Abstract
Iron uptake in mitochondria and fractionated mitochondria compartments was studied to understand iron transport in heart mitochondria. The inner membrane is most active in iron uptake. Mitochondrial uptake was dependent on iron concentration and the amount of mitochondria. Iron transport was inversely proportional to pH in the range of 6.0 to 8.0. Iron transport reached a maximum after 30 min of incubation at 37°C. Iron uptake was inhibited by 1 mM ATP and stimulated by 1 mM ADP. The oxidative phosphorylation inhibitor oligomycin inhibited iron uptake, but rotenone and antimycin A did not. The divalent ions Mg2+, Cu2+, Mn2+, and Zn2+ suppressed iron uptake at 10 µM and stimulated it at 1 mM. The divalent ion Ca2+ stimulated iron uptake at 10 µM and suppressed it at 1 mM, competing with iron. The uptake of calcium was stimulated by 10 to 1000 µM ATP, while iron uptake was stimulated reciprocally by 10 to 1000 µM ADP, suggesting that these ions have movements similar to those of ATP and ADP.
Abbreviations
| EDTA | = |
ethylenediaminetetraacetic acid |
| AAC | = |
ATP/ADP carrier |
| RR | = |
ruthenium red |
| AMPPCP | = |
adenosine 5′-(β,γ-methylene)triphosphate |
| mADP | = |
methylene ADP |
| CCCP | = |
carbonyl cyanide 3-chlorophenylhydrazone |
Introduction
The free radical was first postulated as a causative agent of aging over a half-century ago by Harman (Citation1956). Reactive oxygen species (ROS) are now regarded as an ultimate cause of degenerative diseases including cancer (Samson and Nelson Citation2000). Iron, a powerful generator of free radicals through the Fenton reaction (Halliwell and Gutteridge Citation1984), has also been the subject of similar interest. Mitochondria use oxygen for electron transport, and have been implicated as major sites generating ROS (Harman Citation1972; Chance et al. Citation1979).
The accumulation of iron in mitochondria was reported first in abnormal erythroblasts (Bessis and Jensen Citation1965) and by biochemical studies (Cederbaum and Wainio Citation1972; Romslo Citation1975). Iron uptake was found to be dependent on the membrane potential of the mitochondria (Romslo Citation1975). The biogenesis of the mitochondrial organelle relies on the availability of iron, a critical constituent of the hemes and the iron sulfur centers that make up the enzymes of the electron transport chain and citric acid cycle (Pierrel et al. Citation2007; Levi and Rovida Citation2009). Iron is primarily taken up into the mitochondria and is required most for mitochondrial components (Napier et al. Citation2005). As centers of iron metabolism, mitochondria meet the demands for iron and store surplus by distributing iron to various sites in the cytoplasm such as the nucleus and ferritin. Iron transport and metabolism in mitochondria are known to involve components such as frataxin and mitoferrin (Garrick and Garrick Citation2009). However, the specific details of maintaining iron homeostasis in mitochondria, such as the transport mechanisms and associated factors, are not known.
Iron and mitochondrion are two active components that generate ROS and may exacerbate the damage from ROS if combined together. A study of iron metabolism in mitochondria will aid in comprehending underlying mechanisms of cardiac pathology. ATP depletion in iron-overloaded heart mitochondria was previously observed by our group (Kim et al. Citation2008). The heart sustains a heavy work load, so maintaining the ATP level should be critical there. The loss of ATP from iron overload may change the fate of cells and may be signaled by the ATP/ADP ratio. This work studies the effects of ATP and ADP on the iron uptake of mitochondria to understand how changes in cardiac physiology are associated with severe ATP depletion due to iron overload.
