?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This paper investigates the leaching potential of heavy metals present in stabilised/solidified (S/S) electric arc furnace dust (EAFD). Granular leaching test results showed that Mo was effectively stabilised by Portland cement type I (CEMI) and MgO at 1:2:7 and 3:12:35 ratios. Monolithic leaching test results showed that pulverised fuel ash (PFA) stabilised Zn and Pb but not Mo and Cl. MgO was highly effective in reducing Zn leaching. X-ray diffraction analysis revealed the presence of zinc ferrite in the solidified EAFD products which explains the excellent immobilisation of Zn. The leaching mechanisms analysis demonstrated that few S/S samples exhibited a leaching behaviour dominated by diffusion with the majority displaying a leaching mode dominated by depletion. Although the monoliths’ physical integrity was maintained, Pb, Cl and Mo were not effectively immobilised with PFA and MgO-based systems based on the current granular and monolith leaching test protocols.
1. Introduction
Toxic and hazardous wastes have been a major issue as not all can be destroyed or eliminated. It is possible to chemically rearrange or detoxify them, thus isolating them from the environment and avoiding human, animal and plant exposure to these toxic materials (Griffin, Citation2009). In the last few years, strict regulations have been drawn up in Europe, naturally including the UK, to control the release of heavy metals into the environment. As a consequence, the Waste Acceptance Criteria (WAC) were introduced under the Landfill (England and Wales) Regulations in July 2005 (Environment Agency, Citation2005). According to the WAC, hazardous wastes have to be pre-treated so as to meet the limits specified in the regulations prior to landfill disposal. Many different technologies have been developed for the pre-treatment of various types of hazardous waste, including; (1) additive to cement/concrete industry (de Vargas, Masuero, & Vilela, Citation2006), (2) recovery of valuable metals (iron, zinc and lead) before land disposal (MacRay, Citation1985) and (3) additive to asphalt cement (Alsheyab & Khedaywi, Citation2013; Šturm et al., Citation2009)). Among these available technologies solidification/stabilisation (S/S) has been accepted as the best demonstrated available technology due its ease of use and low cost (Conner, Citation1990).
This paper focuses on the treatment of electric arc furnace baghouse dust (EAFD), a solid waste generated as a by-product in the steel manufacturing industry when metal scrap is electrically melted. This dust contains iron oxide and toxic metals such as Pb, Zn, Cr and Cd, which are dangerous for the environment and human health. These elements are readily soluble in rain water and if the dust is not properly disposed of, these metals can get into rivers and eventually into drinking water. Due to its toxic metals content, this waste has been listed as hazardous waste under the code 10 02 07* in the European Waste Catalogue (EWC) in 2002 (European Union, Citation2002; Orhan, Citation2005; Pereira, Rodrı́guez-Piñero, & Vale, Citation2001; Sofili, Rastovcan-Mioc, Cerjan-Stefanovic, Novosel-Radovic, & Jenko, Citation2004) and hence has to be treated before being disposed of in a landfill according to the WAC (Environment Agency, Citation2005). S/S is one of the most widely used techniques for the pre-treatment of EAFD. It aims to reduce the permeability/mobility of the contaminants, reduce the level of toxicity and limit the solubility of heavy metals present in EAFD. The right choice and effective use of binders in S/S is important since they physically and chemically trap contaminants. Portland cement and lime have been widely used as primary binders while ground granulated blast furnace slag (GGBS), pulverised fuel ash (PFA), natural pozzolans, low grade magnesium oxide and silica fume are widely used as secondary binders (Alsheyab & Khedaywi, Citation2013; Arribas, Santamaría, Ruiz, Ortega-López, & Manso, Citation2015; Chang, Lin, Ko, & Liaw,Citation1999; Chen, Tyrer, Hills, Yang, & Carey, Citation2009; del Valle-Zermeño, Giro-Paloma, Formosa, & Chimenos, Citation2015; Fernandez, Macias, Guerreo, Lorenzo, & Goni, Citation2000; Gervais & Ouki, Citation2000, Citation2002, Lampris, Stegemann, & Cheeseman, Citation2008, Citation2009a, Citation2009b; Paria & Yuet, Citation2006; Pasetto & Baldo, Citation2010; Poon, Lio, & Tang, Citation2001; Rodrfguez-Piñero, Pereira, de Elvira Francoy, & Vale Parapar, Citation1998; Salihoglu & Pinarli, Citation2008; Serjun et al., Citation2015; Shi & Spence, Citation2004; Stegemann & Zhou, Citation2009).
Ordinary Portland cement type I (CEMI) was used as the primary binder alongside with PFA. In addition to well-established binding agents, a less commonly used material, low-grade MgO (MgO hereafter), was also used in this study. A limited data is available on the use of MgO in the S/S of EAFD (Cubukcuoglu & Ouki, Citation2012; Fernández, Chimenos, Raventós, Miralles, & Espiell, Citation2003) whereas wide range of data is available on the use of PFA in the S/S treatment of hazardous wastes. Hence, this paper aims to compare the effectiveness of these two binders in S/S of EAFD by both testing the physical (unconfined compressive strength (UCS) test) and chemical characteristics (via various leaching tests) of samples before and after S/S. Physical testing is required to demonstrate the relative success or failure of the S/S process while chemical testing is used to evaluate the performance of S/S as a treatment method for hazardous waste. Leaching tests are essential to evaluate the waste leachability of the granular and/or monolith wastes. The compliance test protocols required by the landfill regulations were used to evaluate the leaching potential of the S/S products prepared.
2. Materials and methods
2.1. Materials
The waste (EAFD) used in this study was provided by Tata Steel Europe. It was collected from the engineering steels site of the company located in Rotherham, South Yorkshire, UK with a production capacity of 1.3 Mt/year. The dust generation is estimated to around 10 to 20 kg/tonne crude steel (Menad, Ayala, Garcia-Carcedo, Ruiz-Ayúcar, & Hernández, Citation2003) while the worldwide crude steel production in 2011 was reported as 1527 Mt (World Steel Association, Citation2015). The MgO used in the study was supplied by Magnesitas Navarras, S.A. It is a by-product of the calcination of magnesite in a kiln at a temperature of 1100°C. The CEMI, ASTM C150-89 (ASTM C 150-89, Citation1989), was provided by Hanson Cement as commercial cement. PFA, also known as fly ash, is produced in UK power stations and was provided by the United Kingdom Quality Ash Association (UKQAA). The elemental composition and the leaching results obtained from the as-received waste sample subject to BS EN 12457-2 are presented in Table .
Table 1. The chemical composition and leaching results of as-received EAFD.
