ABSTRACT
The mammalian nuclear envelope (NE) can develop complex dynamic membrane-bounded invaginations in response to both physiological and pathological stimuli. Since the formation of these nucleoplasmic reticulum (NR) structures can occur during interphase, without mitotic NE breakdown and reassembly, some other mechanism must drive their development. Here we consider models for deformation of the interphase NE, together with the evidence for their potential roles in NR formation.
Nucleoplasmic reticulum, a widespread organelle
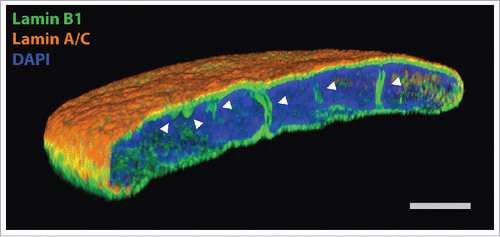
The nuclear envelope (NE) is a unique structure forming a physical barrier between the nucleoplasm and the cytoplasm. It is comprised of 2 phospholipid bilayers, the inner nuclear membrane (INM) and outer nuclear membrane (ONM), with an intervening luminal space between (for a review, see ref. Citation1). Underlying the INM is the nuclear lamina, a proteinaceous meshwork of intermediate filament proteins. It is well established that the structure of the nucleus is more complex than just a membrane-bound spheroid containing chromatin, and pierced by nuclear pore complexes (NPC). Nuclei vary in shape not only in different cell types, but also under different pathological and physiological conditions.Citation2 The NE frequently shows multiple invaginations of the nuclear membrane into the nucleus, forming an often elaborate network of tubules and sheets of INM, and sometimes ONM, continuous with the NE (See ). This feature is termed a nucleoplasmic reticulum (NR), so named for its structural resemblance to the endoplasmic reticulum.Citation3,4 The NR is a widespread feature of many cells and tissues under normal cellular conditions.Citation5-8 In addition, it is also observed in cells grown in 2D and 3D cultures, including many tumor cell types, for example breast, brain, bladder, kidney, ovary, and prostate.Citation9 Moreover, NR abundance is altered in various pathologies such as cancer,Citation10,11 Alzheimer's disease,Citation12 myotonic dystrophy,Citation13 Hutchinson-Gilford Progeria Syndrome,Citation14 and others, suggesting a dysregulation of mechanisms responsible for NR regulation under pathological conditions.
NR structure
NR structures are classified into 2 main classes, type I and type II.Citation3 Type I invaginations are those where the INM alone invaginates into the nucleoplasm, whereas type II involves invagination of both the inner and outer nuclear membranes, hence type II NR contains a cytoplasmic core. Moreover, in the cytoplasmic core of type II NR microtubules, microfilaments, and mitochondria have all been detected.Citation15,16 The NR structure can be more complex though, with type I invaginations branching off type II, both as membrane sheets and as tubules. In addition, these complex invaginations may traverse the nucleus, forming cross-nuclear channels.
NR function
Despite the fact that NR is a widely spread organelle, present in multiple cell types, its exact function is still not fully understood. New reports, however, keep emerging, shedding more light on its role. The NR is thought to provide a structural support for the nucleus, as well as to bring functions of the peripheral NE deep into the interior of nucleus and aid in nuclear import-export due to presence of NPC at the invaginations.Citation17 NR has also been shown to aid in cellular processes such as transcription, DNA repair and lipid metabolism.
Probably the best studied role of the NR is the calcium signaling inside the nucleus. Endoplasmic reticulum, the main reservoir of calcium ions in a cell shares its lumen with the nuclear double membrane, thus it is also continuous with the intervening luminal space of the NR channels. Indeed, it has been shown that calcium can be released from the NE store into the nucleoplasm through channels sensitive to inositol triphosphate (IP3).Citation18,19 Interestingly, both PIP2 and phospholipase C (PLC), required for production of IP3, are also present in the nucleus.Citation20,21 While PLC can associate with the nuclear membrane,Citation22 the nuclear PIP2 was suggested to reside within the nuclear membrane forming NR invaginations.Citation23 Moreover, it has been reported that NR invaginations contain inositol triphosphate receptors (IP3R)Citation4 as well as ryanodine receptors,Citation24 which are involved in a selective calcium release into the nucleus, therefore the NR allows for controlled and spatially localized calcium signaling in nuclear functions,Citation25 including transcription regulation.Citation26 In addition, it has been proposed that presence of NR allows for not only initiation of localized nuclear calcium signaling, but also for its termination due to presence of IP3-kinase isoform B (IP3KB) at nuclear invaginationsCitation27 which can inactivate IP3Citation28,29 as well as sarco/endoplasmic reticulum calcium ATPase (SERCA).Citation30 Moreover, it has been suggested that NR identified in plant cells has a similar role in regulation of nuclear calcium signaling,Citation31,32 hence implying a conserved role for this structure between the two kingdoms.
It has been widely observed that NR invaginations often associate with nucleoliCitation5,33,34 and are found in close proximity to fibrillarin-positive regions or point toward UBF-1-positive nucleolar compartments.Citation35 These are sites of active transcription of ribosomal genes, thus, association of type II NR channels with cytoplasmic core, and pierced by NPC, could suggest a role for the NR in facilitating a nuclear export of rRNA. However, these are microscopy co-localization studies, therefore further experiments are required to determine whether rRNA export truly occurs and dominates such associations. In addition, cells treated with the histone-deacetylase inhibitor trichostatin A show higher abundance of NR,Citation36 thus it may further support the hypothesis that NR helps with increased gene expression and RNA export in general. Moreover, NR channels have been observed to closely associate with repressive complexes such as BMI1-positive Polycomb group proteins (PcG) - related bodies and heterochromatin marker HP1β,Citation35 which could further facilitate NR involvement in chromatin organization and transcription regulation. Indeed, soluble intra-nuclear lamin A/C was shown to interplay and regulate PcG-mediated transcriptional repression,Citation37,38 thus lamin A/C underlying NR channels could offer additional anchor points of PcG compartmentalization. It may seem contradictory that NR channels associate with repressive PcG complexes, and yet become more abundant upon chromatin relaxation induced by a histone-deacetylase inhibitor.Citation36 Association with the NE, however, has also been shown to promote both chromatin activation and silencing, depending on context and interactors,Citation39-41 thus NR as an intranuclear extension of nuclear envelope may exhibit similar properties. Legartova and colleagues also observed a tight association of NR tubules with γH2AX-positive DNA lesions, induced by γ-radiation, and with 53BP1, a regulator of cellular response to DNA damage,Citation42 implying a role for a dynamic NR in DNA damage repair.Citation35
Another cellular process in which NR has been implicated is lipid metabolism. Recently, it was shown that type I NR closely associates with lipid droplets and their number correlates with the amount of type I NR in a promyelocytic leukemia nuclear body-dependent manner.Citation43 Nuclear lipid droplets appear to incorporate newly synthesized lipid esters and stain positively for diacylglycerol O-acyltransferase 2 (DGAT2) and CTP:phosphocholine cytidylyltransferase α (CCTα), the key enzymes in triglyceride and phosphatidylcholine synthesis, respectively.Citation44,45 Interestingly, NR formation can not only be induced by CCTα,Citation46 but also depends on this enzyme.Citation17 Moreover, CCTα upon activation translocates to the NE and the NR, thus, bearing in mind a wide spectrum of functions performed by CCTα in lipid metabolismCitation45 and its close links with NR, it could suggest additional roles for NR in lipid signaling.
