Abstract
SNX27 is a member of the sorting nexin family that plays an important role in the recycling of receptors from endosomes to the cell surface. In addition to a PX (Phox homology) domain that regulates its endosomal localization, SNX27 has a unique PDZ (Psd-95/Dlg/ZO1) domain and an atypical FERM (4.1, ezrin, radixin, moesin) domain that both function to bind short peptide sequence motifs in the cytoplasmic domains of the cargo receptors. Using the T cell immune synapse (IS) as a model for polarized protein recycling, we recently identified an additional mechanism that enhances SNX27 localization to the endosomal recycling compartment (ERC). Our study defined a phosphoinositide (PI) lipid-binding site within the SNX27 FERM domain, with a clear preference for bi- and triphosphorylated PIs, which may promote SNX27 localization to phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) and/or PtdIns(3,4,5)P3-enriched membrane domains. Using fluorescently tagged lipid-binding probes, we studied the kinetics of distinct PIs in living T cells during IS formation. Our results suggest that PtdIns(3,4,5)P3 accumulates at the contact site simultaneously with early SNX27 recruitment to the plasma membrane (PM), and this is partly controlled by by lipid binding through the FERM domain. These studies define 2 independent binding sites for PtdIns-derived lipids in SNX27, that contribute to the dynamic recruitment of SNX27 to distinct membranes during T cell activation.
Introduction
Sorting nexin (SNX) proteins are a family of molecules that regulate protein sorting and trafficking. All members of the SNX family contain a phox homology (PX) domain that mediates membrane recruitment, most commonly binding to PtdIns3P (phosphatidylinositol-3-phosphate)-enriched endosomal membranes. SNX27 is a unique SNX, in that it has a PDZ (Psd-95/Dlg/ZO1) domain that mediates sorting from endosomes to the plasma membrane (PM) of proteins that bear a specific PDZ-binding motif (PDZ-bm). Proteins with a PDZ domain are often found in the postsynaptic density of neuronal synapses, where SNX27 participates specifically in glutamate and β-adrenoreceptor trafficking.Citation1–3 In other polarized models such as activated T cells, the PDZ domain mediates SNX27 interaction with diacylglycerol kinase zeta (DGKζ), a negative regulator of T cell activation that attenuates diacylglycerol (DAG)-mediated signals by catalyzing DAG conversion into phosphatidic acid (PA).Citation4 In addition, SNX27 binds ubiquitously expressed transmembrane cargos such as glucose and metal ion transporters that have a class I PDZ binding motif (PDZ-bm).Citation5
With SNX17 and SNX31, SNX27 is classified as a SNX-FERM protein. These proteins have an atypical FERM domain (4.1, ezrin, radixin, moesin) responsible for binding cargos bearing a Phe-Xaa-Asn-Pro-Xaa-Tyr (FxNPxY) sequence for recycling.Citation6 In vitro and in vivo studies have determined a role for SNX17 and SNX31 in β-integrin recycling to prevent their lysosomal degradation.Citation7–10 Although in peptide array experiments, SNX27 bound a large variety of transmembrane proteins harboring FxNPxY sequences,Citation9 in cells, no specific cargos have yet been reported to interact with this protein via its FxNPxY-binding site. To date SNX27 appears to contribute to cargo recycling primarily through PDZ-dependent mechanisms.Citation5 In addition to cargo recognition, the SNX27 PDZ domain mediates interaction with the vacuolar sorting 26A (VPS26A) retromer subunit.Citation5,11 As a result, SNX27 mediates endosomal recycling together with the retromer protein assembly, and the actin remodeling Wiskott-Aldrich syndrome and SCAR-homolog (WASH)-containing multiprotein complex called the WASH Regulatory Complex (SHRC).