2.2. Sample preparation
Cubic samples 5 × 5 × 5 cm in dimension were prepared in triplicates with CEMI:MgO:EAFD and CEMI:PFA:EAFD combinations at different mix ratios and water-to-solid (w/s) ratios between 0.2 and 0.4. The design matrix of mixes and w/s ratios are given in Table . The samples were mixed (dry mixes prior to water addition), moulded and de-moulded according to BS EN 196-1 (Citation2005). They were then covered with a damp cloth and placed into sealed polyethylene bags to avoid any possible carbonation of the cementitious matrix during the curing period by maintaining a high humidity environment (at a temperature of 20 ± 2 °C and 95% humidity). Physical durability of the S/S products was tested by UCS before and after water immersion (WI) as a function of time at 28 days’ curing age (UCS – BS EN 196-1, Citation2005). The samples were cured for 21 days and then immersed in water for 7 days for WI testing. Granular leaching tests were carried out before and after S/S processes. The samples were dried at 60°C for 48 h, stood for 1 h at room temperature and were finally grounded and sieved to less than 150 μm to produce a waste in a homogenous form. Monolithic leaching tests were performed on cubic monoliths which were immersed in water for 64 days after 28 days of curing.
Table 2. Design matrix of binders, EAFD and water mixes.
2.3. Product testing protocols
2.3.1. Physical properties
The physical properties of S/S EAFD are determined through the use of certain experimental techniques which were reported earlier (Cubukcuoglu & Ouki, Citation2012) with performance threshold values identified by Stegemann and Zhou (Citation2009). UCS results are presented only for mix designs at 28 days (before and after WI) with the threshold value at UCS28days > 1 MPa. All of the test samples were prepared in triplicates.
2.3.2. Leaching properties
The leaching behaviour of S/S products was tested to provide measures by which to judge the results of treatability studies and full-scale application. Moreover, pH measurements were made in order to predict reaction conditions and impact on leaching characteristics.
2.3.2.1. Granular leaching test
The leaching tests were conducted only on samples with UCS28d higher than 1 MPa. The granular leaching test was carried out according to akin-BS EN 12457-2 (Citation2002). Contaminant solubility was identified by the chemical analysis of leachates without acid addition. A grinded and sieved sample weighing 5.0 ± 0.2 g was mixed with deionised water. The tubes were sealed and agitated for 48 h using an orbital platform (IKA KS501 Digital) rotating at 250 cycles/min and subjected to centrifuge at 6500 rpm for 10 min. The fluid was then extracted, filtered and poured into bottles with 0.45 μm syringe filters. The pH values were determined from a solid-to-liquid ratio of 1:10 water leaching test.
2.3.2.2. Monolithic leaching test
The monolithic leaching test, also known as the tank test, was conducted according to NEN 7375 – diffusion test (EA NEN 7375, Citation2004). The samples cured for 28 days were immersed into a tank filled with deionised water at a liquid-to-solid ratio equal to 4 L/kg. A 120 mL aliquot was collected on 8 different occasions over a total period of 64 days and filtered through a 0.45 μm syringe filter before pH measurements and chemical analysis for the determination of cumulative releases of heavy metals of concern. This test was used to control the contaminant release from the monolith. It is an important parameter used for testing monolithic wastes as it provides an indication of the real-time leaching potential of contaminants by diffusion from inside the monolith to the outside surface of the monolith. In this test, leaching is related to the surface area of the waste material which is in a monolithic form (Van der Sloot, Citation2007). The measured cumulative leaching in each of the periods n = 1 to N was calculated as follows:(1)
(1)
where is the measured leaching (mg/m2) of the component in fraction i;
is the measured cumulative leaching (mg/m2) of a component during the period n including fraction i = 1 to n and N is the number of specified replenishment times (N = 8).
The following formula was used to determine the derived cumulative leaching of a component:(2)
(2)
where is the derived cumulative leaching (mg/m2) of a component for period n including fraction i = 1 to n;
is the measured leaching (mg/m2) of the component in fraction i and N is the number of specified replenishment times (N = 8); t
i
is the replenishment time of fraction i and t
i−1 is the replenishment time of fraction i−1. e
n
indicates only the cumulative leaching up to and including period i on the basis of the measured leaching up to and including period i. It is important to note that
always includes the measured leaching of previous periods since any deviations in a period (like wash-off effects) could affect the following periods, which may make interpretation difficult (EA NEN 7375, Citation2004). These values help to determine whether the leaching is affected by diffusion. Heavy metals’ leaching data are evaluated as the log-log plots of derived cumulative leaching (ɛ
n
) vs. time. The leaching mechanisms are determined based on the slope values, hence a slope < 0.35 indicates surface wash-off or depletion, 0.35 < slope < 0.65 indicates diffusion and a slope > 0.65 indicates dissolution.
2.4. Mineralogical and micro-structural analyses
The surface and micro-structural characteristics of the EAFD, binders and S/S products were analysed using X-ray diffraction (XRD) and energy-dispersive X-ray spectroscopy (SEM-EDX). XRD gives an insight into the different mineralogical phases of the materials and products. A Bruker D8 advance Cu anode 2.2 kW tube at 40 kV and 40 mA were used for XRD analyses. 0.4 s counting time per 0.02° 2θ step; with 176 channels active equivalent to 70.4 s per step were used at 5–70° 2θ. Data was collected with the DiffracPlus XRD commander software.
SEM-EDX identifies the presence of trace metals in particular solid phases. A Hitachi S3200 N electron microscope fitted with a secondary electron and backscattered electron (BSE) imaging system with dispersive analytical capabilities was used for SEM-EDX observations.
3. Results and discussion
3.1. Physical properties
UCS plays a vital role in examining the leaching potential of heavy metals from solidified wastes. A curing age of 28 days is required to test S/S products as two-thirds of the cement hydration processed is completed over this period of time (Glasser, Citation1997). The UCS values for CEMI:PFA:EAFD and CEMI:MgO:EAFD blended samples at 28 days before and after immersion are given in Table . The compressive strengths of CEMI:MgO:EAFD mix combinations ranged between 2.9 ± 0.2 and 14.9 ± 0.9 MPa where UCS ranged between 4.3 ± 0.2 and 27.8 ± 0.7 MPa for CEMI:PFA:EAFD blended samples. Hence, all ratios studied at 28 days’ curing age (both before and after WI) met the UCS performance threshold value (>1 MPa) (Stegemann & Zhou, Citation2009). Even though promising strength development was achieved at almost all ratios studied, it was observed that waste addition had a damaging effect on the calcium-silicate-hydrate (C-S-H) hydration, which resulted in lower strength. This finding is valid for all ratios of PFA blended samples. However, an increase was observed for MgO-blended samples when waste was first incorporated at 40%. This was mainly due to the lower w/s ratios used in those samples. Lower w/s ratios lead to better strength development and durability. As confirmed by other researchers, UCS values for products containing smaller amounts of waste and binder were greater than for products containing a higher proportion of waste and binders (Dermatas, Menounou, & Meng, Citation2006). The UCS after immersion is slightly lower than before immersion for only a few mix ratios studied. As it is also mentioned in Goumans, Senden, & van der Sloot, Citation1997; UCS after immersion is expected to increase. The addition of larger proportions of water in the S/S process should be investigated to provide improved strength formation. Cement content and curing conditions (with or without moisture) tend to be the major factors to have an increasing effect on strength development after immersion. Hence, this explains the reason why there are lots of mixtures which UCS has increased after immersion. Since the decrease in UCS after immersion only exhibited for a few samples and the maximal diminution was only observed up to 2 MPa, it can be said that it is caused due to the expansion or non-uniform saturation of the waste.