NR formation
NR tubules can be a result of post-mitotic reassembly of the nucleus, when the fusion of recruited NE sheets is imperfect and some get trapped within the nucleus.Citation47 However, a number of reports showed clearly that new NR channels can form in a cell-cycle-independent manner in post-mitotic primary cells,Citation48 in cycle-arrested cells,Citation5,17 and during interphase in free-cycling cells,Citation17 thus suggesting the existence of a controlling mechanism for NR formation in an interphase nucleus.
Nuclear architecture is complex and various physical forces affect the organization and shape of the NE, both from within the nucleus and/or from the cytoplasm.Citation49-54 Proliferation of highly curved NR channels is an energy demanding process, because pure lipid bilayers, a major component of NE, remain flat unless energy is provided to stimulate them to curve.Citation55 The energy that aids in curvature introduction to the cellular membranes could be sourced from either modification of lipid composition or bilayer asymmetry, or from membrane-associated proteins (for a review, see ref. Citation56). Protein mechanisms vary and can rely purely on the shape of transmembrane proteins, further enhanced by partitioning or crowding effects of the protein insertion, or on docking of hydrophobic protein domains in the membrane. Oligomerization of protein monomers and formation of protein coats can greatly enhance and stabilize membrane curvature (for a review, see ref. Citation57).
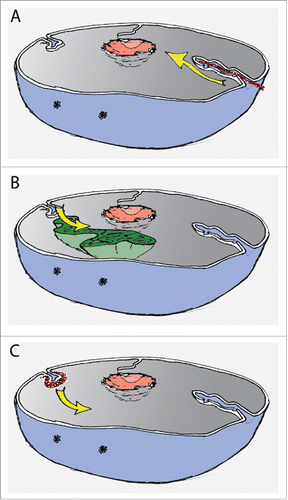
This diversity of regulated membrane curvature inducing processes leads to a range of possible mechanisms that could cause the drastic alterations to the properties of the NE and drive NR formation (See ). Although relying on different principles, these models are not mutually exclusive.
Figure 2. Schematic representation of nucleus with possible mechanisms driving NR formation. (A) Pushing the NE by cytoskeleton (red) as visualised by yellow arrow; (B) Pulling the NE by chromatin movement (green), movement indicated by yellow arrow; (C) Focal and de novo assembly and growth (yellow arrow) of NR invaginations (red) by dedicated machinery.
Pulling in
Nuclear architecture may be defined by interactions between chromatin and the NE, as a substantial literature on chromatin-lamina and chromatin-INM protein interactions attests (for reviews, see refs. Citation58-60). It is well established that chromatin organization is not random and higher order chromosomal territories exist (for reviews, see refs. Citation61,62), although their organization seem to be cell-type specific. Distribution of chromosomes can be dictated either by their sizeCitation63 or gene density.Citation64 In addition, in an interphase nucleus, dynamic chromatin movements occur as a result of chromosome condensation.Citation65,66 Thus, NR invaginations could be driven by rearrangements of chromatin tethered to the NE and pulling in the nuclear membrane (). This observation was made for NR formation in polytene nuclei from Drosophila melanogaster salivary glands.Citation67 Conversely, however, a link between NR and chromatin decondensation could be implied by the observation that mouse embryonic fibroblasts treated with histone-deacetylase inhibitor show an increased abundance of type II NR.Citation36
Pushing in
Alternatively, the pressure on the NE that curves the membrane and induces NR formation may come from outside of the nucleus (). It is well established that the cytoskeleton can counterbalance internal forces of chromatin and the nuclear lamina, thus playing a pivotal role in stabilization of nuclear architecture.Citation50,51,68 It is possible then that the cytoskeleton exerts a force on the NE and pushes it in, driving invagination. In fact, it has been shown that type II NR invaginations contain microtubules and microfilaments in their cytoplasmic core.Citation3,5,69,70 In addition, a close proximity of centrioles to major nuclear invaginations of granulocytic cells was shown, with the suggestion that centrosomes exhibit the tensor force curving the NE through cytoskeletal proteins.Citation71 Although likely to be relevant under some conditions, a putative cytoskeleton-driven formation of NR cannot explain proliferation of type I NR, consisting of the INM only.
Selective recruitment
The final scenario suggests the existence of dedicated machinery that assembles the NR structure de novo, rather than through rearrangement of pre-existing NE (). In fact many cellular machineries exist dedicated specifically to induction of lipid bilayer curvature and cellular membrane invaginations.Citation56
One of the best studied is clathrin-mediated endocytosis, which is initiated by focal assembly of a clathrin lattice at a flat membrane area, a process orchestrated by adapter proteins in conjunction with actin polymerisation.Citation72-74 Several other clathrin-independent mechanisms of plasma membrane invagination have been characterized as well. They also require specific interactions of mediator proteins and can lead to varying membrane morphologies such as tubular or vesicular structures. Clathrin-independent carriers/glycosylphosphatidylinositol-enriched early endosomal compartment pathway,Citation75 endophilin-mediated endocytosisCitation76 or caveolae formation.Citation77,78 are just a few of them.