Proteins possessing PDZ and FERM modules with PI-binding characteristics have been reported (Reviewed in Balla et al.Citation12). In our recent publication, we examined the lipid-binding ability of full-length SNX27, and defined a new PtdIns-binding site at the FERM domain with a definite preference for bi- and triphosphorylated PIs. Homology modeling and sequence alignment of the SNX27-FERM domain F3 module revealed a basic patch constituted by positively charged amino acids that were absent in SNX17 and SNX31. The interaction was confirmed through biophysical, mutagenesis and modeling approaches.Citation13
The SNX27 FERM domain PI binding site is entirely distinct from those found in other FERM-domains that recognize PtdIns phosphorylated forms, such as those present in talin or radixin. The PI binding pocket in SNX27 is close to the FxNPxY cargo recognition site, suggesting that cargo and lipid binding may be allosterically linked or favor coincidence detection of specific membrane sites. The discovery of distinct binding of SNX27 to negatively charged phosphoinositides via its FERM domain suggests a potential mechanism for SNX27 distribution to membrane domains independently of the classical interaction of the PX domain with PtdIns3P-enriched endosomes.
Static, Dynamic and Polarized Models for Vesicle Trafficking
Localization and dynamic studies of vesicular proteins are generally conducted in cell lines whose large, flat cell morphologies facilitate microscopy techniques. COS-7, HeLa and RPE1 cells have been used to address the precise SNX27 localization to distinct vesicular compartments and to identify cargos and their PDZ-mediated stabilizationCitation3,5,14; Our identification of a lipid binding property of the SNX27-FERM domain raised questions regarding its possible contribution to SNX27 localization. Based on our results in HeLa cells, the FERM-located PtdIns-binding site is not necessary for SNX27 accumulation at the endoplasmic recycling compartment (ERC), a property dependent exclusively on the PX domain.Citation13 This coincides with results from Tseng et al., who compared SNX27 vesicle-binding abilities to those of other SNX-FERM proteins, and suggested an additional lipid-binding site in SNX27 that was absent in both SNX17 and SNX31.Citation8 The relevance of this alternative lipid binding site for SNX27 endosomal localization was only apparent in HeLa cells treated with the PI3K inhibitor wortmannin, or when the PX domain was mutated.Citation8
Jurkat T cells in contact with SEE-loaded Raji B cells are widely used models of polarized membrane trafficking, in which continuous recycling is sustained by receptor-triggered signals. When T lymphocytes encounter antigen-presenting cells (APC), activation of the T cell receptor (TCR) triggers a complex rearrangement of molecules at the T cell-APC contact area termed the immune synapse (IS).Citation15,16 Although first applied to T and B cells of the adaptive immune system, the concept of the IS has expanded to cells of the innate immune system such as NK cell or phagocytes.Citation17,18 During IS formation, the cytoskeleton is remodeled and T cells reorient the microtubule-organizing center (MTOC), the Golgi apparatus, and endosomal compartments toward the contact site.Citation19–22 Lipid composition is also altered to allow the signaling, adhesion, and membrane trafficking adjustments needed for conversion to an activated state.Citation23,24 The IS thus presents a unique and powerful model for studying the role and dynamics of different molecules in activation-induced polarized recycling.Citation25
SNX27 displays PX-dependent endosomal localization that was positive for early endosomes (EE) and ERC in resting T lymphocytes. During IS assembly, we observed polarization of SNX27 enriched endosomal compartments and dynamic partitioning of SNX27 between the ERC and the plasma membrane (PM).Citation4 In contrast to what was observed in HeLa cells, endosomal localization of the SNX27-FERM PI-binding mutant was markedly altered in the Jurkat model. Diminished vesicle association was evident in basal conditions, and this phenotype was more pronounced in Jurkat cells challenged by superantigen-loaded APC. This suggests additional control mechanisms for SNX27 localization during polarized traffic that involves lipid binding through the FERM domain.