Table 3. CEMI:MgO:EAFD and CEMI:PFA:EAFD mix combinations for UCS 28 days before and after Water Immersion (WI).
It is suspected that certain chemical elements have an influence on the strength development. The decreasing UCS values after immersion indicate possible matrix disruption reactions. Caldwell, Stegemann, and Shi (Citation1999) explained the weaker strength values after immersion with the expansion or non-uniform saturation of the waste. The results of the present study clearly indicate that the differences in UCS before and after immersion result from the curing environment employed. It is well known that MgO (periclase crystalline form) undergoes a delayed and expansive hydration that causes damage to the integrity of cement structures (Liwu, Tang, & Al-Tabbaa, Citation2014). For that reason, the amount of MgO is limited in several cementititous applications. Indeed, the low compressive strength achieved even in the waste-free sample could point to this behaviour. MgO itself is a very reactive material which was observed during the mixing process. A high amount of heat was released when MgO was reacted with water and cement. However, the expected reactivity and possible foreseen problems related to this behaviour at long-term was investigated by testing UCS of samples after longer periods (i.e. 56 days and longer). Even though a significant reduction was observed in the samples with MgO incorporation, an increase in UCS was observed after 28 days of curing. Hence, no more significant detrimental impact is expected during long-term period on the physical integrity of MgO incorporated samples.
3.2. Granular leaching test results
The relationships between the leachability of the contaminants and the leachate pH in the different mixes shed light on the immobilisation mechanism(s) for the various binders. This is due to the importance of pH factor in controlling the release potential of heavy metals. PFA is an alkaline material with pH > 9 and EAFD has a high natural pH of about 12. Portland cement is an alkaline material with a high buffering capacity and MgO is also an alkaline material with pH values around 11–12. Higher pH values mean an alkaline environment, which is the most suitable for the immobilisation of most heavy metals. However, some metal hydroxides (such as Pb and Zn) may have high solubility rates at a high pH range of about 12 due to their amphoteric properties. As discussed in a previous study (Cubukcuoglu & Ouki, Citation2012), metal leaching is normally limited in the pH range 7–12. However, due to the amphoteric behaviour of some metals, their solubility and hence an increase in their leaching potential would not be unexpected. For instance, Zn may be completely stabilised at pH 7 whereas its stability is low at a pH range between 9 and 11 (Bhatty, Citation1987; Srivastava, Chaudhary, & Khale, Citation2008).
The landfill WAC for granular wastes was taken into consideration for the evaluation of the results achieved. The granular leaching test results for CEMI:MgO:EAFD and CEMI:PFA:EAFD combinations with 40 and 70% waste addition are shown in Table . These results showed that Pb leaching was within the WAC limits for both MgO and PFA-blended samples. This may be linked to the possible incorporation of Pb into the un-dissolved C-S-H matrix or its precipitation as Pb silicate compounds (Kogbara & Al-Tabbaa, Citation2011). According to Cheeseman, Asavapisit, and Knight (Citation1998), Pb(OH)3 is sorbed on C-S-H surfaces and hence not released back into the solution. Malviya and Chaudhary (Citation2006) achieved the lowest Pb leaching at the pH range of 8 to 9 where 8.5 is accepted as the theoretical solubility point for Pb. Pb leachability increases when pH > 11 due to the formation of . The findings of this study shows that less Pb leachability is achieved at pH values lower than 11.96 regardless of the amount of waste incorporated into the mixture and a sharp increase in Pb leaching rates was observed as soon as pH reaches the level of 12.19. Soluble Pb hydroxide complexes can form at pH > 12 thus increases Pb mobility. Voglar and Leštan (Citation2010) reported a similar observation.
Table 4. Granular leaching test results – release of contaminants – 28 days (mg/kg).
Mo leaching rates exceeded the limits for almost all ratios studied except CEMI:MgO:EAFD 1:2:2 and 3:12:10 mix ratios. Lower Mo leaching rates were achieved at these two ratios mostly because of the reduced amount of Mo in the solidified waste samples due to the lower waste addition. Therefore, 40% waste addition could be accepted as the optimum level to achieve the best immobilisation of most of the heavy metals investigated. Molybdate () is the main speciation of Mo which is affected by leachate pH. It is soluble and formed when minerals containing Mo come in contact with water. Mo solubility is pH-dependent and Mo can exist in different valences and oxidation states as water soluble molybdate salts. Mo leaching mostly increased as pH increased. This was due to the lack of
ions because of OH− ions that replaced
ions. A study by Kindness, Macias, and Glasser (Citation1994) on Mo immobilisation mentions that, in Portland cement, the insoluble Mo is distributed between powellite (CaMoO4) and a calcium aluminate sulphate hydrate with a very small amount in cement gel, a hydration calcium silicate. Alkaline conditions increase the mobility and solubility of Mo (Calderone & Frankenberger, Citation1990). It is believed that the Mo leaching rate may be controlled by CaMoO4 precipitation (Johnson, Kersten, Ziegler, & Moor, Citation1996).
Zn leaching rates for the PFA blends were much lower than the WAC limits. Zn is amphoteric and hence soluble both under acidic and/or alkaline conditions. The leachability of the metal in the treated EAFD increased at pH > 11 in line with the solubility profile of Zn(OH)2. Zn generally displays an amphoteric behaviour when it is found as an oxide or hydroxide. Its solubility increases with respect to zinc hydroxide formation and may dissolve in a basic environment as zincate (Pereira et al., Citation2001). According to the results obtained in the present study, in a basic environment, where the pH was around 11.6–11.9 for PFA-blended mixes and 11.1–12.4 for MgO-blended samples, Zn leaching was low. In other words, the solubility of Zn was controlled in a basic environment. The same behaviour was observed in a study by Salihoglu and Pinarli (Citation2008).