Phospholipid bilayer deformation is not limited to the plasma membrane. Many intracellular structures exist as membrane-bound organelles compartmentalising the cell interior and rely on membrane curvature in order to perform their functions, such as the endoplasmic reticulum. Reticulons and DP1/YOP1 (defective in polyposis/yeast ortholog) proteins are regulators of membrane curvature, involved in the formation of tubular ER in animals, fungi, and plants.Citation79-81 Moreover, reticulons can generate arc-shaped scaffolds by an oligomerization process further contributing not only to induction, but also to the maintenance of high membrane curvature.Citation82 Therefore, reticulons can influence the balance between the ER sheet and ER tubule proliferation, favoring conversion of sheets into tubules. Interestingly, some reticulons have been suggested to be involved in NPC insertion at the NE.Citation83 Moreover, reticulon 4a was found in junctions between ER and the edges of growing NE in both Xenopus oocytes and in in vitro nuclei assembly system,Citation84 thus, this protein appears be involved in facilitating NE growth by stabilizing high curvature where new membrane is added to the re-forming nuclei. Therefore, it is tempting to speculate that reticulons might also aid in NR development through positive curvature generation in the nuclear membrane.
Coatomer protein complex I and II (COPI and COPII) have well defined roles in vesicle budding from the Golgi and ER.Citation85,86 It is a process requiring membrane deformation in which COPI and COPII are orchestrated by small GTPases Arf1 and Sar1, respectively, and stabilize curvature.Citation87,88 Interestingly, it was shown that COPI may be involved in NE mitotic breakdown in Xenopus by formation of vesicles and tubular structures with the ER.Citation89 Components of COPI are recruited by nucleoporins and are critical for disassembly of the NE.Citation90,91 Of note, it was proposed that nucleoporins share a common ancestor with COPI, COPII and clathrin/adaptin complexes and diverged during the evolution of internal membrane systems that ultimately led to the acquisition of the nucleus.Citation92,93 Moreover, depletion of Rab5, a GTPase with a well established role in endocytosis,Citation94,95 was also shown to impair mitotic NE breakdown and membrane remodelling.Citation96 Post-mitotic NE reassembly is a process requiring a massive rearrangement of membrane as well. Recently, it was reported that the endosomal sorting complex required for transport (ESCRT)-III proteins, classically involved in membrane fission during formation of multivesicular endosomes, enveloped virus budding and cytokinetic abscission,Citation97 are also responsible for annular fusion in the reassembling NE.Citation98,99 These observations indicate a wider role of membrane bending and modifying proteins at the NE, potentially also involved in the NR regulation.
Cells have developed a multitude of membrane deformation mechanisms. They appear to be tightly regulated and multistage, with an array of molecular sensors of curvature and machineries allowing for controlled proliferation of membrane tubules and vesicle formation. Thus, it is very likely that selective mechanisms also exist in the process of NR induction and stabilization of intranuclear channels.
Role of proteins and lipids in NR development
Over-expression of some NE proteins has been reported to increase NR abundance. Most notably, overexpression of lamins harbouring C-terminal CaaX motif was a strong inducer of NR proliferation.Citation100 In agreement, progerin, a lamin A mutant expressed in Hutchinson-Gilford Progeria Syndrome which exhibits farnesylation of the cysteine in a retained CaaX motif, causes NR proliferation.Citation101 Inhibition of the lamin A maturation process which leads to build up of precursor prelamin A, retaining farnesylation at the cysteine, was also shown to induce NR proliferation.Citation17,102 The presence of the isoprenylated cysteine at the protein C-terminus most likely increases its affinity for the INM and may affect membrane curvature by exhibiting additional physical strain on the nuclear membrane.Citation103 Blocking lamin A farnesylation, by using farnesyl transferase inhibitors, improves the dysmorphic nuclear shape by displacing prelamin A to the nuclear interior; processing of the protein to its mature form still fails, but the product is not held at the NE by a persistent hydrophobic interaction.Citation103,104 Similar observations were made for progerin.Citation105 It is also possible that retention of farnesylation at the C-terminus of lamin A impacts its interaction with the lamin B network, which normally remains permanently farnesylated and forms a closely associated, but separate fiber meshwork.Citation106 Thus mixing of the 2 and potential perturbation of their normal assembly could also prove relevant in regard to formation of nuclear membrane invaginations. Interestingly, other lamin A mutants associated with Emery–Dreifuss muscular dystrophy or Dunnigan-type familial partial lipodystrophy have also been shown to increase NR prevalence.Citation107
Other inducers of NR proliferation such as overexpression of INM protein LAP2βCitation108 or nucleolar shuttling protein NOPP140Citation109 have also been reported. It should be noted, however, that overexpression of any nuclear membrane protein will perturb the nuclear envelope by changing protein overload and access for interactions, which may result in a distorted nuclear rim, but not necessarily in the NR structures as defined earlier. Interestingly though, in certain cell types knock-down of SUN proteins, components of the linker of nucleoskeleton and cytoskeleton (LINC) complex,Citation110 can also increase abundance of type I NR,Citation43 presumably by decoupling the INM and ONM, permitting a morphogenic process to operate on either membrane alone.
NR development seems to depend on the enzyme choline-phosphate cytidylyltransferase α (CCTα).Citation46 It is the rate limiting enzyme in phosphatidylcholine synthesis, and is crucial for membrane biosynthesis.Citation45 CCTα is also believed to introduce positive membrane curvature by its insertion into the INM.Citation111 This causes infolding of the INM, and may further support tubulation of the NR. Interestingly, some interplay between nuclear lamina and CCTα appears to occur in the process of NR formation; knocking-down of either lamin A/C or B1 expression significantly reduced NR development, even after CCTα stimulation.Citation112
Despite a clear requirement for new membrane synthesis in order to form NR, as shown by experiments investigating CCTα role, our knowledge of how phospholipids are added to an expanding NE and NR is rather limited. It would be of particular interest to determine whether NR expansion is a result of the free flow of lipids between peripheral endoplasmic reticulum and NE, resulting in rearrangement of pre-existing membranes, or a focal assembly process more akin to coated pit formation exists.