If we combine the results in both cell models, we can conclude that an additional lipid anchor in SNX27 is advantageous to its maintenance at the ERC when membrane lipid compositions are altered, whether induced by inhibitor treatment or by transition to an activated state. In the case of APC-stimulated Jurkat T cells we also observed marked alterations in the partitioning of the SNX27-FERM mutant between different membrane domains. Although initial recruitment of the mutant to the IS was similar compared to that of wild type SNX27, deficiency in lipid binding by the FERM domain impaired the subsequent retrieval of the mutant into endosomal compartments following IS maturation. These results correlate with previous studies showing that PM localization of SNX27 during IS formation is absolutely PDZ domain-dependent,Citation4 and suggest that this additional lipid binding site with a preference for the bi- and triphosphorylated PtdIns derivatives known to be involved in endocytosis,Citation13 favors SNX27 recycling.
There are other biologically relevant models of polarized recycling in which the role of SNX27 has not yet been explored. In migrating cells, signaling and trafficking are coordinated to direct recycling toward the leading cell edge. In metastatic cancer cells, invadopodium structures are sites at which membranes undergo rapid recycling to promote increased secretion, matrix degradation and overall aggressive behavior.Citation26 Given the molecular similarities of the IS and invadopodia (), some of the findings in the IS model might be usefully applied to other systems. Recent studies suggest that both structures are similar and both are exosome-targeting sites that might be determined by the same fundamental molecular combination of actin, microtubules and integrinsCitation27,28; other studies years ago highlighted significant parallels between the mechanisms that regulate the formation of IS and of podosomes, a invadopodium-like structure.Citation29 Similar reasoning led Griffiths et al. to state that the IS bears a resemblance to other structures in which an area of the PM becomes a focal zone for endo- and exocytosis, for example during cilia formation and cytokinesis.Citation30
Phosphoinositide Dynamics During IS Formation
The analysis of SNX27 requirements for subcellular distribution in different cell models suggests that additional lipid interaction mechanisms are needed for adequate SNX27 organelle distribution during polarized responses. The additional lipid-binding site at the FERM domain showed a clear preference for bi- and triphosphorylated PI such as PtdIns(4,5)P2 and PtdIns(3,4,5)P3. We next analyzed the contribution of these lipids to SNX27 recruitment during IS formation by investigating lipid dynamics using fluorescent probes for these lipids to track their subcellular distribution in living T cells. PI3K mediated generation of local PtdIns(3,4,5)P3 at the PM is one of the earliest signals observed in IS formation.Citation31 Transfection of Jurkat T cells with the PtdIns(3,4,5)P3-binding AKT pleckstrin homology (PH)-domain allowed us to distinguish between initial and mature stages of IS formation (). We confirmed PtdIns(3,4,5)P3 accumulation at the T cell-APC contact area during early IS formation; at later times, the AKT-PH domain translocated to distal and peripheral areas that correspond to annular PtdIns(3,4,5)P3 accumulation, which controls actin architecture and thus facilitates cell adhesion and polarized secretion.Citation32 Cotransfection of the PtdIns(3,4,5)P3 sensor with wild type or the PtdIns-binding SNX27 mutant revealed impaired mutant protein colocalization with PtdIns(3,4,5)P3 during initial recruitment. Our result suggests that the very early SNX27 translocation to the PM is enhanced by FERM domain binding to lipids, a mechanism reminiscent of that of other crucial signaling molecules at the ERC. For example, initial recruitment of the membrane adaptor protein LAT (linker for activation of T cells) is mediated by cholesterol-sphingolipid raft domains at the IS.Citation33 Although we did not study this question further, it is intriguing that SNX27 was also identified as a protein with a putative CRAC (cholesterol recognition amino acid consensus) motif on its PDZ domain,Citation34 as these motifs can also mediate membrane localization.
Figure 1. Molecular similarities between the immune synapse and invadopodia. Scheme of immune synapse (IS) and invadopodium structures, showing a central core containing functional molecules specific for each model (TCR or MMP, respectively), surrounded by an integrin-enriched adhesive ring (adapted from Wenimont et al.Citation29). Both are pictured as active sites of actin polymerization and constitute focal zones for endo- and exocytosis (not shown). Top view (A); front view (B).
