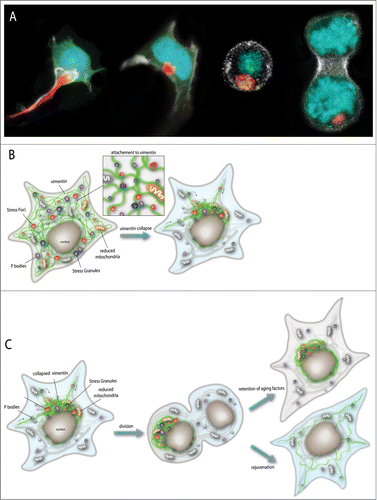
Figure 1. (A) 3D time-lapse (4D imaging) of a live neuronal-derived cell line entering asymmetric mitosis. Vimentin IF is red, Histone-2b is blue, alpha-Tubulin is green and F-Actin is white. Misfolded proteins in the collapsed VIF JUNQ are inherited by the aging cell. (B) Model of VIF attachment to aging determinants, including stress foci (misfolded proteins), p-bodies (RNA), stress granules (RNA), and old mitochondria. (C) Model of asymmetric inheritance of collapsed VIF during mitosis. Cells which avoid inheriting collapsed VIF, which traps aging determinants, are more youthful and rejuvenated.
However, yeast is merely the simplest and most tractable example of a cell that uses mitosis for rejuvenation. Studies published within the last year have established that replicative rejuvenation is utilized in multiple examples of cell divisions in multicellular organisms and human cells.Citation6,35,36 This is true for divisions of cells that are known to be polar (e. g. old centrosome partitioning to renewing stem cell), as well as divisions where polarity is not immediately apparent (e. g. dividing immortal cancer cells, stem cells differentiating to different tissues, pluripotent stem cells, etc.).Citation37,38 Thus, just as yeast, multicellular organisms rejuvenate replicatively and the chronological lifespan, as opposed to cell type, determines the level of damaged proteins and other components present in the cell.Citation39 A list of asymmetrically partitioning factors is beginning to emerge in yeast, though it is far from comprehensive.Citation3 We know even less about what determines the organelles, proteins, and membrane compartments that partition asymmetrically in dividing mammalian somatic cells and stem cells, nor has it been explored how this happens.
Mechanisms of replicative rejuvenation
Four general mechanisms are thought to maintain asymmetric partitioning during mitosis (and thereby facilitate rejuvenation). These include 1. motor-driven transport (via actin/myosin and microtubules); 2. direct or indirect association with one of the 2 centrosomes; 3. confinement by diffusion barriers; and 4. spatial sequestration into earmarked deposits that are attached to membranesCitation12,21,25,30,Citation40-42 or are large enough in volume that their movement is significantly constrained. All of these have been clearly shown in budding yeast, but almost nothing is known about which of these mechanisms operate in mammalian cells. Since the yeast division is a priori polar (in every division there is a pre-defined mother cell and daughter cell) directed transport can pull new oxidized mitochondriaCitation43 and high proton gradient vacuolesCitation44 into the bud as it emerges from the mother cell. The nuclear membrane remains intact during the yeast mitosis, and hence can be used as a platform for retention of misfolded proteins (in the JUNQ quality control compartment).Citation25 Insoluble aggregates (in the IPOD insoluble aggregate compartment) are also retained by virtue of spatial sequestration and adhesion to vacuoles earmarked for the mother cell.Citation25 During closed mitosis the nuclear membrane also contains diffusion barriers that have been shown by the Barral group to ensure the retention of extra-chromosomal rDNA circles (ERCs) in the mother cell.Citation45 For these retention mechanisms, the specific adaptors that designate “old” vs. “new” are not yet known. It is unclear how reduced mitochondria and unfit vacuoles are recognized and transported. ERCs have been proposed to attach to old nuclear pores and thus retained during division, however this is far from clear.Citation46
Asymmetry in multicellular organisms