3.3. Monolithic leaching test results
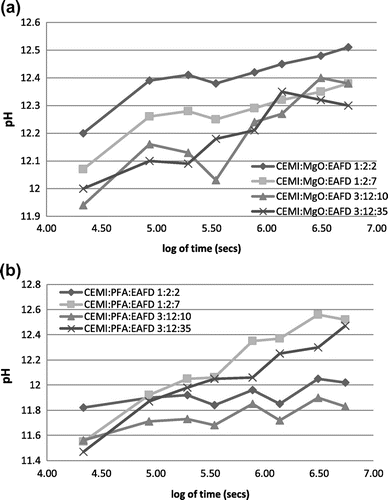
The pH values of the solution for different combinations of the monolithic leaching test for the samples prepared are determined and presented in Figure (a) and (b), respectively. As already discussed within granular leaching test results section of the paper, pH is an important factor in controlling the release potential of heavy metals. All CEMI:MgO:EAFD combinations exhibited pH values in the range of 11.9–12.5 while CEMI:PFA:EAFD mixes had similar pH values ranging around 11.5–12.6. According to the results shown in Figure (a), pH gradually increased with time with only a slight decrease on the 4th (log104 = 5.539) days of leaching for all CEMI:MgO:EAFD mixes and on the 64th days of leaching for CEMI:MgO:EAFD 3:12:10 and 3:12:35 mix designs. According to Figure (b), a slight pH decrease was observed on the 4th (log104 = 5.539); 16th (log1016 = 6.141) and 64th (log1064 = 6.743) days of leaching for CEMI:PFA:EAFD 1:2:2 and 3:12:10 mix design ratios. These trends are similar to those obtained by Stegemann and Cote (Citation1988). It is very well known that the increasing trend of pH of the leachant influenced the leaching potential of metals. In the monolithic leaching test, pH increased with time when more soluble alkali hydroxides were released from the monoliths. This was mainly due to the leachant that flowed through the monoliths. As mentioned by Poon and Chen (Citation1999), the first release is from the surface of the monoliths where the release concentration increases when the leachant saturates the pores of the monoliths with time.
Figure 1. pH variations for the different fractions under the monolithic leaching test conditions (a) CEMI:MgO:EAFD and (b) CEMI:PFA:EAFD.
In this study, the cumulative measured leaching over the test period is accepted as the upper limit of leaching. The leaching potential of 11 different heavy metals have been investigated including As, Cd, Co, Cr, Cu, Sb, Se, Mo, Ni, Pb and Zn. It is important to note that all systems studied were successful at Zn immobilisation, which is considered to be the most problematic component since it has the highest concentration in the waste. According to the leaching analysis data of as-received EAFD provided in Table , Mo and Pb exist in very small amounts but Zn is found in significant amounts. Measured cumulative Pb, Zn, Mo and Cl release () of all mixes tested according to the monolithic leaching test are shown in Figures , respectively. Moreover, the total measured amount of each element leached (Pb, Zn, Mo and Cl), expressed as emission per unit of external surface area, slopes of the log-log plots of derived cumulative leaching vs. time for diffusion controlled increments and the estimated 64-day emissions based on the diffusion-controlled interval are all summarised in Table . Other mentioned elements were also analysed and found to be lower than the limits and hence were not represented in these figures and table. None of these heavy metals’ leaching rates exceeded the monolithic WAC (monWAC) limits for any of the ratios of CEMI:PFA:EAFD studied except Cr, Mo and Cl. The results showed that the metals’ leaching ratio increased as the percentage of EAFD in the mixture increased. MgO effectively stabilised Zn and achieved smaller leaching ratios when compared to PFA. However, MgO was only found effective in reducing Zn leaching but not effective in Pb, Mo and Cl leaching control. Pb, Mo and Cl leaching rates exceeded the monWAC limits (Mo – 20 mg/m2; Zn – 100 mg/m2) at the ratios studied for MgO-blended mixes. Mix designs without any waste addition were also tested and found to contain trace quantities at all mix ratios. Pb leaching rates were extremely high in MgO-blended combinations at all ratios studied with waste addition and exceeded the monWAC limits (Pb – 20 mg/m2) where PFA-blended mix combinations had zero leaching rates for Pb. PFA was sufficiently effective in immobilising Pb. Cl leaching rates were extremely high at all ratios studied and hence exceeded the monWAC limits (20,000 mg/m2). On the other hand, MgO had lower leaching rates than PFA-blended mixtures, but still higher than the required limits.
Figure 2. Cumulative derived Pb leaching plots of (a) CEMI:MgO:EAFD 1:2:2; (b) CEMI:MgO:EAFD 1:2:7; (c) CEMI:MgO:EAFD 3:12:10; (d) CEMI:MgO:EAFD 3:12:35; (e) CEMI:PFA:EAFD 1:2:2; (f) CEMI:PFA:EAFD 1:2:7; (g) CEMI:PFA:EAFD 3:12:10; (h) CEMI:PFA:EAFD 3:12:35.
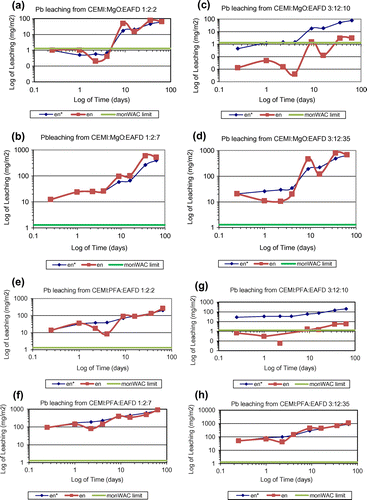
Figure 3. Cumulative derived Zn leaching plots of (a) CEMI:MgO:EAFD 1:2:2; (b) CEMI:MgO:EAFD 1:2:7; (c) CEMI:MgO:EAFD 3:12:10; (d) CEMI:MgO:EAFD 3:12:35; (e) CEMI:PFA:EAFD 1:2:2; (f) CEMI:PFA:EAFD 1:2:7; (g) CEMI:PFA:EAFD 3:12:10 (h) CEMI:PFA:EAFD 3:12:35.