Physiological regulation
The NR appears in many cell types with multiple pathways contributing to its formation. It also occurs as a physiological cellular response to external stimuli. It has long been recognized that a structurally advanced NR, referred to as the nucleolar channel system (NCS) is a hallmark of the endometrium following ovulation.Citation113,114 Its transient presence manifests in human endometrial cells during a 3 to 4 day period during the midluteal, receptive phase of the menstrual cycle.Citation115 The NCS structure forms multilamellar and tubular membrane cisternae within the nucleus that are derived from the INM.Citation109,114 These cisternae exhibit the presence of NPC proteins and a subset of NE-specific components.Citation115 The proposed significance of the NCS is that it is formed in preparation for blastocyst attachment and implantation to the endometrium. This hypothesis is supported by several reports demonstrating the absence or delayed development of NCS in cases of unexplained primary infertility.Citation116,117 It is further supported by observations that oral contraceptives interfere with NCS formation.Citation118,119 It has been demonstrated that the formation of NCS can be elicited by the action of estrogen and progesterone at the time of ovulation.Citation120,121 While NCS represents a unique tubular structure, its development from the INM suggests that it may originate as an NR invagination which, in response to hormones, gains further complexity, possibly representing an advanced and differentiated form of NR. Of note, human leukemic cell line HL-60, after in vitro induced differentiation into granulocytic form, develops highly structured and unique NE invaginations named nuclear envelope-limited chromatin sheets (ELCS).Citation122 ELCS, predominantly observed in haematological malignancies, is also proposed to originate as an INM invagination,Citation123 thus may share with NR similar mechanisms for membrane curving, at least at the initial formation stage.
Recently, is has been demonstrated that rabbit pre-implantation development is accompanied by changes in NR abundance.Citation124 Type II NR, although bountiful in rabbit embryos in general, was consistently present at the highest number at the 4-cell stage, after which the number of NR invaginations declined. Interestingly, it correlates with a significant nuclear volume decline that begins at the 8-cell stage. Moreover, these type II NR channels stained positively for NPC and were in close contact with nucleolar precursor bodies, thus suggesting a transient role for the NR in a high protein import demand of nucleolar precursor bodies during that precise developmental stage. Cell differentiation state has also been shown to affect the abundance of NR.Citation70 Johnson and colleagues not only observed transient NR channels in the nuclei of embryonic cells, but also noted that differentiated cells had significantly fewer nuclear invaginations, than highly de-differentiated or cancerous cells. These observations lend some support to the idea that dynamic NR changes might play a role in the regulation of gene expression programmes.
Concluding remarks
In conclusion, the NR forms a distinct and widespread feature in nuclear organization, therefore gaining further understanding of its form and function is an important aspect of the cell biology of the nucleus. Regulation of the NR is a dynamic process and a number of cellular pathways involved in its regulation have already been identified. However, many questions still remain unanswered. It would be of particular interest to see if dedicated membrane bending machineries are also involved in NR induction/stabilization. The origin of components, such as phospholipid bilayer or nuclear lamina which are the building blocks for the NR is also not well defined, and would certainly repay further research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the Micron Imaging Facility, Oxford, for access to the super-resolution LM microscope.
Funding
Work in the author's lab in this area is funded by BBSRC grant BB/L002159/1, a BBSRC studentship and an Edward Penley Abraham Trust award.
References
- Prunuske AJ, Ullman KS. The nuclear envelope: form and reformation. Curr Opin Cell Biol 2006; 18:108-16; PMID:16364623; http://dx.doi.org/10.1016/j.ceb.2005.12.004
- Kamei H. Relationship of nuclear invaginations to perinuclear rings composed of intermediate filaments in MIA PaCa-2 and some other cells. Cell Structure Function 1994; 19:123-32; PMID:7954871; http://dx.doi.org/10.1247/csf.19.123
- Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: form and function. Trends Cell Biol 2011; 21:362-73; PMID:21514163; http://dx.doi.org/10.1016/j.tcb.2011.03.008
- Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 2003; 5:440-6; PMID:12717445; http://dx.doi.org/10.1038/ncb980
- Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol 1997; 136:531-44; PMID:9024685; http://dx.doi.org/10.1083/jcb.136.3.531
- Langevin HM, Storch KN, Snapp RR, Bouffard NA, Badger GJ, Howe AK, Taatjes DJ. Tissue stretch induces nuclear remodeling in connective tissue fibroblasts. Histochem Cell Biol 2010; 133:405-15; PMID:20237796; http://dx.doi.org/10.1007/s00418-010-0680-3
- Storch K, Taatjes D, Bouffard N, Locknar S, Bishop N, Langevin H. Alpha smooth muscle actin distribution in cytoplasm and nuclear invaginations of connective tissue fibroblasts. Histochem Cell Biol 2007; 127:523-30; PMID:17310383; http://dx.doi.org/10.1007/s00418-007-0275-9
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 2008; 320:1332-6; PMID:18535242; http://dx.doi.org/10.1126/science.1156947
- True LD, Jordan CD. The cancer nuclear microenvironment: interface between light microscopic cytology and molecular phenotype. J Cell Biochem 2008; 104:1994-2003; PMID:18041766; http://dx.doi.org/10.1002/jcb.21478