Asymmetry in mammalian cells is more difficult to track than in a budding organism like yeast because every division is a priori symmetrical in multicellular organisms and cultured cells. Asymmetry has been studied by following the fate of each cell (proliferation versus death; self-renewal vs. differentiation; etc.)Citation38,39,47,48 or by tracking specific components (e. g. protein aggregates; ubiquitinated proteins; reduced mitochondria).8,Citation49-51 These studies point to many instances of asymmetry (only one cell inheriting an aggregate) without necessarily observing polarity that designates one cell as “old” and the other as “young.” However, organism-level studies in Drosophila have also observed asymmetric partitioning of damaged proteins and aggregates to specific cell lineages.Citation52 Intestinal cells, for example, partition damage to differentiating progeny and away from self-renewing cells.Citation12 One notable example of a characterized asymmetry mechanism in multi-cellular organisms is the inheritance of P granules during C. elegans development. P granules are membraneless organelle-like dynamic droplets packed with RNA and proteins that segregate to germ cell precursors during development.Citation53,54 Although not anchored to other organelles, P granules are retained asymmetrically by the dividing one-cell embryo by virtue of its assembly and disassembly dynamics and their large volume. In the posterior side of the embryo, destined to become the P granule inheriting cell, assembly kinetics are rapid, driven by high concentrations of P granule components. Conversely, at the anterior of the cell, disassembly is rapid, leading to rapidly diffusing components which are free to assemble at the posterior.
Cytoskeleton: Vehicle for Replicative Rejuvenation
The cytoskeleton mediates cell division, and also plays a key role in maintenance of division asymmetry.Citation55 In both yeast and mammalian cells the spindle pole body or the centrosome establish the polarity of division.Citation37,56,57 The old spindle pole body is always inherited by the bug in yeast, and the old mother centrosome is also inherited in a conserved manner in divisions where an axis of polarity exists (such as in development).Citation58
The precise role of specific cytoskeletal components in yeast asymmetric aging and rejuvenation is a matter of some controversy, with actin, tubulin, and constrains on the movement of misfolded proteins all implicated to various degrees. The actin cytoskeleton is one of the major regulators of asymmetry and replicative rejuvenation in yeast.Citation41,59,60 The Nystrom group has demonstrated that deletion of the Sir2 aging regulator, which decreases replicative lifespan, acts via the actin cytoskeleton by decreasing actin production and thus the rate of retrograde transport of aggregates and other aging determinants.Citation41,60,61 In fact, a key experiment showed that even temporary pharmacological disruption of actin leads to daughter cell contamination by aging determinants, and that this daughter cell has a shorter replicative lifespan than subsequent daughter cells which were budded once F-actin was restored.Citation41 F-actin cables and associated myosin motors have also been implicated in the transport of fit mitochondria and vacuoles to the bud.Citation34
The requirement of actin for rejuvenation and fitness appears to be highly conserved, though it remains to be seen whether the mechanism of its involvement is as well. In C. elegans, a recent study discovered that increasing the stability of the actin cytoskeleton by over-expressing the pat-10 protein was sufficient to extend the lifespan of the nematode and to improve tolerance of heat stress.Citation62 Conversely, disrupting F-actin lead to decreased lifespan and heat tolerance in yeast,Citation41 C. elegans, and mammalian cell lines.Citation63-65 Not all of these effects can be attributed to the role of actin in replicative rejuvenation – clearly actin is also essential for maintaining cellular homeostasis, proper protein movement, and cytoplasmic organization.Citation66 However, the above-mentioned experiments clearly demonstrate an essential role for actin-based rejuvenation in maintaining cellular fitness. Hence, in looking for the mechanism of mammalian cell asymmetry, the cytoskeleton is an obvious target.