Materials and methods
Animal treatment and preparation of mitochondria
All animal work was reviewed by an ethical board at Sookmyung Women's University. Male Sprague-Dawley rats weighing 260 to 280 g (Orient Biology Inc., Seongnam, Korea) were fed regular solid food (#38057) (Purina Mills, Seongnam, Korea) and water ad libitum. After rats were anethetized with ether, their hearts were perfused with phosphate-buffered saline (PBS) with heparin (500 U/kg) and quickly excised. The excised tissues were washed with cold 0.25 M sucrose and chopped into small pieces. The sliced tissues were incubated for 5 min in isolation buffer that contained 20 mM Tris-Cl at pH 7.4, 0.25 M sucrose, 1 mM EDTA, 1 mM EGTA, 0.5% protease inhibitor cocktail (Sigma, St. Louis, MO, USA), 10 mM NaF, 1 mM Na3VO4 and 0.05% saponin. The tissues were then homogenized with a glass homogenizer (3 strokes×5 sec) and then a Teflon homogenizer (4 strokes×5 sec). The homogenate was separated by differential centrifugation (Pande and Blanchaer Citation1971). The mitochondrial pellet was resuspended with isolation buffer, washed twice, and kept at −80°C until use.
Preparation of vesicles from mitochondrial fractions
Isolated mitochondria were resuspended in 10 mM Tris-phosphate buffer at pH 7.5 containing 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, and 1 µl protease inhibitor cocktail (Sigma). The mitochondria were briefly homogenized and then kept on ice for 5 min, then resuspended in one volume of 1.8 M sucrose, 2 mM ATP, 2 mM MgSO4, and kept on ice for 10 min. The suspended mitochondria were next sonicated 5 sec, three times in a row, with a Branson sonifier (Arlington Heights, IL, USA). The mitochondria were subfractionated by gradient centrifugation at 85,000×g for 60 min at 4°C in sucrose solutions of 0.45 M, 1.0 M, and 1.2 M (Whereat et al. Citation1969). This resulted in four major fractions: intermembrane space, outer membrane, and mitoplast inner membrane and matrix. The soluble intermembrane space particles were in the 0.45 M sucrose layer, the outer membrane fraction was between the 0.45 M and 1.0 M sucrose layers, and the mitoplast was the pellet. The mitoplast was further fractionated into the inner membrane and the matrix by solubilization using 0.1% Tx-100. The identity of each fraction was confirmed by assaying the activity of these marker enzymes: monoamine oxidase for the outer membrane (Chuang et al. Citation1974), adenylate kinase for the intermembrane space (Russell et al. Citation1974), cytochrome c oxidase for the inner membrane (Barrientos Citation2002) and malate dehydrogenase for the matrix (Mullinax et al. Citation1982).
Small unilamellar vesicles can be prepared by using asolectin, which is soybean phospholipids, and cardiolipin at a ratio of 9:1 (Hackenbrock and Chazotte Citation1986). A total of 900 mg dry asolectin was added to 100 mg cardiolipin in 21 ml ethanol for mixing, and then nitrogen gas at 0°C evaporated the ethanol. The dried mixture was redissolved in 2 ml chloroform and evaporated with nitrogen at 0°C. The mixture of asolectin and cardiolipin was then suspended in 1 ml of 50 mM Tris-Cl at pH 8.0, 100 mM KCl, 2 mM MgCl2, 1% w/v cholate and dispersed under nitrogen at 0°C by sonification. The solution of phospholipids was combined with each of four major subfractions of mitochondria to prepare corresponding vesicles (1 mg/ml) at a 25:1 lipid to protein ratio and incubated for 2 h at room temperature. Finally, the mixture was incubated for 2 h at 4°C with Bio-Beads XAD-4 (Sigma, St. Louis, MO, USA) to remove detergent. To determine the effect of components of the oxidative phosphorylation system on iron uptake, which occurs in the inner membrane, oligomycin (10 µM), as inhibitor of F1Fo ATP synthase, rotenone (5 µM), as inhibitor of complex I, antimycin A (5 µM), as inhibitor of complex III, and carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (10 µM), the uncoupler, were tested.