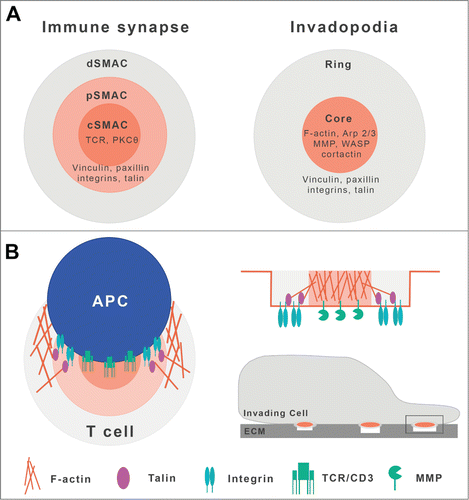
Figure 2. SNX27 and phophoinositide lipid dynamics during immune synapse formation. (A) Scheme of lipid dynamics and SNX27 partitioning during IS formation. In basal conditions, SNX27 localizes to the PtdIns3P-enriched ERC. Initial contact of the Jurkat T cell with antigen-loaded APC triggers SNX27 accumulation at the T cell-APC contact area simultaneously with PtdIns(3,4,5)P3 production from PtdIns(4,5)P2. Formation of a mature supramolecular adhesion complex (SMAC) comprises lipid segregation to central, peripheral and distal SMAC, maintaining focal points of adhesion and endo/exocytosis by recruitment of lipid-binding proteins. (B) Summary of our proposed models for SNX27 FERM and PDZ mutant behavior during IS formation. The structure of GFP-tagged constructs used is shown, and their partitioning is represented in green. For details, see text.
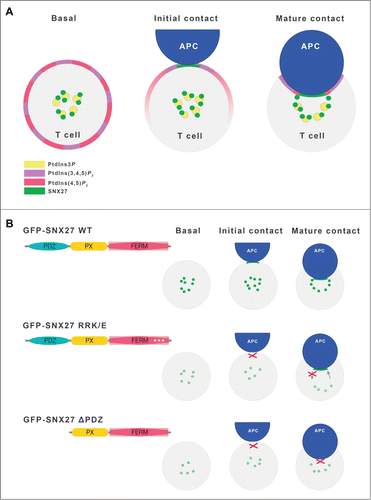
Together with its phosphorylated product PtdIns(3,4,5)P3, PtdIns(4,5)P2 is also widely recognized as a regulator of polarized trafficking. The spatiotemporal generation and accumulation of this lipid is precisely modulated to activate effectors or signaling molecules. In stimulated T cells, PtdIns(4,5)P2 is converted to PtdIns(3,4,5)P3 by PI3K action, or is hydrolyzed by phospholipase C (PLC) to produce inositol 1,4,5-triphosphate (Ins(1,4,5)P3) and DAG. As a result, PtdIns(4,5)P2 levels decrease substantially following APC contact, and transfection of the PLCδ PtdIns(4,5)P2-binding PH domain did not allow clear analysis of PtdIns(4,5)P2 dynamics in activated T cells. Rigorous studies have nonetheless determined that local PtdIns(4,5)P2 concentrations are sufficient to recruit endocytic machinery proteins such as AP-2 (adaptor protein complex 2), which regulates vesicle sorting to intracellular sitesCitation35; a similar mechanism is observed in neuronal and immunological synapses.Citation35–37 Studies in cytotoxic T lymphocytes have shown that proteins involved in polarized secretion use lipid binding domains to translocate to PtdIns(4,5)P2-enriched membrane domains for endocytosis.Citation35 The correct accumulation of the SNX27 PI-binding mutant at the contact membrane during IS formation and the increased fluorescence loss at the ERC during IS maturation, suggest that PtdIns(4,5)P2 recognition by the SNX27 FERM domain helps to promote SNX27 localization to active endocytosis sites at the T cell-APC contact area.