Replicative rejuvenation in mammalian cells
What is the role of the cytoskeleton in replicative rejuvenation and maintenance of asymmetry in mammalian cells? Besides the polarity of the division of the centrosome, not much is known about a possible role of the cytoskeleton in ensuring the asymmetric segregation of damage. It is possible that, as in yeast, mammalian cells use retrograde actin-based movement of aging determinants from one cell to the other during division. Another recent candidate for regulating replicative rejuvenation in mammalian cells is the intermediate filament vimentin (VIF). VIF is a versatile intermediate filament, which has been implicated in regulating differentiation, senescence, and immortalization.Citation67,68 Fibroblasts lacking VIF exhibited an inability to become immortal, and VIF has been shown to protect cells from oxidative damage.Citation68,69
Recent work from our group has demonstrated that a specific (collapsed) form of VIF consistently undergoes asymmetric partitioning in dividing immortal mammalian cell lines.Citation6 We hypothesize that VIF binds to certain aggregates, ribonuclear protein (RNP) granules, reduced mitochondria, and to misfolded proteins, including ones that are mobile and relatively soluble, and promotes their asymmetric inheritance by trapping them in collapsed VIF structure. We call these structures JUNQs (for juxta-nuclear quality control compartments) because they contain mobile misfolded proteins (as opposed to aggregates) and because these misfolded proteins undergo proteasomal degradation within the inclusions.Citation6,70 These structures are distinct from insoluble aggregates (IPODs) or aggresomes, which we observe to form later in the aggregation process upon exposure to stress or higher levels of misfolded proteins.Citation70,71 JUNQ inclusions form very rapidly in response to high levels of misfolded proteins. This can be triggered by pharmacological proteasome inhibition, or (in our hands) simply by over-expression of model misfolded proteins including von-Hippel Lindau protein (VHL), the CL1 hydrophobic peptide, or a thermosensitive version of Ubc9 or Luciferase.Citation70,72,73 JUNQ structures are extremely dynamic and have high turnover rates. When we observe cells for longer periods of time, we see the appearance of small cytoplasmic foci, which we call stress foci because their appearance can be triggered by acute stress including heat shock, arsenite, chaperone inhibition, and disruption of the cytoskeleton.Citation25,70 Over time, stress foci are transported toward the JUNQ and accumulate around it. This corresponds to VIF collapse, and a transition from a dynamic JUNQ inclusion, to an immobile aggresome in place of the JUNQ. The collapsed VIF remains intact during mitosis, and is attached to the microtubule organizing center (MTOC) which mediates polar inheritance (example division shown in ). Hence, this study implicates the MTOC, tubulin, and VIF in the asymmetric aging of mammalian cells.
Implications for cellular fitness and development
We observed that cells which avoid inheriting the collapsed VIF are more “fit” than cells which do inherit it. Using our low phototoxicity 4D imaging approach we were able to follow multiple divisions of HEK and N2a cells, tracking the localization of the collapsed VIF inclusions. Interestingly, we observed that cells which fail to inherit the inclusion always divide before cells which do inherit it (6).
Although the reasons for the increased fitness of the cells which do not inherit the VIF are still unclear, our preliminary results indicate that VIF binds multiple aging determinants in addition to misfolded proteins. Similar to misfolded proteins (e. g. CL1 peptide and VHL) we also observe reduced mitochondria, p-body markers, and stress granule markers associating with VIF and trapped in collapsed VIF (model – ).
VIF – a potential master-regulator of replicative rejuvenation in mammalian cells
VIF is upregulated early in differentiation, is usually expressed together with tissue-specific IFs, and is then downregulated.Citation74,75 Hence, its expression may be an early cleaning mechanism that mediates cell specification and the generation of pristine lineages. Conversely, VIF is an important player in immortalization and carcinogenesis, and may act to prevent senescence in rapidly dividing, metabolically active, and damage-prone cancer cells.Citation68,Citation76-78 Contrary to past models of mitosis, we posit that many if not most mammalian mitotic divisions are asymmetric.Citation13 This asymmetry has sweeping implications for immortalization, carcinogenesis, stem cell maintenance and differentiation, aging, and induced pluripotency (or rejuvenation).Citation3,38,79
Our model is that the expression of VIF in immortalized cells and differentiating stem cells regulates the asymmetric inheritance of aging determinants, including damaged, misfolded, and aggregated proteins and reduced mitochondria. VIF collapse (regulated by Rho kinase and p21-activated kinaseCitation80-82) traps aging determinants, as well as specific RNP granules, and mediates their asymmetric partitioning between 2 daughter cells during mitosis. We posit that this asymmetry may function to rejuvenate specific lineages that are meant to be pristine (such as germ-line precursors and immortalized cells) and may also function to give specific cells a fitness advantage in the face of metabolic, oxidative, or protein folding stress.