Measurement of uptake of iron and calcium
To measure the uptake of labile iron, isolated mitochondria were suspended in incubation medium containing 250 mM sucrose, 10 mM K2PO4 pH 7.4, 20 mM Tris-Cl pH 7.4, 5 mM MgCl2, 5 mM KCl and 5 mM succinate. Then, 20 µM FeSO4 was added to the medium and the mixture was incubated for 15 min at 37°C. The mitochondria were collected by centrifugation. The collected mitochondria were suspended in 0.1 mM Tris-Cl pH 7.4, 1% Tx-100 containing 500 nM calcein (Invitrogen, Carlsbad, CA, USA). The fluorescence of calcein was measured at 485 nm (excitation) and at 535 nm (emission) with a JA-750 fluorometer (Jasco, Tokyo, Japan) after 5 min. Calcein fluorescence is quenched by bound iron, so the reference fluorescence was measured in the absence of iron. The concentration of labile iron was calculated using a standard curve based on the difference in fluorescence when iron was present or absent (Lange et al. Citation1999). To measure the calcium uptake, mitochondria were incubated similarly in the same incubation medium as before except 10 µM CaCl2 was included instead of 20 µM FeSO4. Then the collected mitochondria were suspended in 0.1 mM Tris-Cl pH 7.4, 1% Tx-100 containing 500 nM fluo-3 (Invitrogen, Carlsbad, CA, USA) instead of calcein. The fluorescence of fluo-3 was measured at 506 nm (excitation) and at 526 nm (emission) with a JA-750 fluorometer immediately (Minta et al. Citation1989). To measure the iron uptake by the vesicles of mitochondrial subfractions, the vesicles were incubated in 250 mM sucrose, 10 mM K2PO4 pH 7.4, 20 mM Tris-Cl pH 7.4, 5 mM MgCl2, 5 mM KCl, 5 mM succinate and 500 nM calcein. Vesicles were incubated for 15 min after addition of iron. Because calcein was not impermeable to vesicles, the fluorescence of calcein after 15 min was regarded as the iron remaining which was not taken up inside the vesicles. The iron uptake of vesicles was calculated by the difference between the fluorescence of calcein at the initial time of iron addition and the iron remaining outside the vesicles after incubation for 15 min. The iron content was calculated using a standard curve obtained under identical conditions except for mitochondrial fractions in the concentration range of FeSO4.
Results
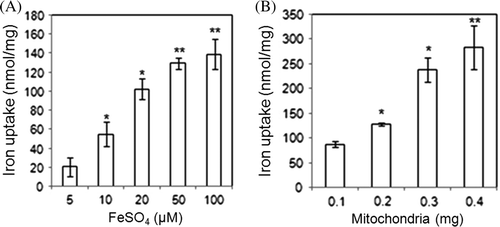
Iron uptake is dependent on iron concentration and the quantity of mitochondria
Iron concentrations from 5 to 100 µM determined the capacity of mitochondria to uptake iron. Iron uptake by mitochondria increased proportionally to the increase in iron concentration (A). When increasing amounts of isolated mitochondria were treated with 20 µM FeSO4, the uptake of iron increased proportionately to the amount of mitochondria (B). This result was applied to set the optimum condition for the later investigations with FeSO4 as 20 µM and mitochondria as 250 µg.
Figure 1. Iron uptake of mitochondria is dependent on the concentration of added iron (A) and the amount of mitochondria (B). Isolated mitochondria were suspended in 250 mM sucrose, 10 mM K2PO4 at pH 7.4, 20 mM Tris-Cl at pH 7.4, 5 mM MgCl2, 5 mM KCl, and 5 mM succinate. Then, extra iron was added and the mixture was incubated 15 min at 37°C. The mitochondria were collected by centrifugation. Calcein fluorescence was used to measure mitochondrial iron and remnants in the buffer (data not shown). The iron concentration was calculated based on a standard curve of FeSO4. The uptake of the indicated amounts of iron as FeSO4 by 100 mg mitochondria was measured (A). Data from four experiments is presented. In comparison to the 5 µM FeSO4 treatment, * indicates P<0.05 and ** indicates P<0.0001. The indicated amounts of mitochondria were each treated with 20 µM FeSO4 (B). Data from four experiments are presented. In comparison to the 0.1 mg mitochondria control group, *P<0.001 and **P<0.005.