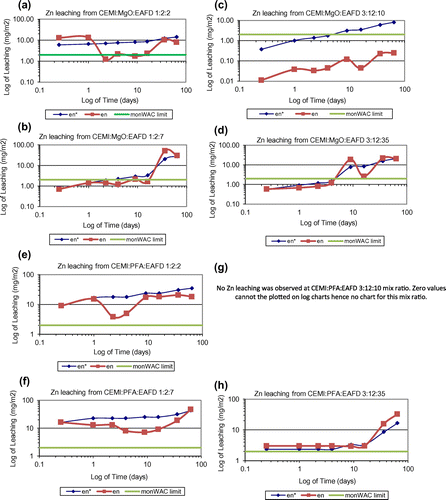
Figure 4. Cumulative derived Mo leaching plots of (a) CEMI:MgO:EAFD 1:2:2; (b) CEMI:MgO:EAFD 1:2:7;(c) CEMI:MgO:EAFD 3:12:10; (d) CEMI:MgO:EAFD 3:12:35; (e) CEMI:PFA:EAFD 1:2:2; (f) CEMI:PFA:EAFD 1:2:7; (g) CEMI:PFA:EAFD 3:12:10; (h) CEMI:PFA:EAFD 3:12:35.
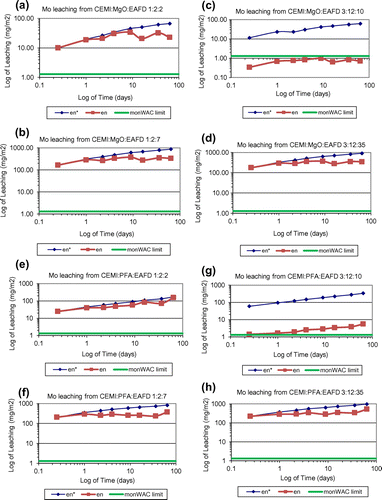
Figure 5. Cumulative derived Cl leaching plots of (a) CEMI:MgO:EAFD 1:2:2; (b) CEMI:MgO:EAFD 1:2:7; (c) CEMI:MgO:EAFD 3:12:10; (d) CEMI:MgO:EAFD 3:12:35; (e) CEMI:PFA:EAFD 1:2:2; (f) CEMI:PFA:EAFD 1:2:7; (g) CEMI:PFA:EAFD 3:12:10; (h) CEMI:PFA:EAFD 3:12:35.
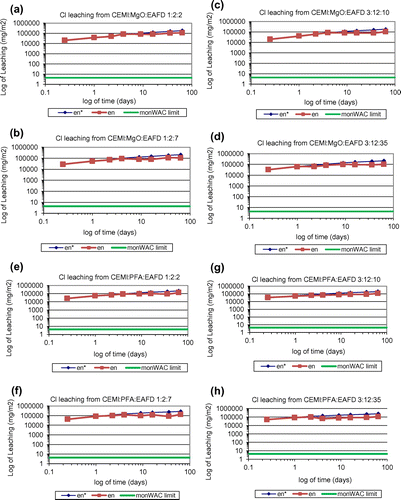
Table 5. Diffusion controlled increments and slopes of the log-log plots.
In overall, Mo and Cl leaching rates were above the monWAC limits and hence, these metals’ fixation was not effective with PFA and MgO-based s/s systems.
As it is clearly shown in Figure (e)–(h), the release of Cl occurred during the first 2 days of leaching with very little leaching during the remaining period of the test for PFA-blended samples. Cl is a weakly bounded species of the matrix. Cl leaching may be due to the formation of mono-chloroaluminates of Ca. It is also important to note that, although digital photographs of the monolith blocks with various binder and EAFD additions are not presented here, expansion, cracking and precipitation were not observed in samples after the 64 days of the leaching test.
The gradient of a log-log plot of cumulative leaching vs. time indicates the predominant leaching mechanism according to the standard method followed (EA NEN 7375, Citation2004). Linear regression analysis was used to calculate the slope of the cumulative log-log plot of leaching. Gradients less than 0.35 indicates wash-off, between 0.35 and 0.65 indicate diffusion controlled release, whereas gradients greater than 0.65 indicate dissolution. Examination of the slopes for different leaching intervals in Figures shows diffusion control in early leaching intervals (1–4). These trends are similar to findings of Lu, Wei, Tang, and Giesy (Citation2016) and Lampris, Stegemann, Pellizon-Birelli, Fowler, and Cheeseman (Citation2011).
For CEMI:PFA:EAFD blends, no leaching mechanism could be determined for Zn whereas diffusion control was dominant in the early intervals (1–4) and depletion in the later intervals for Cl. For CEMI:PFA:EAFD 1:2:7 mix ratio, wash-off was observed for Pb and Mo and diffusion for Cl. However, depletion was observed in later intervals for all samples. For CEMI:PFA:EAFD 3:12:10 mix ratio, wash-off was predominant in the early intervals for Mo and Cl. In the later intervals, depletion was the controlling mechanism for Mo and Cl. For CEMI:PFA:EAFD 3:12:35, wash-off was the driving leaching mechanism for Pb and Mo. For CEMI:MgO:EAFD 1:2:2 mix ratio, mostly diffusion-controlled leaching was observed for Cl whereas in later leaching intervals depletion-dominant leaching was observed for Zn and Mo. Diffusion was observed in the early leaching intervals for Mo and Cl for CEMI:MgO:EAFD 1:2:7 whereas dissolution was observed for Pb and Zn at later leaching intervals. Wash-off was observed at early leaching intervals for Pb and Zn and dissolution at later leaching intervals. In later leaching intervals, Mo and Cl depletion was observed. For CEMI:MgO:EAFD 3:12:10, wash-off was observed for Pb in early leaching intervals and dissolution in later intervals. Zn and Cl showed wash-off in early leaching intervals and dissolution for Pb and Zn in later intervals.
For all mixes studies, it is clear from the data provided in Figures and Table , Pb and Cl release were found to be diffusion controlled.
In general, the slope values were less than 0.5 and varied at all ratios and intervals studied. This demonstrates that the leaching mechanisms of the samples continuously change, as described earlier. It is clear from the results obtained that only very few of the pieces tested displayed a leaching behaviour mainly dominated by diffusion (the mode that the test is designed to record). A very high proportion of the samples showed a mode dominated mostly by depletion. The 64-day emissions are estimated from the results of the diffusion control period. However, in the presence of depletion, the estimated 64-day emissions might provide an overestimate of the actual leaching (Lampris et al., Citation2009b). In the present study, depletion was the dominant leaching mechanism at most of the mix ratios and leaching intervals for the heavy metals investigated.
The estimated 64-day emissions are determined by the diffusion-controlled interval. Hence, the differences between the measured and derived 64-days emissions can be easily observed. Measured emissions during the test may be influenced or controlled by other mechanisms whereas calculation of the derived emission always assumes pure diffusion control. Based on the data achieved it can be concluded that for elements under diffusion control, the estimated release is close the measured release.
In conclusion, the results showed that the most problematic element in this study was Cl. It is not present in the waste at very high levels as other metals do but it leaches at extremely high ratios. Overall, MgO showed a better performance than PFA in that it yielded lower Mo leaching rates. The monoliths’ leaching tests showed that the release of Pb, Cl and Mo exceeded the UK limits for compliance for cement-based waste forms to be disposed of in landfills for hazardous wastes. However, the limits set for compliance of granular waste were met by crushed S/S products with waste loads of up to 70%.