Future Directions
The study of aging asymmetry or replicative rejuvenation is rapidly expanding in search of parallel mechanisms in mammalian cells to those that have been characterized in yeast, the specific elements that designate certain cellular components as “old,” as well as the machinery that affects their selective retention. VIF provides a supple and elegant solution to the asymmetry problem, interacting with the actin/tubulin cytoskeleton as well as most organelles and with misfolded proteins. The asymmetric partitioning of collapsed VIF raises 2 key questions that should be the topic of future investigations. How does VIF interact with organelles and “old” dynamic droplets? An attractive model is that VIF acts as a “sponge” for dynamics droplets and misfolded proteins via its disordered regions. Another question is: what is the cost and benefit to cellular fitness of inheriting collapsed VIF with all of its associated aging factors? One possibility which should be investigated is that inheriting aging components is beneficial in the short term (since many of them can be re-used and there is a cost to removing them from the cell); whereas it may be costly in the long term, since they are ultimately less fit. As in previous studies, comparing and contrasting the mechanisms utilized by different organisms toward similar goals will likely prove useful in future investigations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Triana Amen, Mark Kaganovich, and members of the Kaganovich lab for discussion, help, and comments on the manuscript. We apologize to any colleagues if we unintentionally missed their studies or were unable to mention them due to space limitations.
Funding
This work was supported by the European Research Council under the European Union's Seventh Framework Program (FP/2007-2013)/ERC-StG2013 337713 DarkSide starting grant, an Israel Science Foundation Grant ISF 843/11, a German Israel Foundation Grant GIFI-1201-242.13/2012 (D.K.); an Israel Health Ministry grant under the framework of E-Rare-2, a Niedersachsen-Israel Research Program grant, and a grant from the American Federation for Aging Research.
References
- Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 2011; 25:2248-53; PMID:22056670; http://dx.doi.org/10.1101/gad.173922.111
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663-76; http://dx.doi.org/10.1016/j.cell.2006.07.024
- Nystrom T, Liu B. The mystery of aging and rejuvenation–a budding topic. Curr Opin Microbiol 2014; 18:61-7; http://dx.doi.org/10.1016/j.mib.2014.02.003
- Lynch MD. Replicative aging in E. coli. Rejuvenation Res 2005; 8:79-81; PMID:15929714; http://dx.doi.org/10.1089/rej.2005.8.79
- Nystrom T. A bacterial kind of aging. PLoS Genet 2007; 3:e224; PMID:18085827; http://dx.doi.org/10.1371/journal.pgen.0030224
- Ogrodnik M, Salmonowicz H, Brown R, Turkowska J, Średniawa W, Pattabiraman S, Amen T, Abraham AC, Eichler N, Lyakhovetsky R, et al. Dynamic JUNQ inclusion bodies are asymmetrically inherited in mammalian cell lines through the asymmetric partitioning of vimentin. Proc Natl Acad Sci U S A 2014; 111:8049-54; PMID:24843142; http://dx.doi.org/10.1073/pnas.1324035111
- Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol 2011; 193:257-66; PMID:21502357; http://dx.doi.org/10.1083/jcb.201010131
- Katajisto P, Döhla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, Zoncu R, Chen W, Weinberg RA, Sabatini DM. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015 Apr 17;348(6232):340-3. doi: 10.1126/science.1260384. Epub 2015 Apr 2. PMID:25837514.