- Bussolati G, Marchi∫ C, Gaetano L, Lupo R, Sapino A. Pleomorphism of the nuclear envelope in breast cancer: a new approach to an old problem. J Cell Mol Med 2008; 12:209-18; PMID:18053086; http://dx.doi.org/10.1111/j.1582-4934.2007.00176.x
- Malhas A, Vaux D. Nuclear envelope invaginations and cancer. In: Schirmer EC, de las Heras JI, eds. Cancer Biology and the Nuclear Envelope: Springer New York, 2014:523-35
- Frost B, Bardai FH, Feany MB. Lamin dysfunction mediates neurodegeneration in tauopathies. Curr Biol 2016; 26:129-36; PMID:26725200; http://dx.doi.org/10.1016/j.cub.2015.11.039
- Rodríguez R, Hernández-Hernández O, Magaña JJ, González-Ramírez R, García-López ES, Cisneros B. Altered nuclear structure in myotonic dystrophy type 1-derived fibroblasts. Mol Biol Reports 2015; 42:479-88; http://dx.doi.org/10.1007/s11033-014-3791-4
- McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One 2007; 2:e1269; PMID:18060063; http://dx.doi.org/10.1371/journal.pone.0001269
- Brandes D, Schofield BH, Anton E. Nuclear Mitochondria? Science 1965; 149:1373-4; PMID:5889956; http://dx.doi.org/10.1126/science.149.3690.1373
- Clubb BH, Locke M. 3T3 cells have nuclear invaginations containing F-actin. Tissue Cell 1998; 30:684-91; PMID:10189322; http://dx.doi.org/10.1016/S0040-8166(98)80087-6
- Goulbourne CN, Malhas AN, Vaux DJ. The induction of a nucleoplasmic reticulum by prelamin A accumulation requires CTP:phosphocholine cytidylyltransferase-α. J Cell Sci 2011; 124:4253-66; PMID:22223883; http://dx.doi.org/10.1242/jcs.091009
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 1995; 80:439-44; PMID:7859285; http://dx.doi.org/10.1016/0092-8674(95)90494-8
- Nicotera P, McConkey DJ, Jones DP, Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A 1989; 86:453-7; PMID:2911591; http://dx.doi.org/10.1073/pnas.86.2.453
- Maraldi NM, Cocco L, Capitani S, Mazzotti G, Barnabei O, Manzoli FA. Lipid-dependent nuclear signalling: morphological and functional features. Adv Enzyme Regulation 1994; 34:129-43; PMID:7942270; http://dx.doi.org/10.1016/0065-2571(94)90013-2
- Divecha N, Banfić H, Irvine RF. Inositides and the nucleus and inositides in the nucleus. Cell 1993; 74:405-7; PMID:8394217; http://dx.doi.org/10.1016/0092-8674(93)80041-C
- Manzoli L, Martelli AM, Billi AM, Faenza I, Fiume R, Cocco L. Nuclear phospholipase C: involvement in signal transduction. Progress Lipid Res 2005; 44:185-206; PMID:15896848; http://dx.doi.org/10.1016/j.plipres.2005.04.003
- Irvine RF. Nuclear inositide signalling-expansion, structures and clarification. Biochim Et Biophys Acta - Mol Cell Biol Lipids 2006; 1761:505-8; ; http://dx.doi.org/10.1016/j.bbalip.2006.02.008
- Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium 2006; 39:65-73; PMID:16289270; http://dx.doi.org/10.1016/j.ceca.2005.09.010
- Oliveira AG, Guimarães ES, Andrade LM, Menezes GB, Leite MF. Decoding calcium signaling across the nucleus. Physiol 2014; 29:361-8; PMID:25180265; http://dx.doi.org/10.1152/physiol.00056.2013
- Zhang SJ, Zou M, Lu L, Lau D, Ditzel DAW, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. Nuclear calcium signaling controls expression of a large gene pool: Identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genetics 2009; 5:e1000604
- Nalaskowski MM, Fliegert R, Ernst O, Brehm MA, Fanick W, Windhorst S, Lin H, Giehler S, Hein J, Yuan-Na Lin, et al. Human inositol 1,4,5-trisphosphate 3-kinase isoform B (IP3KB) is a nucleocytoplasmic shuttling protein specifically enriched at cortical actin filaments and at invaginations of the nuclear envelope. J Biol Chem 2011; 286:4500-10; PMID:21148483; http://dx.doi.org/10.1074/jbc.M110.173062
- Dewaste V, Moreau C, De Smedt F, Bex F, De Smedt H, Wuytack F, Missiaen L, Erneux C. The three isoenzymes of human inositol-1,4,5-trisphosphate 3-kinase show specific intracellular localization but comparable Ca2+ responses on transfection in COS-7 cells. Biochem J 2003; 374:41-9; PMID:12747803; http://dx.doi.org/10.1042/bj20021963
- Yu JCH, Lloyd-Burton SM, Irvine RF, Schell MJ. Regulation of the localization and activity of inositol 1,4,5-trisphosphate 3-kinase B in intact cells by proteolysis. Biochem J 2005; 392:435-41; PMID:16173920; http://dx.doi.org/10.1042/BJ20050829
- Collado-Hilly M, Shirvani H, Jaillard D, Mauger J-P. Differential redistribution of Ca2+-handling proteins during polarisation of MDCK cells: Effects on Ca2+ signalling. Cell Calcium 2010; 48:215-24; PMID:20932574; http://dx.doi.org/10.1016/j.ceca.2010.09.003
- Pauly N, Knight MR, Thuleau P, Van Der Luit AH, Moreau M, Trewavas AJ, Ranjeva R, Mazars C. Control of free calcium in plant cell nuclei. Nature 2000; 405:754-5; PMID:10866186; http://dx.doi.org/10.1038/35015671
- Collings DA, Carter CN, Rink JC, Scott AC, Wyatt SE, Allen NS. Plant nuclei can contain extensive grooves and invaginations. Plant Cell 2000; 12:2425-39; PMID:11148288; http://dx.doi.org/10.1105/tpc.12.12.2425
- McNamara LE, Burchmore R, Riehle MO, Herzyk P, Biggs MJP, Wilkinson CDW, Curtis AS, Dalby MJ. The role of microtopography in cellular mechanotransduction. Biomaterials 2012; 33:2835-47; PMID:22248989; http://dx.doi.org/10.1016/j.biomaterials.2011.11.047
- Bourgeois CA, Hemon D, Bouteille M. Structural relationship between the nucleolus and the nuclear envelope. J Ultrastructure Res 1979; 68:328-40; PMID:490761; http://dx.doi.org/10.1016/S0022-5320(79)90165-5
- Legartová S, Stixová L, Laur O, Kozubek S, Sehnalová P, Bártová E. Nuclear structures surrounding internal lamin invaginations. J Cell Biochem 2014; 115:476-87; http://dx.doi.org/10.1002/jcb.24681
- Galiová G, Bártová E, Raška I, Krejčí J, Kozubek S. Chromatin changes induced by lamin A/C deficiency and the histone deacetylase inhibitor trichostatin A. Eur J Cell Biol 2008; 87:291-303; http://dx.doi.org/10.1016/j.ejcb.2008.01.013