3.4. Mineralogy and micro-structure
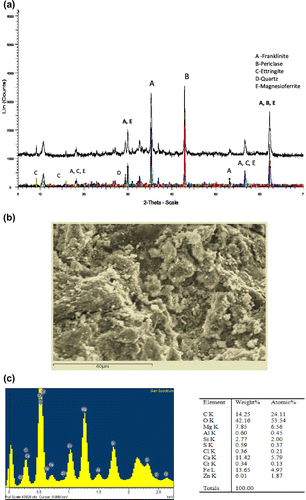
Iron oxides are the main components of EAFD. However, considerable amounts of volatile components (such as Zn, Pb, Cd and other metallic compounds) are also transferred into the dust during the melting of scrap metal. Hence, the mineralogical structure is composed of those elements added during the production process of steel. Fe is present in EAFD mostly as magnetite whereas Zn is present as franklinite or franklinite with isomorphous substituted metals and zincite (Havlik, Souza, Bernardes, Schneider, & Miskufova, Citation2006). The XRD analysis of this waste reveals the presence of zincite and franklinite as the two main phases.
PFA is a well-established binding agent so XRD results for PFA-blended samples are not presented in this paper. On the other hand, the XRD analysis and micrograph of a selected mix combination (CEMI:MgO:EAFD 1:2:2) is presented to provide an overall idea on the phases identified when waste is incorporated into a cement-MgO blended mix matrix. Crystalline phases identified in the S/S product are presented in Figure (a) with a micrograph and sum spectrum in Figure (b) and (c), respectively. SEM- EDX analysis reveals the presence of O, C, Fe, Ca, Mg and Zn, which are attributed to periclase, calcite, ettringite, franklinite, larnite (β-C2S); with periclase, quartz and vaterite as the dominant phases in the sample. In addition, a γ-C2S phase was detected with dolomite. The existence of those phases was also confirmed by XRD analysis. The reflection peaks observed in the XRD patterns are in agreement with the Rietveld refinement results. Accordingly, periclase, franklinite and C2S-β are the dominant phases present in this sample while periclase and franklinite phases were also found in the as-received EAFD. Franklinite had reflection peaks at 30°, 35.5°, 43° and 62°, periclase at 43° and 62.2°. The content of the crystalline phases is directly proportional to the reflection peak intensity. Results show that when MgO is blended with cement and EAFD, periclase and dolomite are the major crystalline phases dominating the XRD pattern of the paste. As a result of EAFD addition, a hydration retardation of cementitious materials was expected. This would cause the appearance of the un-reacted di- and tri-calcium silicate phases as reported by Asavapisit, Naksrichum, and Harnwajanawong (Citation2005). The formation of franklinite was expected and reported during the Portland cement hydration in the presence of Zn. It is well known that Zn is present as ZnFe2O4 (franklinite) and ZnO (zincite) in EAFD while Fe is present mostly as Fe3O4 (magnetite). Zincite is considered as a problematic phase when it comes to leaching. When Zn is present in the form of zincite, it is easily leached/dissolved into the solution whereas zinc ferrite is less likely to cause leaching problems (Oustadakis, Tsakiridis, Katsiapi, & Agatzini-Leonardou, Citation2010). The XRD analysis revealed the presence of zinc ferrite in the S/S of EAFD; this explains the excellent immobilisation of Zn as illustrated in the leaching test results.
4. Conclusions
A durable cement-based matrix is produced as a result of hydration. The presence of a considerable amount of Zn in the waste has to be considered as a major concern for the effectiveness of the S/S process, since this compound is a well-known retarding agent for cement hydration. However, results showed that Zn can be successfully immobilised in S/S products and the leaching ratios are very low when compared to other metals that exist in very small quantities in the waste (e.g. Mo). To meet the UK WAC requirement for being a solid (UCS28d > 1 MPa), a maximum of 40% waste load for CEMI:MgO:EAFD and CEMI:PFA:EAFD is allowed. UCS failure started at waste additions in excess of those mentioned above and the S/S product could not ensure the necessary integrity of the waste form. An analysis of heavy metals is required in order to decide the exact effect of pH changes of the solution on the leaching of heavy metals. Monolithic tests showed Cl, Mo, Pb and Zn leached from most mix formulations. On the other hand, granular test results showed that most of the mix formulations were successful in stabilising most metals. The monolithic leaching test was derived from the diffusion-controlled leaching mechanism. The effective diffusion coefficient changed with time and so did the release mechanisms for some of the contaminants. The physical integrity of the matrix, the release mechanism, and hence the leachate composition can be adversely affected by the high concentrations of soluble contaminants in the long term. Therefore, as mentioned by Lampris et al. (Citation2011), it is difficult to follow monWAC requirements as exactly as intended.
Overall, PFA-blended samples demonstrated good physical integrity but exhibited poor chemical immobilisation properties for heavy metals. In particular, Mo leaching did not meet the requirements at all ratios studied in any of the leaching tests concluded. In summary, although the granular leaching test is known as the worst case scenario where the mix matrix is spiked with acid additions, a condition that leads to the dissolution of metals, the granular leaching demonstrated much better performance in heavy metal retention when compared to rates of metals leached out of the monolithic leaching test samples.
The results clearly pointed out the fact that the use of both PFA and MgO as an alternative binder for EAFD S/S exhibited much better performance when the granular leaching test was used compared to the monolithic one.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research is funded by the Technology Strategy Board (TSB) UK. It is also supplemented by a grant provided by both TRNC Ministry of National Education and Culture and Funds for Women Graduates (FfWG).
References
- Alsheyab, M. A.T. , & Khedaywi, T. S. (2013). Effect of electric arc furnace dust (EAFD) on properties of asphalt cement mixture. Resources, Conservation and Recycling , 70 , 38–43.10.1016/j.resconrec.2012.10.003
- Arribas, I. , Santamaría, A. , Ruiz, E. , Ortega-López, V. , & Manso, J. M. (2015). Electric arc furnace slag and its use in hydraulic concrete. Construction and Building Materials , 90 , 68–79.10.1016/j.conbuildmat.2015.05.003
- ASTM C 150-89 . (1989). Annual book of ASTM standards . Philadelphia, PA : Standard Specification for Portland Cement.
- Asavapisit, S. , Naksrichum, S. , & Harnwajanawong, N. (2005). Strength, leachability and microstructure characteristics of cement-based solidified plating sludge. Cement and Concrete Research , 35 , 1042–1049.10.1016/j.cemconres.2004.07.041
- Bhatty, M. S. Y. (1987, March 16–18). Fixation of metallic ions in Portland cement. In Proceedings of the 4th International Conference on Hazardous Waste Materials (pp. 140–145). Washington, DC: Silver Springs.