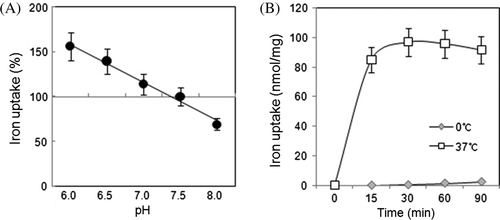
Iron uptake depends on pH and temperature
The iron uptake was inversely proportional to pH over the range of pH 6.0 to 8.0 (A). Uptake of iron was facilitated by protons at pH 6.0, indicating the involvement of protons in iron uptake and possible symport of iron and proton. At 37°C, iron uptake increased with incubation times through 30 min (B), and the slight decrease with longer incubations may be caused by the release or export of accumulated iron. At 0°C, iron uptake was negligible, with a slight increase at 60 to 90 min that may have been nonspecific uptake or adsorption. The dependence of uptake on the ambient temperature agrees with typical energy-related reactions. Notably, ATP synthesis depends heavily on temperature.
Inner membrane exhibits high iron uptake activity and is affected by inhibitors of the electron transport system
The transport system for iron was identified by subfractionating mitochondria into the four major parts: the outer membrane (OM), the inner membrane (IM), the intermembrane space (IMS) and the matrix (M). Each specific compartment was confirmed by assaying an enzyme known to be specifically located in each (A). Proteoliposome vesicles were prepared from each fraction using asolectin mixed with either cardiolipin or asolectin. These fraction vesicles had clear differences in their capacities to uptake iron (B), with the IM vesicle having the highest capacity.
Figure 3. Iron uptake of vesicles containing submitochondrial fractions was examined by separating the mitochondria into outer membrane (OM), intermembrane space (IMS), inner membrane (IM), matrix (M) fractions. (A) The mitochondrial fractions were assessed with the marker enzymes exclusively present in OM, IMS, IM, and M, by monoamine oxidase (white bars), adenylate kinase (black bars), cytochrome c oxidase (gray bars), and malate dehydrogenase (hatch marked bars), respectively. Enzyme activity is expressed as arbitrary unit (a.u.). (B) Vesicles were constructed from the subfractionated mitochondrial fractions with phospholipid. The ability of vesicles containing each subfractionated mitochondrial fractions for iron uptake in the presence of 20 µM FeSO4 alone (control, black bars), with 1 mM ADP (white bars), or 1 mM ATP (gray bars) was measured.
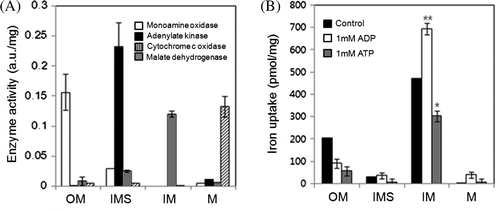
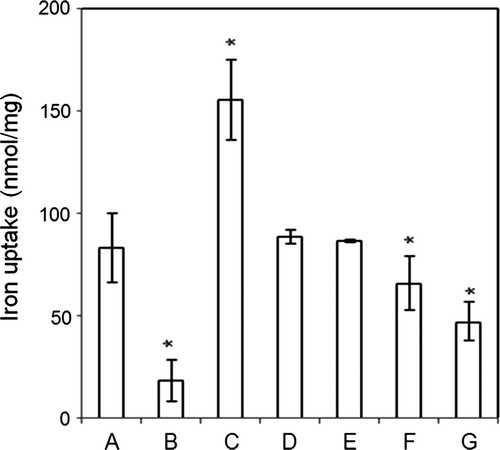
Inhibitors of oxidative phosphorylation, which occurs in the IM, were tested (). Oligomycin, which inhibits F1Fo ATP synthase, inhibited mitochondrial iron uptake significantly, but rotenone, which inhibits complex I, and antimycin A, which inhibits complex III, did not. The uncoupler meta-chloro carbonyl cyanide phenylhydrazone (CCCP) inhibited iron uptake, an effect consistent with the concept that iron uptake is dependent on membrane potential (Romslo and Flatmark Citation1973). The involvement of the F1Fo ATP synthase in iron uptake is strongly suggested by the effects of oligomycin and of the adenine nucleotides, with ATP attenuating and ADP enhancing.
Figure 4. Inhibitors of electron transport system, ATP, and ADP affected iron uptake by vesicles containing inner membrane fractions. The uptake of iron by inner membrane vesicles was measured in the presence of 20 µM Fe2_ alone (A); or with 1 mM ATP (B); 1 mM ADP (C); 5 µM rotenone (D), which inhibits complex I; 5 µM antimycin A (E), which inhibits complex III; with 10 µM oligomycin (F), which inhibits complex V; or 10 µM CCCP (G), which uncouples mitochondria. When compared to 20 µM FeSO4, *P<0.001.