- Erjavec N, Nystrom T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2007; 104:10877-81; PMID:17581878; http://dx.doi.org/10.1073/pnas.0701634104
- Coelho M, Lade SJ, Alberti S, Gross T, Tolic IM. Fusion of protein aggregates facilitates asymmetric damage segregation. PLoS Biol 2014; 12:e1001886; PMID:24936793; http://dx.doi.org/10.1371/journal.pbio.1001886PBIOLOGY-D-13-04860
- Nystrom T. Aging: filtering out bad mitochondria. Curr Biol 2013; 23:R1037-9; PMID:24309277; http://dx.doi.org/10.1016/j.cub.2013.10.049
- Rujano MA, Bosveld F, Salomons FA, Dijk F, van Waarde MA, van der Want JJ, de Vos RA, Brunt ER, Sibon OC, Kampinga HH. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. Plos Biol 2006; 4:2325-35; PMID:17147470; http://dx.doi.org/10.1371/journal.pbio.0040417
- Knoblich JA. Asymmetric cell division during animal development. Nat Rev Mol Cell Biol 2001; 2:11-20; http://dx.doi.org/10.1038/3504808535048085
- Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol 2008; 9:355-66; PMID:18431399; http://dx.doi.org/10.1038/nrm2388
- Salzmann V, Chen C, Chiang CY, Tiyaboonchai A, Mayer M, Yamashita YM. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol Biol Cell 2014; 25:267-275; PMID:24227883; http://dx.doi.org/10.1091/mbc.E13-09-0541
- England JL. Statistical physics of self-replication. J Chem Phys 2013; 139:121923; PMID:24089735; http://dx.doi.org/10.1063/1.4818538
- Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol 2002; 20:682-8; PMID:12089552; http://dx.doi.org/10.1038/nbt0702-682
- Lindner AB, Madden R, Dernarez A, Stewart EJ, Taddei F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. P Natl Acad Sci U S A 2008; 105:3076-81; http://dx.doi.org/10.1073/pnas.0708931105
- Amen T, Kaganovich D. Dynamic droplets: the role of cytoplasmic inclusions in stress, function, and disease. Cell Mol Life Sci 2015; 72:401-15; PMID:25283146; http://dx.doi.org/10.1007/s00018-014-1740-y
- Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nyström T, et al. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell 2010; 140:257-67; PMID:20141839; http://dx.doi.org/10.1016/j.cell.2009.12.031
- Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature 2008; 454:728-U764; PMID:18660802; http://dx.doi.org/10.1038/Nature07212;
- Erjavec N, Larsson L, Grantham J, Nystrom T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev 2007; 21:2410-21; PMID:17908928; http://dx.doi.org/10.1101/gad.439307
- Fehrmann S, Paoletti C, Goulev Y, Ungureanu A, Aguilaniu H, Charvin G. Aging yeast cells undergo a sharp entry into senescence unrelated to the loss of mitochondrial membrane potential. Cell Rep 2013; 5:1589-99; PMID:24332850; http://dx.doi.org/10.1016/j.celrep.2013.11.013
- Longo VD, Shadel GS., Kaeberlein M, Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab 2012; 16:18-31; PMID:22768836; http://dx.doi.org/10.1016/j.cmet.2012.06.002
- Spokoini R, Moldavski O, Nahmias Y, England JL, Schuldiner M, Kaganovich D. Confinement to organelle-associated inclusion structures mediates asymmetric inheritance of aggregated protein in budding yeast. Cell Rep 2012; 2:738-47; PMID:23022486; http://dx.doi.org/10.1016/j.celrep.2012.08.024
- Lippuner AD, Julou T, Barral Y. Budding yeast as a model organism to study the effects of age. Fems Microbiol Rev 2014; 38:300-25; PMID:24484434; http://dx.doi.org/10.1111/1574-6976.12060
- Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell 2012; 23:3041-56; PMID:22718905; http://dx.doi.org/10.1091/mbc.E12-03-0194
- Hill SM, Hao XX, Liu BD, Nystrom T. Life-span extension by a metacaspase in the yeast Saccharomyces cerevisiae. Science 2014; 344:1389-92; PMID:24855027; http://dx.doi.org/10.1126/science.1252634
- Alberti S. Molecular mechanisms of spatial protein quality control. Prion 2012; 6:437-42; PMID:23051707; http://dx.doi.org/10.4161/pri.2247022470
- Kaeberlein M. Lessons on longevity from budding yeast (vol 464, pg 513, 2010). Nature 2010; 464:513-9; PMID:20336133; http://dx.doi.org/10.1038/Nature09046;
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles – A cause of aging in yeast. Cell 1997; 91: 1033-42; PMID:9428525; http://dx.doi.org/10.1016/S0092-8674(00)80493-6
- Spokoini R, Shamir M, Keness A, Kaganovich D. 4D imaging of protein aggregation in live cells. J Vis Exp 2013; PMID:23608881; http://dx.doi.org/10.3791/50083
- Nystrom T, Liu B. Protein quality control in time and space – links to cellular aging. FEMS Yeast Res 2013; http://dx.doi.org/10.1111/1567-1364.12095 1:40–8;
- Vevea JD, Swayne TC, Boldogh IR, Pon LA. Inheritance of the fittest mitochondria in yeast. Trends Cell Biol 2014; 24: 53-60; PMID:23932848; http://dx.doi.org/10.1016/j.tcb.2013.07.003
- Strandkvist C, Juul J, Bendtsen KM. Asymmetric segregation of damaged cellular components in spatially structured multicellular organisms. PLoS One 2014; 9: e87917; http://dx.doi.org/10.1371/journal.pone.0087917 PONE-D-13-36280 [pii]
- Volovik Y, Marques FC, Cohen E. The nematode Caenorhabditis elegans: a versatile model for the study of proteotoxicity and aging. Methods 2014; 68:458-64; PMID:24794346; http://dx.doi.org/10.1016/j.ymeth.2014.04.014
- Yamashita YM. The centrosome and asymmetric cell division. Prion 2009; 3:84-88.
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol 2005; 17:648-57; PMID:16243506; http://dx.doi.org/10.1016/j.ceb.2005.10.005
- Bufalino MR, DeVeale B, van der Kooy D. The asymmetric segregation of damaged proteins is stem cell-type dependent. J Cell Biol 2013; 201:523-30; PMID:23649805; http://dx.doi.org/10.1083/jcb.201207052
- Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 2014; 15:634-46; PMID:25237825; http://dx.doi.org/10.1038/nrm3877
- Liu BD, Larsson L, Franssens V, Hao X, Hill SM, Andersson V, Höglund D, Song J, Yang X, Öling D, et al. Segregation of Protein Aggregates Involves Actin and the Polarity Machinery. Cell 2011; 147:959-61; PMID:22118450; http://dx.doi.org/10.1016/j.cell.2011.11.018
- Clay L, Caudron F, Denoth-Lippuner A, Boettcher B, Buvelot Frei S, Snapp EL, Barral Y. A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. Elife 2014; 3:e01883; PMID:24843009; http://dx.doi.org/10.7554/eLife.01883
- Higuchi-Sanabria R, Pernice WM, Vevea JD, Alessi Wolken DM, Boldogh IR, Pon LA. Role of asymmetric cell division in lifespan control in Saccharomyces cerevisiae. FEMS Yeast Res 2014; 14:1133-46; PMID:25263578; http://dx.doi.org/10.1111/1567-1364.12216
- Henderson KA, Hughes AL, Gottschling DE. Mother-daughter asymmetry of pH underlies aging and rejuvenation in yeast. Elife 2014; 3:e03504; PMID:25190112; http://dx.doi.org/10.7554/eLife.03504.