- BS EN 12457-2 . (2002). Characterisation of Waste. Leaching. Compliance test for leaching of granular waste materials and sludges . London: BSI.
- BS EN 196-1 . (2005). Methods of testing cement—Part 1: Determination of strength . London: BSI.
- Calderone, S. J. , & Frankenberger, W. T. (1990). Influence of organic amendments on the mobilization of molybdenum in soils. Bulletin of Environmental Contamination and Toxicology , 45 , 228–231.10.1007/BF01700188
- Caldwell, R. J. , Stegemann, J. A. , & Shi, C. (1999). Effect of curing on field-solidified waste properties. Part 1: Physical properties. Waste Manage . doi:10.1177/0734242X9901700106
- Chang, J. , Lin, T. , Ko, M. , & Liaw, D. (1999). Stabilization/solidification of sludges containing heavy metals by using cement and waste pozzolans. Journal of Environmental Science and Health, Part A , 34 , 1143–1160.10.1080/10934529909376887
- Cheeseman, C. R. , Asavapisit, S. , & Knight, J. (1998). Effect of uniaxially pressing ordinary Portland cement pastes containing metal hydroxides on porosity, density, and leaching. Cement and Concrete Research , 28 , 1639–1653.
- Chen, Q. Y. , Tyrer, M. , Hills, C. D. , Yang, X. M. , & Carey, P. (2009). Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Management , 29 , 390–403.10.1016/j.wasman.2008.01.019
- Conner, J. R. (1990). Chemical fixation and solidification of hazardous wastes . New York, NY : Van Nostrand Reinhold.
- Cubukcuoglu, B. , & Ouki, S. K. (2012). Solidification/stabilisation of electric arc furnace waste using low grade MgO. Chemosphere , 86 , 789–796.10.1016/j.chemosphere.2011.11.007
- de Vargas, A. S. , Masuero, Ângela B. , & Vilela, A. C. F. (2006). Investigations on the use of electric-arc furnace dust (EAFD) in Pozzolan-modified Portland cement I (MP) pastes. Cement and Concrete Research , 36 , 1833–1841.10.1016/j.cemconres.2006.06.003
- del Valle-Zermeño, R. , Giro-Paloma, J. , Formosa, J. , & Chimenos, J. M. (2015). Low-grade magnesium oxide by-products for environmental solutions: Characterization and geochemical performance. Journal of Geochemical Exploration , 152 , 134–144.10.1016/j.gexplo.2015.02.007
- Dermatas, D. , Menounou, N. , & Meng, X. G. (2006). Mechanisms of lead immobilization in treated soils. Land Contamination & Reclamation , 14 , 43–56.10.2462/09670513.702
- EA NEN 7375 . (2004). Leaching characteristics of moulded or monolithic building and waste materials. Determination of leaching of inorganic components with the diffusion Test. ‘The tank test’ based on the translation of the Netherlands Normalisation Institute Standard, Version 1.0. United Kingdom, UK: Environment Agency.
- Environment Agency . (2005). The landfill (England and Wales) (amendment) regulations . Bristol: Author.
- European Union . (2002). European waste catalogue and hazardous waste list by commission decision 2002/532/EC . Ireland: Environmental Protection Agency.
- Fernández, A. I. , Chimenos, J. M. , Raventós, N. , Miralles, L. , & Espiell, F. (2003). Stabilisation of electrical arc furnace dust with Low-grade MgO prior to landfill. Journal of Environmental Engineering , 129 , 275–279.10.1061/(ASCE)0733-9372(2003)129:3(275)
- Fernandez E. , Macias A. , Guerreo, A. , Lorenzo, M. P. , & Goni, S. (2000, May 31, June 1–2). Influence of the cement type on the stabilisation of fly ashes from municipal solid waste incineration. In Proceedings of the International Conference on the Science and Engineering of Recycling for Environmental Protection. Waste Materials in Construction-Wascon . Harrogate: Elsevier Science.
- Gervais, C., & Ouki, S. K. (2000). Effects of foundry dusts on the mechanical, microstructural and leaching characteristics of a cementitious system. Waste Manage Series , 1 , 782–790.
- Gervais, C. , & Ouki, S. K. (2002). Performance study of cementitious systems containing zeolite and silica fume: Effects of four metal nitrates on the setting time, strength and leaching characteristics. Journal of Hazardous Materials , 93 , 187–200.10.1016/S0304-3894(02)00005-5
- Glasser, F. P. (1997). Fundamental aspects of cement solidification and stabilisation. Journal of Hazardous Materials , 52 , 151–170.10.1016/S0304-3894(96)01805-5
- Goumans, J. J. J. M. , Senden, G. J. , & van der Sloot, H. A. (1997). Waste materials in construction: Putting theory in practice. Studies in Environmental Science (Vol. 71). Amsterdam, Netherlands: Elsevier Science.
- Griffin, R. D. (2009). Principles of hazardous materials management . CRC Press. Retrieved March 15, 2012, from http://www.crcnetbase.com/doi/abs/10.1201/9781420089714-c9
- Havlik, T. , Souza, B. V. E. , Bernardes, A. M. , Schneider, I. A. H. , & Miskufova, A. (2006). Hydrometallurgical processing of carbon steel EAF dust. Journal of Hazardous Materials , 135 , 311–318.10.1016/j.jhazmat.2005.11.067
- Johnson, C. A. , Kersten, M. , Ziegler, F. , & Moor, H. C. (1996). Leaching behaviour and solubility—Controlling solid phases of heavy metals in municipal solid waste incinerator ash. Waste Management , 16 , 129–134.10.1016/S0956-053X(96)00036-0
- Kindness, A. , Macias, A. , & Glasser, F. P. (1994). Immobilization of chromium in cement matrices. Waste Management , 14 , 3–11.10.1016/0956-053X(94)90016-7
- Kogbara, R. B. , & Al-Tabbaa, A. (2011). Mechanical and leaching behaviour of slag-cement and lime-activated slag stabilised/solidified contaminated soil. Science of The Total Environment , 409 , 2325–2335.10.1016/j.scitotenv.2011.02.037
- Lampris, C. , Stegemann, J. A. , & Cheeseman, C. R. (2008). Chloride leaching from air pollution control residues solidified using ground granulated blast furnace slag. Chemosphere , 73 , 1544–1549.10.1016/j.chemosphere.2008.08.003
- Lampris, C. , Stegemann, J. A. , & Cheeseman, C. R. (2009a). Metal leaching from APC residues solidified using Portland cement and ground granulated blast furnace slag. In Proceedings of WASCON-Sustainable Management of Waste and Recycled Materials in Construction , June 3–5. Lyon.