Effect of ATP and ADP on the iron uptake
The cellular energy state was represented by comparing the effects of ATP and ADP on iron uptake in mitochondria. ADP stimulated iron uptake but ATP suppressed it (B and 4, ). The roles of ADP and ATP in iron uptake may depend on their ratio. Their contrasting effects, along with the pH dependence and the inhibition by oligomycin, suggests that the iron transport system may be associated with ATP/ADP carrier (AAC) of the ATP synthasome that consists of the F1Fo ATP synthase and the Pi transporter (Ko et al. Citation2003).
Table 1. ATP and ADP affect the iron and calcium uptake of mitochondria.
Effect of divalent ions on iron uptake of mitochondria
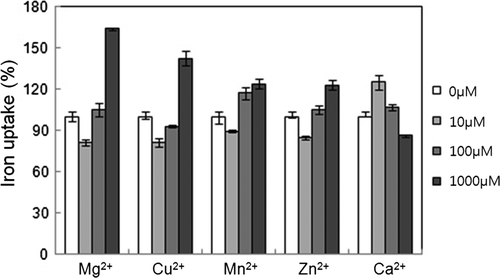
The effects of other divalent ions on iron uptake of mitochondria were tested to explore the iron transport system in mitochondria (). As the concentrations of Mg2+, Cu2+, Mn2+ and Zn2+ were increased from 10 to 1000 µM, iron uptake also increased. These ions inhibited uptake at concentrations below 100 µM and stimulated uptake at mM concentrations. Because these divalent ions behaved in a manner similar to iron, there may be a common transport system shared by iron and these minor divalent ions. The ion Ca2+ was an exception, as the highest iron uptake with Ca2+ occurred at 10 µM Ca2+ and increasing Ca2+ concentrations inhibited iron uptake. Because mitochondria play a major role in Ca2+ accumulation (Saris and Carafoli Citation2005), the relationship between mitochondrial iron and calcium transport might be significant.
Figure 5. Divalent metal ions affected iron uptake. Each metal ion at described concentration was added in the presence of 20 µM FeSO4. The control was taken as iron uptake measured in the presence of 20 µMFeSO4 alone in the absence of any divalent metal ion. The uptake ratio (%) was shown relative to control (100%).
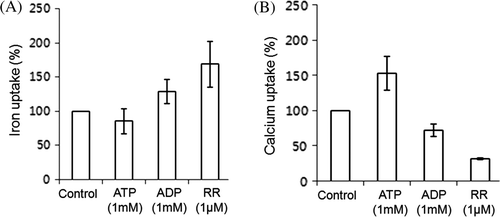
The inhibitor of Ca2+ uniporter, ruthenium red (RR), was tested to compare the effects on iron and calcium uptake (). Iron uptake was markedly increased by RR (A) and Ca2+ uptake was decreased as expected (B). The uniporter may be associated with the iron uptake machinery, balancing the iron and calcium of mitochondria similarly to the balance of ATP and ADP. Interestingly, iron uptake is proportionally dependent on ADP concentration and inversely dependent on ATP concentration (). However, increases in ADP inhibited calcium uptake while increases in ATP concentration stimulated calcium uptake. The exchange of ATP and ADP through AAC may be accompanied by simultaneous exchange of calcium and iron in heart mitochondria. The effect of the ATP analog AMPPCP mimicked that of ATP so that calcium uptake was stimulated greatly but iron uptake was minimal. Meanwhile the typical ADP analog mADP did not behave like ADP. Stimulatory uptake for both calcium and iron in the presence of CCCP, the uncoupler, was observed, implying a supplementary transport function of CCCP for divalent metal ions.
Figure 6. Iron and calcium uptake by mitochondria are affected by ATP, ADP, and RR. Mitochondrial uptake of iron in the presence of 20 µM FeSO4 (A) and calcium of 10 µM CaCl2, (B) to which 1 mM ATP, 1 mM ADP, or 1 µM ruthenium red (RR) was added. Control was set in the presence of either 20 µM FeSO4 (A) or of 10 µM CaCl2 (B) alone in the absence of any effector. The uptake ratio (%) was displayed relative to the control in which iron uptake was considered 100%.