As the IS is a focal point not only for endocytosis, but also for exocytosis, we should consider that vesicle fusion to the PM could account for some SNX27 accumulation at the synapse. To track the dynamics of ERC enriched PtdIns3P, we transfected Jurkat T cells with a sensor composed of 2 tandem FYVE (Fab1p, YOTB, Vac1, EEA1) domains fused to a fluorescent protein (Cherry-FYVE). APC-challenged T cells did not accumulate PtdIns3P at the synapse; when T cells were cotransfected with Cherry-FYVE and the SNX27 constructs, PtdIns3P-enriched vesicles accumulated at discrete locations that delimited the SNX27-positive compartment, often at 2 foci at the synapse periphery. This once again confirmed that PDZ binding to an as yet uncharacterized protein(s) and/or PtdIns is the main driver of SNX27 accumulation at the IS.
Concluding Remarks
Our findings reveal another unique property of SNX27 and demonstrate that, in addition to PtdIns3P recognition, binding to higher phosphorylated PI forms may contribute to SNX27 functions in cargo sorting, in particular at polarized sites of endocytic recycling. The study of polarized trafficking during IS formation suggest a model in which the PtdIns binding region at the FERM domain is needed for the rapid, initial SNX27 accumulation at the PM, and also promotes SNX27 endocytosis back to the ERC. These data coincide with our earlier work showing that the PDZ domain is responsible for subsequent IS accumulation (). The identity of the specific cargo(s) that mediate SNX27 recruitment to the IS remains unknown. Videomicroscopy studies of living T cells suggest that DGKζ translocation kinetics do not mirror those of SNX27 during IS formation, which implies that this SNX27-binding lipid kinase is not responsible for specific SNX27 recruitment to the T cell-APC contact area. Attenuation of SNX27 expression nonetheless mirrors the effect of DGKζ silencing on Ras activation during T cell activation, suggesting that SNX27 interaction helps to regulate DGKζ-dependent DAG regulationCitation4 SNX27 recognition of lipids and lipid modifying enzymes might thus contribute to vesicle trafficking by altering the biophysical properties of membranes.
Besides providing a better understanding of the IS, the study of SNX27 dynamics and function in this model offers interesting possibilities to extrapolate some findings to models of polarized trafficking. From the neurological synapse to invadopodium formation, all share fundamental structures necessary for correct rearrangement of actin, microtubules, adhesion molecules and lipids that facilitate vesicle trafficking. SNX27 is proving to be a critical recycling protein in the nervous system, and its down-regulation is associated with pathological conditions characteristic of Alzheimer disease or Down syndrome.Citation2,38 A comprehensive study of SNX27 function at the IS, including PDZ-binding cargos, may thus help elucidate the role of SNX27 in the immune and other cellular systems.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
I.M. is supported in part by grants from the Spanish Ministry of Economy and Competitivity BFU2013–47640-P; Spanish Ministry of Health (Instituto de Salud Carlos III; RD12/0036/0059) and the Madrid regional government (IMMUNOTHERCAM Consortium S2010/BMD-2326). M.T. receives an FPI fellowship from the Spanish Ministry of Economy and Competitivity. B.C. is supported by project funding and a Career Development Fellowship from the National Health and Medical Research Council (NHMRC) of Australia (APP1058734; APP1061574).
References
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 2011 13, 715-721; PMID:21602791; http://dx.doi.org/10.1038/ncb2252.
- Wang X, Zhao Y, Zhang X, Badie H, Zhou Y, Mu Y, Loo LS, Cai L, Thompson RC, Yang B, et al. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down syndrome. Nat Med 2013 19, 473-480; PMID:23524343; http://dx.doi.org/10.1038/nm.3117.