- Cesarini E, Mozzetta C, Marullo F, Gregoretti F, Gargiulo A, Columbaro M, Cortesi A, Antonelli L, Di Pelino S, Squarzoni S, et al. Lamin A/C sustains PcG protein architecture, maintaining transcriptional repression at target genes. J Cell Biol 2015; 211:533-51; PMID:26553927; http://dx.doi.org/10.1083/jcb.201504035
- Marullo F, Cesarini E, Antonelli L, Gregoretti F, Oliva G, Lanzuolo C. Nucleoplasmic Lamin A/C and Polycomb group of proteins: An evolutionarily conserved interplay. Nucleus 2016; 7:103-11; PMID:26930442; http://dx.doi.org/10.1080/19491034.2016.1157675
- Finlan LE. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genetics 2008; 4:e1000039; PMID:18369458; http://dx.doi.org/10.1371/journal.pgen.1000039
- Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol 2008; 180:51-65; PMID:18195101; http://dx.doi.org/10.1083/jcb.200706060
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 2008; 452:243-7; PMID:18272965; http://dx.doi.org/10.1038/nature06727
- Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 2014; 15:7-18; PMID:24326623; http://dx.doi.org/10.1038/nrm3719
- Ohsaki Y, Kawai T, Yoshikawa Y, Cheng J, Jokitalo E, Fujimoto T. PML isoform II plays a critical role in nuclear lipid droplet formation. J Cell Biol 2016; 212:29-38; PMID:26728854; http://dx.doi.org/10.1083/jcb.201507122
- Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Fröhlich F, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell 2013; 24:384-99; PMID:23415954; http://dx.doi.org/10.1016/j.devcel.2013.01.013
- Cornell RB, Ridgway ND. CTP:phosphocholine cytidylyltransferase: Function, regulation, and structure of an amphitropic enzyme required for membrane biogenesis. Progress Lipid Res 2015; 59:147-71; PMID:26165797; http://dx.doi.org/10.1016/j.plipres.2015.07.001
- Lagace TA, Ridgway ND. The rate-limiting enzyme in phosphatidylcholine synthesis regulates proliferation of the nucleoplasmic reticulum. Mol Biol Cell 2005; 16:1120-30; PMID:15635091; http://dx.doi.org/10.1091/mbc.E04-10-0874
- Gupton SL, Collings DA, Allen NS. Endoplasmic reticulum targeted GFP reveals ER organization in tobacco NT-1 cells during cell division. Plant Physiol Biochem 2006; 44:95-105; PMID:16647266; http://dx.doi.org/10.1016/j.plaphy.2006.03.003
- Broers JLV, Ramaekers FCS, Bonne G, Ben Yaou R, Hutchison CJ. Nuclear lamins: Laminopathies and their role in premature ageing. Physiological Rev 2006; 86:967-1008; PMID:16816143; http://dx.doi.org/10.1152/physrev.00047.2005
- Funkhouser CM, Sknepnek R, Shimi T, Goldman AE, Goldman RD, Olvera de la Cruz M. Mechanical model of blebbing in nuclear lamin meshworks. Proc Natl Acad Sci U S A 2013; 110:3248-53; PMID:23401537; http://dx.doi.org/10.1073/pnas.1300215110
- Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not Lamin B1 regulate nuclear mechanics. J Biol Chem 2006; 281:25768-80; PMID:16825190; http://dx.doi.org/10.1074/jbc.M513511200
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 1997; 94:849-54; PMID:9023345; http://dx.doi.org/10.1073/pnas.94.3.849
- Mazumder A, Roopa T, Kumar A, Iyer KV, Ramdas NM, Shivashankar GV. Chapter 10 - Prestressed Nuclear Organization in Living Cells. In: Shivashankar GV, ed. Methods in Cell Biology: Academic Press, 2010:221-39
- Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development 2010; 137:1407-20; PMID:20388652; http://dx.doi.org/10.1242/dev.024166
- Rowat AC, Lammerding J, Herrmann H, Aebi U. Towards an integrated understanding of the structure and mechanics of the cell nucleus. BioEssays 2008; 30:226-36; PMID:18293361; http://dx.doi.org/10.1002/bies.20720
- Helfrich P, Jakobsson E. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys J 1990; 57:1075-84; PMID:1692748; http://dx.doi.org/10.1016/S0006-3495(90)82625-4
- Jarsch IK, Daste F, Gallop JL. Membrane curvature in cell biology: An integration of molecular mechanisms. J Cell Biol 2016; 214:375-87; PMID:27528656; http://dx.doi.org/10.1083/jcb.201604003
- McMahon HT, Boucrot E. Membrane curvature at a glance. J Cell Sci 2015; 128:1065-70; PMID:25774051; http://dx.doi.org/10.1242/jcs.114454
- Pombo A, Dillon N. Three-dimensional genome architecture: players and mechanisms. Nat Rev Mol Cell Biol 2015; 16:245-57; PMID:25757416; http://dx.doi.org/10.1038/nrm3965
- Amendola M, Van Steensel B. Mechanisms and dynamics of nuclear lamina-genome interactions. Curr Opin Cell Biol 2014; 28:61-8; PMID:24694724; http://dx.doi.org/10.1016/j.ceb.2014.03.003
- Towbin BD, Gonzalez-Sandoval A, Gasser SM. Mechanisms of heterochromatin subnuclear localization. Trends Biochem Sci 2013; 38:356-63; PMID:23746617; http://dx.doi.org/10.1016/j.tibs.2013.04.004
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genetics 2001; 2:292-301; PMID:11283701; http://dx.doi.org/10.1038/35066075
- Bickmore WA. The spatial organization of the human genome. Annual Rev Genomics Hum Genetics 2013; 14:67-84; PMID:23875797; http://dx.doi.org/10.1146/annurev-genom-091212-153515
- Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Müller S, Eils R, Cremer C, Speicher MR, et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol 2005; 3:e157; PMID:15839726; http://dx.doi.org/10.1371/journal.pbio.0030157
- Boyle S, Gilchrist S, Bridger JM, Mahy NL, Ellis JA, Bickmore WA. The spatial organization of human chromosomes within the nuclei of normal and emerin-mutant cells. Hum Mol Genetics 2001; 10:211-9; PMID:11159939; http://dx.doi.org/10.1093/hmg/10.3.211
- Hartl TA, Smith HF, Bosco G. Chromosome alignment and transvection are antagonized by condensin II. Science 2008; 322:1384-7; PMID:19039137; http://dx.doi.org/10.1126/science.1164216
- Bauer CR, Hartl TA, Bosco G. Condensin II promotes the formation of chromosome territories by inducing axial compaction of polyploid interphase chromosomes. PLoS Genetics 2012; 8:e1002873; PMID:22956908; http://dx.doi.org/10.1371/journal.pgen.1002873