Discussion
The associations between iron metabolism and mitochondrial ATP/ADP metabolism have not been actively studied except for the active chelation of iron with ATP and ADP (Vile et al. Citation1987). Because mitochondria accumulate iron, the metabolism of iron may be closely associated with the balance of ATP and ADP, and these nucleotides may affect the uptake of iron by mitochondria as carried out by membrane potential. This biochemical study of the effects of ATP and ADP on iron uptake sought to characterize the iron transport system in mitochondria (Romslo Citation1975).
Consistent with the finding that the inner membrane is capable of iron accumulation (Romslo and Flatmark, Citation1974), proteoliposome containing inner membrane exhibited active iron uptake (Fig.A). However, a significant amount of iron was found with the outer membrane. It is observed that the outer membrane, where pores are located, constructs a nonselective membrane barrier, through which small molecules of mol. wt. (up to ~ 5 kDa) can easily pass (De Pinto and Palmieri, Citation1992; Salnikov et al., Citation2007). Possibly, iron will enter the intermembrane space and distribute equally throughout the cytosol and intermembrane space outside the inner membrane. Moreover, it is suggested that there are unidentified structures composed of outer membrane linked to inner membrane to form a continuous path (Benz et al., 19 Citation1990). Meanwhile, in the presence of ATP and ADP, iron uptake was inhibited. In general, ATP makes a stronger chelate of iron than ADP (Anghileri et al., Citation1994), to retard (or inhibit) the iron movement into a different extent (A). However, under the specific uptake system of ATP synthase linked to AAC of IM driven actively by ADP, more iron may be transported by ADP than by ATP.
Our finding that rotenone and antimycin A have little effect on iron uptake indicates that the electron transport chain does not significantly contribute to iron uptake. In contrast, oligomycin and CCCP inhibit iron uptake, which indicates that the iron uptake system is associated with the F1Fo ATP synthase or with membrane potential, in agreement with an earlier report (Romslo Citation1975). The dependence of iron uptake on pH suggests that proton transport is related to the unidentified components of iron uptake. The inhibition by oligomycin and dependence on pH suggest the involvement of the F1Fo ATP synthase in iron uptake. The dependence of iron uptake on both ATP and ADP, which both bind to the F1Fo ATP synthase, is consistent with the earlier report of that enzyme's role in iron uptake (Kim and Song Citation2010).
Iron uptake was markedly augmented in the presence of ADP and suppressed in the presence of ATP. Both ATP and ADP are able to form chelation complexes with divalent ions, but chelation cannot explain the stimulatory or suppressive effects of these nucleotides because they can both complex with iron in a similar way. High levels of ADP in a cell are a molecular signal that stimulates energy synthesis in the form of ATP. This results in the activation of oxidative phosphorylation and increased uptake of ADP to synthesize ATP. The transport of ADP acts in a reciprocal mode with ATP transport and is a notable feature of AAC in mitochondria. The AAC and the F1Fo ATP synthase in mitochondria are two major components whose activities contribute to the balance of ATP and ADP. The ATP synthasome is a supercomplex of assembled AAC, F1Fo ATP synthase and Pi transporter (Ko et al. Citation2003). The presence of these as a supercomplex indicates that the three protein components, functionally closely related, are assembled together tightly to better coordinate their efforts to meet the energy demands of a cell. Hence, ATP/ADP transport is correlated with ATP synthesis and hydrolysis, proton transport and phosphate transport. The import of ADP and Pi with protons should occur together for the ATP synthesis, and be balanced by ATP export. The increase of iron uptake by ADP and by low pH suggests the cotransport of iron with protons. The cotransport implies participation of the proton channel of F1Fo ATP synthase under these conditions (Kim and Song Citation2010). Our results in and show that the degree of iron uptake was dependent on the ratio of ATP and ADP, and on the ADP concentration. The ATP/ADP ratio controls AAC (Klingenberg Citation2008), and iron uptake may be related to AAC activity. The activation of AAC by ADP may be coupled to the proton channel of the F1Fo ATP synthase in the supercomplex structure. Since the ATP synthase can transport iron, as reported earlier (Kim and Song Citation2010), the uptake of iron in proportion to ADP uptake seems plausible through the coordinated operation of this supercomplex.