- Denoth-Lippuner A, Krzyzanowski MK, Stober C, Barral Y. Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. Elife 2014; 3:E1; PMID:20651645; http://dx.doi.org/10.7554/eLife.03790;
- Khmelinskii A, Keller PJ, Lorenz H, Schiebel E, Knop M. Segregation of yeast nuclear pores. Nature 2010; 466:E1; http://dx.doi.org/10.1038/nature09255
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized wnt signal orients asymmetric stem cell division in vitro. Science 2013; 339:1445-8; PMID:23520113; http://dx.doi.org/10.1126/science.1231077
- Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 2011; 470:353-8; PMID:21331036; http://dx.doi.org/10.1038/nature09793
- Fuentealba LC, Eivers E, Geissert D, Taelman V, De Robertis EM. Asymmetric mitosis: unequal segregation of proteins destined for degradation. P Natl Acad Sci USA 2008; 105: 7732-7; http://dx.doi.org/10.1073/pnas.0803027105
- McFaline-Figueroa JR, Vevea J, Swayne TC, Zhou C, Liu C, Leung G, Boldogh IR, Pon LA. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell 2011; 10:885-95; PMID:21726403; http://dx.doi.org/10.1111/j.1474-9726.2011.00731.x
- Bufalino MR, van der Kooy D. The aggregation and inheritance of damaged proteins determines cell fate during mitosis. Cell Cycle 2014; 13:1201-7; PMID:24553116; http://dx.doi.org/10.4161/cc.28106
- Neumuller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 2009; 23:2675-99; PMID:19952104; http://dx.doi.org/10.1101/gad.1850809
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009; 324:1729-32; PMID:19460965; http://dx.doi.org/10.1126/science.1172046
- Hyman AA, Weber CA, Julicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 2014; 30:39-58; PMID:25288112; http://dx.doi.org/10.1146/annurev-cellbio-100913-013325
- Cooley J, Whitaker S, Sweeney S, Fraser S, Davidson B. Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nat Cell Biol 2011; 13:952-U176; PMID:21785423; http://dx.doi.org/10.1038/ncb2291
- Bornens M. Organelle positioning and cell polarity. Nat Rev Mol Cell Bio 2008; 9:874-886; http://dx.doi.org/10.1038/Nrm2524
- Yamashita YM. Regulation of asymmetric stem cell division: spindle orientation and the centrosome. Front Biosci 2009; 14:3003-11; http://dx.doi.org/10.2741/4030
- Winey M, O'Toole ET. The spindle cycle in budding yeast. Nat Cell Biol 2001; 3:E23-7; PMID:11146646; http://dx.doi.org/10.1038/35050663
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 2003; 299:1751-3; PMID:12610228; http://dx.doi.org/10.1126/science.1080418
- Erjavec N, Nystrom T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. P Natl Acad Sci USA 2007; 104:10877-81; http://dx.doi.org/10.1073/pnas.0701634104
- Song J, Yang Q, Yang J, Larsson L, Hao X, Zhu X, Malmgren-Hill S, Cvijovic M, Fernandez-Rodriguez J, Grantham J, et al. Essential genetic interactors of SIR2 required for spatial sequestration and asymmetrical inheritance of protein aggregates. Plos Genet 2014; 10:e1004539; http://dx.doi.org/10.1371/journal.pgen.1004539
- Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, Yates JR 3rd, Manning G, Dillin A. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science 2014; 346:360-3; PMID:25324391; http://dx.doi.org/10.1126/science.1253168
- Cohen E. Aging, protein aggregation, chaperones, and neurodegenerative disorders: mechanisms of coupling and therapeutic opportunities. Rambam Maimonides Med J 2012; 3:e0021; http://dx.doi.org/10.5041/RMMJ.10088rmmj-3-4-e0021
- Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A 2009; 106:14914-9; PMID:19706382; http://dx.doi.org/10.1073/pnas.0902882106
- Kirstein-Miles J, Morimoto RI. Caenorhabditis elegans as a model system to study intercompartmental proteostasis: interrelation of mitochondrial function, longevity, and neurodegenerative diseases. Dev Dynam 2010; 239:1529-38; PMID:20419784; http://dx.doi.org/10.1002/dvdy.22292
- Kasza KE, Broedersz CP, Koenderink GH, Lin YC, Messner W, Millman EA, Nakamura F, Stossel TP, Mackintosh FC, Weitz DA. Actin Filament Length Tunes Elasticity of Flexibly Cross-Linked Actin Networks. Biophys J 2010; 99:1091-100; PMID:20712992; http://dx.doi.org/10.1016/j.bpj.2010.06.025
- Nishio K, Inoue A, Qiao SL, Kondo H, Mimura A. Senescence and cytoskeleton: overproduction of vimentin induces senescent-like morphology in human fibroblasts. Histochem Cell Biol 2001; 116:321-7; PMID:11702190; http://dx.doi.org/10.1007/s004180100325
- Tolstonog GV, Shoeman RL, Traub U, Traub P. Role of the intermediate filament protein vimentin in delaying senescence and in the spontaneous immortalization of mouse embryo fibroblasts. DNA Cell Biol 2001; 20:509-29; PMID:11747604; http://dx.doi.org/10.1089/104454901317094945
- Matveeva EA, Chernoivanenko IS, Minin AA. Vimentin intermediate filaments protect mitochondria from oxidative stress. Biol Membrany 2010; 27:471-81.
- Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature 2008; 454:1088-95; PMID:18756251; http://dx.doi.org/10.1038/nature07195
- England JL, Kaganovich D. Polyglutamine shows a urea-like affinity for unfolded cytosolic protein. Febs Lett 2011; 585:381-4; PMID:21176779; http://dx.doi.org/10.1016/j.febslet.2010.12.023
- Betting J, Seufert W. A yeast Ubc9 mutant protein with temperature-sensitive in vivo function is subject to conditional proteolysis by a ubiquitin- and proteasome-dependent pathway. J Biol Chem 1996; 271:25790-6; PMID:8824207; http://dx.doi.org/10.1074/jbc.271.42.25790
- Gupta R, Kasturi P, Bracher A, Loew C, Zheng M, Villella A, Garza D, Hartl FU, Raychaudhuri S. Firefly luciferase mutants as sensors of proteome stress. Nature Methods 2011; 8:879-U155; PMID:21892152; http://dx.doi.org/10.1038/nmeth.1697
- Tsuru A, Nakamura N, Takayama E, Suzuki Y, Hirayoshi K, Nagata K. Regulation of the expression of vimentin gene during the differentiation of mouse myeloid-leukemia cells. J Cell Biol 1990; 110:1655-64; PMID:1970825; http://dx.doi.org/10.1083/jcb.110.5.1655
- Honke K, Wada Y. Regulation of vimentin expression and protease-mediated vimentin degradation during differentiation of human monocytic leukemia cells. Jap J Cancer Res 1997; 88:484-91; PMID:9247605; http://dx.doi.org/10.1111/j.1349-7006.1997.tb00407.x
- Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol 2005; 15:608-17; PMID:16202602; http://dx.doi.org/10.1016/j.tcb.2005.09.004
- Styers ML, Salazar G, Love R, Peden AA, Kowalczyk AP, Faundez V. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell 2004; 15:5369-82; PMID:15456899; http://dx.doi.org/10.1091/mbc.E04-03-0272
- Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 2007; 313:2050-62; PMID:17512929; http://dx.doi.org/10.1016/j.yexcr.2007.03.040
- Strandkvist C, Juul J, Bendtsen KM. Asymmetric segregation of damaged cellular components in spatially structured multicellular organisms. PLoS One 2014; 9:e87917; PMID:24551071; http://dx.doi.org/10.1371/journal.pone.0087917
- Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 2002; 7:91-7; PMID:11895474; http://dx.doi.org/10.1046/j.1356-9597.2001.00504.x
- Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol 1998; 18:6325-39; PMID:9774649
- Robert A, Herrmann H, Davidson MW, Gelfand VI. Microtubule-dependent transport of vimentin filament precursors is regulated by actin and by the concerted action of Rho- and p21-activated kinases. Faseb J 2014; 28:2879-90; PMID:24652946; http://dx.doi.org/10.1096/fj.14-250019