- Bozler J, Nguyen HQ, Rogers GC, Bosco G. Condensins exert force on chromatin-nuclear envelope tethers to mediate nucleoplasmic reticulum formation in Drosophila melanogaster. G3: Genes|Genomes|Genetics 2015; 5:341-52
- Mazumder A, Roopa T, Basu A, Mahadevan L, Shivashankar GV. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys J 2008; 95:3028-35; PMID:18556763; http://dx.doi.org/10.1529/biophysj.108.132274
- Bol'shakova AV, Petukhova OA, Pinaev GP, Magnusson KE. Comparative analysis of subcellular fractionation methods for revealing α-actinin 1 and α-actinin 4 in A431 cells. Cell Tissue Biol 2009; 3:188-97; http://dx.doi.org/10.1134/S1990519X09020114
- Johnson N, Krebs M, Boudreau R, Giorgi G, LeGros M, Larabell C. Actin-filled nuclear invaginations indicate degree of cell de-differentiation. Differentiation 2003; 71:414-24; PMID:12969334; http://dx.doi.org/10.1046/j.1432-0436.2003.7107003.x
- Olins AL, Olins DE. The mechanism of granulocyte nuclear shape determination: possible involvement of the centrosome. Eur J Cell Biol 2005; 84:181-8; PMID:15819399; http://dx.doi.org/10.1016/j.ejcb.2004.12.021
- Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 2004; 432:573-9; PMID:15502812; http://dx.doi.org/10.1038/nature03079
- Avinoam O, Schorb M, Beese CJ, Briggs JAG, Kaksonen M. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science 2015; 348:1369-72; PMID:26089517; http://dx.doi.org/10.1126/science.aaa9555
- Lewellyn EB, Pedersen RTA, Hong J, Lu R, Morrison HM, Drubin DG. An engineered minimal WASP-myosin fusion protein reveals essential functions for endocytosis. Dev Cell 2015; 35:281-94; PMID:26555049; http://dx.doi.org/10.1016/j.devcel.2015.10.007
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, McMahon HT. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol 2008; 18:1802-8; PMID:19036340; http://dx.doi.org/10.1016/j.cub.2008.10.044
- Boucrot E, Ferreira APA, Almeida-Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N, McMahon HT. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015; 517:460-5; PMID:25517094; http://dx.doi.org/10.1038/nature14067
- Morén B, Shah C, Howes MT, Schieber NL, McMahon HT, Parton RG, Daumke O, Lundmark R. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol Biol Cell 2012; 23:1316-29; http://dx.doi.org/10.1091/mbc.E11-09-0787
- Senju Y, Itoh Y, Takano K, Hamada S, Suetsugu S. Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. J Cell Sci 2011; 124:2032-40; PMID:21610094; http://dx.doi.org/10.1242/jcs.086264
- Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol 2003; 13:187-94; PMID:12667756; http://dx.doi.org/10.1016/S0962-8924(03)00035-7
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 2006; 124:573-86; PMID:16469703; http://dx.doi.org/10.1016/j.cell.2005.11.047
- Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 2008; 319:1247-50; PMID:18309084; http://dx.doi.org/10.1126/science.1153634
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA. Mechanisms determining the morphology of the peripheral ER. Cell 2010; 143:774-88; PMID:21111237; http://dx.doi.org/10.1016/j.cell.2010.11.007
- Antonin W. Nuclear Envelope: membrane bending for pore formation? Curr Biol 2009; 19:R410-R2; PMID:19467209; http://dx.doi.org/10.1016/j.cub.2009.03.053
- Kiseleva E, Morozova KN, Voeltz GK, Allen TD, Goldberg MW. Reticulon 4a/NogoA locates to regions of high membrane curvature and may have a role in nuclear envelope growth. J Structural Biol 2007; 160:224-35; PMID:17889556; http://dx.doi.org/10.1016/j.jsb.2007.08.005
- Nickel W, Brügger B, Wieland FT. Vesicular transport: the core machinery of COPI recruitment and budding. J Cell Sci 2002; 115:3235-40; PMID:12140255
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol 2012; 14:20-8; http://dx.doi.org/10.1038/ncb2390
- Beck R, Sun Z, Adolf F, Rutz C, Bassler J, Wild K, Sinning I, Hurt E, Brügger B, Béthune J, et al. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci U S A 2008; 105:11731-6; PMID:18689681; http://dx.doi.org/10.1073/pnas.0805182105
- Lee MCS, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 2005; 122:605-17; PMID:16122427; http://dx.doi.org/10.1016/j.cell.2005.07.025
- Cotter L, Allen TD, Kiseleva E, Goldberg MW. Nuclear membrane disassembly and rupture. J Mol Biol 2007; 369:683-95; PMID:17467734; http://dx.doi.org/10.1016/j.jmb.2007.03.051
- Liu J, Prunuske AJ, Fager AM, Ullman KS. The COPI complex functions in nuclear envelope breakdown and is recruited by the nucleoporin Nup153. Dev Cell 2003; 5:487-98; PMID:12967567; http://dx.doi.org/10.1016/S1534-5807(03)00262-4
- Prunuske AJ, Liu J, Elgort S, Joseph J, Dasso M, Ullman KS. Nuclear envelope breakdown is coordinated by both Nup358/RanBP2 and Nup153, two nucleoporins with zinc finger modules. Mol Biol Cell 2006; 17:760-9; PMID:16314393; http://dx.doi.org/10.1091/mbc.E05-06-0485
- Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol 2004; 2:e380; PMID:15523559; http://dx.doi.org/10.1371/journal.pbio.0020380
- Brohawn SG, Leksa NC, Spear ED, Rajashankar KR, Schwartz TU. Structural evidence for common ancestry of the nuclear pore complex and vesicle coats. Science 2008; 322:1369-73; PMID:18974315; http://dx.doi.org/10.1126/science.1165886
- Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci 2000; 113:183-92; PMID:10633070
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 2013; 25:414-9; PMID:23639309; http://dx.doi.org/10.1016/j.ceb.2013.04.002
- Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol 2007; 178:43-56; PMID:17591921; http://dx.doi.org/10.1083/jcb.200701139
- McCullough J, Colf LA, Sundquist WI. Membrane fission reactions of the mammalian ESCRT pathway. Annual Rev Biochem 2013; 82:663-92; http://dx.doi.org/10.1146/annurev-biochem-072909-101058
- Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. ESCRT-III controls nuclear envelope reformation. Nature 2015; 522:236-9; PMID:26040713; http://dx.doi.org/10.1038/nature14503
- Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 2015; 522:231-5; PMID:26040712; http://dx.doi.org/10.1038/nature14408
- Prüfert K, Vogel A, Krohne G. The lamin CxxM motif promotes nuclear membrane growth. J Cell Sci 2004; 117:6105-16; PMID:15546914; http://dx.doi.org/10.1242/jcs.01532
- McClintock D, Gordon LB, Djabali K. Hutchinson–Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-lamin A G608G antibody. Proc Natl Acad Sci USA 2006; 103:2154-9; PMID:16461887; http://dx.doi.org/10.1073/pnas.0511133103
- Goulbourne CN, Vaux DJ. HIV protease inhibitors inhibit FACE1/ZMPSTE24: a mechanism for acquired lipodystrophy in patients on highly active antiretroviral therapy? Biochem Society Transactions 2010; 38:292-6; PMID:20074077; http://dx.doi.org/10.1042/BST0380292
- Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, Miner JH, Young SG, Fong LG. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci U S A 2005; 102:12873-8; PMID:16129834; http://dx.doi.org/10.1073/pnas.0505767102
- Fong LG, Frost D, Meta M, Qiao X, Yang SH, Coffinier C, et al. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 2006; 311:1621-3; PMID:16484451; http://dx.doi.org/10.1126/science.1124875
- Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 2005; 102:14416-21; PMID:16186497; http://dx.doi.org/10.1073/pnas.0503712102
- Shimi T, Kittisopikul M, Tran J, Goldman AE, Adam SA, Zheng Y, Jaqaman K, Goldman RD. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol Biol Cell 2015; 26:4075-86; PMID:26310440; http://dx.doi.org/10.1091/mbc.E15-07-0461
- Roblek M, Schüchner S, Huber V, Ollram K, Vlcek-Vesely S, Foisner R, Wehnert M, Ogris E. Monoclonal antibodies specific for disease-associated point-mutants: Lamin A/C R453W and R482W. PLoS One 2010; 5:e10604; PMID:20498701; http://dx.doi.org/10.1371/journal.pone.0010604
- Gant TM, Harris CA, Wilson KL. Roles of LAP2 proteins in nuclear assembly and DNA replication: Truncated LAP2β proteins alter lamina assembly, envelope formation, nuclear size, and DNA replication efficiency in Xenopus laevis extracts. J Cell Biol 1999; 144:1083-96; PMID:10087255; http://dx.doi.org/10.1083/jcb.144.6.1083
- Isaac C, Pollard JW, Meier UT. Intranuclear endoplasmic reticulum induced by Nopp140 mimics the nucleolar channel system of human endometrium. J Cell Sci 2001; 114:4253-64; PMID:11739657
- Chang W, Worman HJ, Gundersen GG. Accessorizing and anchoring the LINC complex for multifunctionality. J Cell Biol 2015; 208:11-22; PMID:25559183; http://dx.doi.org/10.1083/jcb.201409047
- Gehrig K, Lagace TA, Ridgway ND. Oxysterol activation of phosphatidylcholine synthesis involves CTP:phosphocholine cytidylyltransferase α translocation to the nuclear envelope. Biochem J 2009; 418:209-17; PMID:18980580; http://dx.doi.org/10.1042/BJ20081923
- Gehrig K, Cornell RB, Ridgway ND. Expansion of the nucleoplasmic reticulum requires the coordinated activity of lamins and CTP:phosphocholine cytidylyltransferase α. Mol Biol Cell 2008; 19:237-47; PMID:17959832; http://dx.doi.org/10.1091/mbc.E07-02-0179
- Terzakis JA. The nucleolar channel system of the human endometrium. J Cell Biol 1965; 27:293-304; PMID:5884628; http://dx.doi.org/10.1083/jcb.27.2.293
- Kittur N, Zapantis G, Aubuchon M, Santoro N, Bazett-Jones DP, Meier UT. The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings. Mol Biol Cell 2007; 18:2296-304; PMID:17429075; http://dx.doi.org/10.1091/mbc.E07-02-0154
- Guffanti E, Kittur N, Brodt ZN, Polotsky AJ, Kuokkanen SM, Heller DS, Young SL, Santoro N, Thomas Meier U. Nuclear pore complex proteins mark the implantation window in human endometrium. J Cell Sci 2008; 121:2037-45; PMID:18505792; http://dx.doi.org/10.1242/jcs.030437
- Kohorn EI, Rice SI, Hemperly S, Gordon M. The relation of the structure of progestational steroids to nucleolar differentiation in human endometrium. J Clin Endocrinol Metab 1972; 34:257-64; PMID:4110445; http://dx.doi.org/10.1210/jcem-34-2-257
- Dockery P, Pritchard K, Warren MA, Li TC, Cooke ID. Uterus and endometrium: Changes in nuclear morphology in the human endometrial glandular epithelium in women with unexplained infertility. Hum Repro 1996; 11:2251-6; PMID:8943538; http://dx.doi.org/10.1093/oxfordjournals.humrep.a019085
- Wynn RM. Intrauterine devices: effects on ultrastructure of human endometrium. Science 1967; 156:1508-10; PMID:5611026; http://dx.doi.org/10.1126/science.156.3781.1508
- Feria-Velasco A, Aznar-Ramos R, González-Angulo A. Ultrastructural changes found in the endometrium of women using megestrol acetate for contraception. Contraception 1972; 5:187-201; PMID:4650649; http://dx.doi.org/10.1016/0010-7824(72)90045-5
- Pryse-Davies J, Ryder TA, Lynn MacKenzie M. In vivo production of the nucleolar channel system in post menopausal endometrium. Cell Tissue Res 1979; 203:493-8; PMID:519737; http://dx.doi.org/10.1007/BF00233277
- Nejat EJ, Szmyga MJ, Zapantis G, Meier UT. Progesterone threshold determines nucleolar channel system formation in human endometrium. Repro Sci 2014; 21:915-20; PMID:24458483; http://dx.doi.org/10.1177/1933719113519177
- Olins AL, Buendia B, Herrmann H, Lichter P, Olins DE. Retinoic acid induction of nuclear envelope-limited chromatin sheets in HL-60. Exp Cell Res 1998; 245:91-104; PMID:9828104; http://dx.doi.org/10.1006/excr.1998.4210
- Olins D, Olins A. Nuclear envelope-limited chromatin sheets (ELCS) and heterochromatin higher order structure. Chromosoma 2009; 118:537-48; PMID:19521714; http://dx.doi.org/10.1007/s00412-009-0219-3
- Popken J, Schmid VJ, Strauss A, Guengoer T, Wolf E, Zakhartchenko V. Stage-dependent remodeling of the nuclear envelope and lamina during rabbit early embryonic development. J Repro Dev 2016; 62:127-35; PMID:26640117; http://dx.doi.org/10.1262/jrd.2015-100