- Lampris, C. , Stegemann, J. A. , & Cheeseman, C. R. (2009b). Solidification/stabilisation of air pollution control residues using Portland cement: Physical properties and chloride leaching. Waste Management , 29 , 1067–1075.10.1016/j.wasman.2008.08.006
- Lampris, C. , Stegemann, J. A. , Pellizon-Birelli, M. , Fowler, G. D. , & Cheeseman, C. R. (2011). Metal leaching from monolithic stabilised/solidified air pollution control residues. Journal of Hazardous Materials , 185 , 1115–1123.10.1016/j.jhazmat.2010.10.021
- Liwu, M. , Tang, M. , & Al-Tabbaa, A. (2014). Analysis of damage development in cement paste due to ice nucleation at different temperatures. Cement and Concrete Composites , 53 , 1–9.10.1016/j.cemconcomp.2014.06.007
- Lu, H. , Wei, F. , Tang, J. , & Giesy, J. P. (2016). Leaching of metals from cement under simulated environmental conditions. Journal of Environmental Management , 169 , 319–327.10.1016/j.jenvman.2015.12.008
- MacRay, D. R. (1985). Electric arc furnace dust disposal recycle and recovery (pp. 85–85). Pitssburggh, PA: Center for Metals Production.
- Malviya, R. , & Chaudhary, R. (2006). Factors affecting hazardous waste solidification/stabilization: A review. Journal of Hazardous Materials , 137 , 267–276.10.1016/j.jhazmat.2006.01.065
- Menad, N. , Ayala, J. N. , Garcia-Carcedo, F. , Ruiz-Ayúcar, E. , & Hernández, A. (2003). Study of the presence of fluorine in the recycled fractions during carbothermal treatment of EAF dust. Waste Management , 23 , 483–491.10.1016/S0956-053X(02)00151-4
- Orhan, G. (2005). Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium. Hydrometallurgy , 78 , 236–245.10.1016/j.hydromet.2005.03.002
- Oustadakis, P. , Tsakiridis, P. E. , Katsiapi, A. , & Agatzini-Leonardou, S. (2010). Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Journal of Hazardous Materials , 179 , 1–7.10.1016/j.jhazmat.2010.01.059
- Paria, S. , & Yuet, P. K. (2006). Solidification–stabilization of organic and inorganic contaminants using portland cement: a literature review. Environmental Reviews , 14 , 217–255.10.1139/a06-004
- Pasetto, M. , & Baldo, N. (2010). Experimental evaluation of high performance base course and road base asphalt concrete with electric arc furnace steel slags. Journal of Hazardous Materials , 181 , 938–948.10.1016/j.jhazmat.2010.05.104
- Pereira, C. F. , Rodrı́guez-Piñero, M. , & Vale, J. (2001). Solidification/stabilization of electric arc furnace dust using coal fly ash. Journal of Hazardous Materials , 82 , 183–195.10.1016/S0304-3894(00)00359-9
- Poon, C. S. , & Chen, Z. Q. (1999). Comparison of the characteristics of flow through and flow-around leaching tests of solidified heavy metal wastes. Chemosphere , 38 , 663–680.10.1016/S0045-6535(98)00203-3
- Poon, C. S. , Lio, K. W. , & Tang, C. I. (2001). A systematic study of cement/PFA chemical stabilisation/solidification process for the treatment of heavy metal waste. Waste Management & Research , 19 , 276–283.10.1177/0734242X0101900403
- Rodrfguez-Piñero, M. , Pereira, C. F. , de Elvira Francoy, C. R. E. , & Vale Parapar, J. F. V. (1998). Stabilization of a chromium-containing solid waste: Immobilization of hexavelent chromium. Journal of the Air & Waste Management Association , 48 , 1093–1099.
- Salihoglu, G. , & Pinarli, V. (2008). Steel foundry electric arc furnace dust management: Stabilization by using lime and Portland cement. Journal of Hazardous Materials , 153 , 1110–1116.10.1016/j.jhazmat.2007.09.066
- Serjun, V. Z. , Mladenovic, A. , Mirtic, B. , Meden, A. , Scancar, J , & Milacic, R. (2015). Recycling of ladle slag in cement composites: Environmental impacts. Waste Management , 43 , 376–385.
- Shi, C. , & Spence, R. (2004). Designing of cement-based formula for solidification/stabilization of hazardous, radioactive, and mixed wastes. Critical Reviews in Environmental Science and Technology , 34 , 391–417.10.1080/10643380490443281
- Sofili, T. , Rastovcan-Mioc, A. , Cerjan-Stefanovic, S. , Novosel-Radovic, R. V. , & Jenko, M. (2004). Characterization of steel mill electric-arc furnace dust. Journal of Hazardous Materials , 109 , 59–70.10.1016/j.jhazmat.2004.02.032
- Srivastava, S. , Chaudhary, R. , & Khale, D. (2008). Influence of pH, curing time and environmental stress on the immobilization of hazardous waste using activated fly ash. Journal of Hazardous Materials , 153 , 1103–1109.10.1016/j.jhazmat.2007.09.065
- Stegemann, J. A. , & Cote, P. L. (1988). Investigation of leaching from solidified wastes. In Proceedings of the International Solid Wastes Conference , Durham, NH.
- Stegemann, J. A. , & Zhou, Q. (2009). Screening tests for assessing treatability of inorganic industrial wastes by stabilisation/solidification with cement. Journal of Hazardous Materials , 161 , 300–306.10.1016/j.jhazmat.2008.03.090
- Šturm, T. , Milačič, R. , Murko, S. , Vahčič, M. , Mladenovič, A. , Šuput, J. S. , & Ščančar, J. (2009). The use of EAF dust in cement composites: Assessment of environmental impact. Journal of Hazardous Materials , 166 , 277–283.10.1016/j.jhazmat.2008.11.015
- Van der Sloot, H. A. (2007). Leaching . Retrieved March 26, 2012, from http://www.leaching.net/leaching/test-methods/relevance-of-tests/
- Voglar, G. E. , & Leštan, D. (2010). Solidification/stabilisation of metals contaminated industrial soil from former Zn smelter in Celje, Slovenia, using cement as a hydraulic binder. Journal of Hazardous Materials , 178 , 926–933.10.1016/j.jhazmat.2010.02.026
- World Steel Association . (2015). Crude steel production . Retrieved July 31, 2015, from https://www.worldsteel.org/dms/internetDocumentList/bookshop/2015/World-Steel-in-Figures-2015/document/World%20Steel%20in%20Figures%202015.pdf