- Nakagawa T, Asahi M. beta1-adrenergic receptor recycles via a membranous organelle, recycling endosome, by binding with sorting nexin27. J Membr Biol 2013 246, 571-579; PMID:23780416; http://dx.doi.org/10.1007/s00232-013-9571-6.
- Rincon E, Sáez de Guinoa J, Gharbi SI, Sorzano CO, Carrasco YR, Mérida I. Translocation dynamics of sorting nexin 27 in activated T cells. J Cell Sci 2011 124, 776-788; PMID:21303929; http://dx.doi.org/10.1242/jcs.072447.
- Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 2013 15, 461-471; PMID:23563491; http://dx.doi.org/10.1038/ncb2721.
- Ghai R, Collins B. M. PX-FERM proteins: A link between endosomal trafficking and signaling? Small GTPases 2011 2, 259-263; PMID:22292128; http://dx.doi.org/10.4161/sgtp.2.5.17276.
- Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol 2012 197, 219-230; PMID:22492727; http://dx.doi.org/10.1083/jcb.201111121.
- Tseng HY, Thorausch N, Ziegler T, Meves A, Fässler R, Böttcher RT. Sorting nexin 31 binds multiple β integrin cytoplasmic domains and regulates beta1 integrin surface levels and stability. J Mol Biol 2014 426, 3180-3194; PMID:25020227; http://dx.doi.org/10.1016/j.jmb.2014.07.003.
- Ghai, R., et al., Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci U S A, 2013. 110(8): p. E643-52.
- Bottcher RT, Stremmel C, Meves A, Meyer H, Widmaier M, Tseng HY, Fässler R. Sorting nexin 17 prevents lysosomal degradation of beta1 integrins by binding to the beta1-integrin tail. Nat Cell Biol 2012 14, 584-592; PMID:22561348; http://dx.doi.org/10.1038/ncb2501.
- Gallon M, Clairfeuille T, Steinberg F, Mas C, Ghai R, Sessions RB, Teasdale RD, Collins BM, Cullen PJ. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc Natl Acad Sci U S A 2014 111, E3604-3613; PMID:25136126; http://dx.doi.org/10.1073/pnas.1410552111.
- Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J Cell Sci 2005 118, 2093-2104; PMID:15890985; http://dx.doi.org/10.1242/jcs.02387.
- Ghai R, Tello-Lafoz M, Norwood SJ, Yang Z, Clairfeuille T, Teasdale RD, Mérida I, Collins BM. Phosphoinositide binding by the SNX27 FERM domain regulates localisation at the immune synapse of activated T-cells. J Cell Sci 2015; 128:553-565; http://dx.doi.org/10.1242/jcs.158204.
- Hayashi H, Naoi S, Nakagawa T, Nishikawa T, Fukuda H, Imajoh-Ohmi S, Kondo A, Kubo K, Yabuki T, Hattori A, et al. Sorting nexin 27 interacts with multidrug resistance-associated protein 4 (MRP4) and mediates internalization of MRP4. J Biol Chem 2012 287, 15054-15065; PMID:22411990; http://dx.doi.org/10.1074/jbc.M111.337931.
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science 1999 285, 221-227; PMID:10398592; http://dx.doi.org/10.1126/science.285.5425.221.
- Dustin ML, Colman DR. Neural and immunological synaptic relations. Science 2002 298, 785-789; PMID:12399580; http://dx.doi.org/10.1126/science.1076386.
- Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature 2001 411, 489-494; PMID:11373683; http://dx.doi.org/10.1038/35078099.
- Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A 1999 96, 15062-15067; PMID:10611338; http://dx.doi.org/10.1073/pnas.96.26.15062.
- Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med 1989 170, 1697-1713; PMID:2530300; http://dx.doi.org/10.1084/jem.170.5.1697.
- Angus KL, Griffiths GM. Cell polarisation and the immunological synapse. Curr Opin Cell Biol 2013 25, 85-91; PMID:22990072; http://dx.doi.org/10.1016/j.ceb.2012.08.013.
- Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 2004 20, 577-588; PMID:15142526; http://dx.doi.org/10.1016/S1074-7613(04)00106-2.
- Alcover A, Thoulouze MI. Vesicle traffic to the immunological synapse: a multifunctional process targeted by lymphotropic viruses. Curr Top Microbiol Immunol 2010 340, 191-207; PMID:19960315; http://dx.doi.org/10.1007/978-3-642-03858-7_10.
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristán C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol 2010 28, 79-105; PMID:19968559; http://dx.doi.org/10.1146/annurev-immunol-030409-101308.
- Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol 2009 10, 627-635; PMID:19430478; http://dx.doi.org/10.1038/ni.1734.
- Martin-Cofreces NB, Baixauli F, Sanchez-Madrid F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol 2014 24, 61-72; PMID:24119664; http://dx.doi.org/10.1016/j.tcb.2013.09.005.
- Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011 12, 413-426; PMID:21697900; http://dx.doi.org/10.1038/nrm3141.
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 2013 5, 1159-1168; PMID:24290760; http://dx.doi.org/10.1016/j.celrep.2013.10.050.
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011 2, 282; PMID:21505438; http://dx.doi.org/10.1038/ncomms1285.
- Wernimont SA, Cortesio CL, Simonson WT, Huttenlocher A. Adhesions ring: a structural comparison between podosomes and the immune synapse. Eur J Cell Biol 2008 87, 507-515; PMID:18343530; http://dx.doi.org/10.1016/j.ejcb.2008.01.011.
- Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol 2010 189, 399-406; PMID:20439993; http://dx.doi.org/10.1083/jcb.201002027.
- Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol 2002 3, 1082-1089; PMID:12389042; http://dx.doi.org/10.1038/ni848.
- Le Floc'h A, Tanaka Y, Bantilan NS, Voisinne G, Altan-Bonnet G, Fukui Y, Huse M. Annular PtdIns(3,4,5)P3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J Exp Med 2013 210, 2721-2737; PMID:24190432; http://dx.doi.org/10.1084/jem.20131324.
- Bonello G, Blanchard N, Montoya MC, Aguado E, Langlet C, He HT, Nunez-Cruz S, Malissen M, Sanchez-Madrid F, Olive D, et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J Cell Sci 2004 117, 1009-1016; PMID:14996932; http://dx.doi.org/10.1242/jcs.00968.
- Sheng R, Chen Y, Yung Gee H, Stec E, Melowic HR, Blatner NR, Tun MP, Kim Y, Källberg M, Fujiwara TK, et al. Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins. Nat Commun 2012 3, 1249; PMID:23212378; http://dx.doi.org/10.1038/ncomms2221.
- Capuano C, Paolini R, Molfetta R, Frati L, Santoni A, Galandrini R. PtdIns(4,5)P2-dependent regulation of Munc13-4 endocytic recycling: impact on the cytolytic secretory pathway. Blood 2012 119, 2252-2262; PMID:22271450; http://dx.doi.org/10.1182/blood-2010-12-324160.
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol 2003 162, 113-124; PMID:12847086; http://dx.doi.org/10.1083/jcb.200301006.
- Koch M, Holt M. Coupling exo- and endocytosis: an essential role for PIP(2) at the synapse. Biochim Biophys Acta 2012 1821, 1114-1132; PMID:22387937; http://dx.doi.org/10.1016/j.bbalip.2012.02.008.
- Wang X., Huang T, Zhao Y, Zheng Q, Thompson RC, Bu G, Zhang YW, Hong W, Xu H. Sorting Nexin 27 Regulates Abeta Production through Modulating gamma-Secretase Activity. Cell Rep 2014 9, 1023-1033; PMID:25437557; http://dx.doi.org/10.1016/j.celrep.2014.09.037.