Calcium participates in many cellular activities including muscle contraction. Calcium uptake has been observed to be enhanced by endogenous ATP in liver mitochondria (Brand and Lehninger Citation1975), and calcium is credited with determining the ATP/ADP ratio (Magnus and Keizer Citation1997). Conversely, the ratio of ATP/ADP via AAC modulates calcium uptake (Haworth and Hunter Citation2000). AAC can exist in two conformation states, m and c, which can be distinguished by bongkrekic acid, and carboxyatractylate, respectively. The m state of AAC is stabilized by ADP, and the turnover of AAC between the m state and the c state is more easily modulated by ADP than by ATP (Klingenberg Citation2008). Conversely, if ATP in the presence of calcium is easily bound to AAC in the c state, AAC may provide a transport of calcium via ATP/ADP transport. The proton channel of ATP synthase has a pivotal role in these functional movements. As free ATP and ADP bind to AAC and ATP synthase, the concept of the reciprocal uptakes of calcium and iron is supported by the ability of RR () to stimulate the iron uptake and inhibit the calcium uptake of mitochondria. These two divalent ions are important for cellular and mitochondrial activity, and their mobilization accompanies ATP and ADP transport through mitochondria. The pattern of calcium transport was different from other divalent ions, in that Ca2+ transport was inversely proportional to ATP concentration. Iron transport was directly proportional to ADP concentration. As Ca2+ forms a complex with ATP, the Ca2+-ATP complex is exchanged with Fe2+-ADP in a manner that depends on the ATP/ADP ratio. The suppressive effect of increasing ATP concentration against stimulatory ADP on iron uptake () strongly suggests the direct reverse behavior of an unknown transporting system. The inverse relationship of uptake between Ca2+ and Fe2+ consistent with competitive inhibition (Romslo and Flatmark Citation1973) was also found in the presence of RR. Under iron-overload conditions, ATP depletion is accompanied by iron accumulation. Depletion of ATP, which may be associated with impaired AAC, appears during ischemia to cause irreversible damage to the heart (Jennings et al. Citation1978, Citation1985; Regitz et al. Citation1984). A marked drop in ATP is a common feature of both ischemia and iron-overload in heart. In this respect, iron-overload in heart mitochondria causes a serious burden by increasing free radicals and ATP loss. Furthermore, a surge of iron into mitochondria may disturb calcium homeostasis, which should exacerbate the injury to the heart.
The inverse relationship between Ca2+-ATP and Fe2+-ADP indicates that a control center for the exchange of iron and calcium exists, and probably uses proton motive force as the major engine. In summary, the iron uptake of mitochondria is stimulated by ADP and low pH (that is, more protons) and acts reciprocally to the calcium uptake activated by ATP. This is consistent with the role of proton movement in coordinating ATP, ADP, calcium and iron (Moreau and Parekh Citation2008). Future studies of the Pi transporter of the ATP synthasome may clarify the functions of the ATP synthasome with regard to iron and calcium.
Acknowledgements
This work was supported by Sookmyung Women's University.
References
- Anghileri , LJ , Maincent , P , Córdova-Martínez , A and Escanero , JF. 1994 . Effects of albumin and adenosine phosphates on iron transfer from ferric lactate . Biol Trace Elem Res. , 40 : 83 – 88 .
- Barrientos , A. 2002 . In vivo and in organello assessment of oxphos activities . Methods. , 26 : 307 – 316 .
- Benz , R , Kottke , M and Brdiczka , D. 1990 . The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin . Biochim Biophys Acta. , 1022 : 311 – 318 .
- Bessis , MC and Jensen , WN. 1965 . Sideroblastic anaemia, mitochondria and erythroblastic iron . Br J Haematol. , 11 : 49 – 51 .
- Brand , MD and Lehninger , AL. 1975 . Superstoichiometric Ca2+ uptake supported by hydrolysis of endogenous ATP in rat liver mitochondria . J Biol Chem. , 250 : 7958 – 7960 .
- Cederbaum , AI and Wainio , WW. 1972 . Binding of iron and copper to bovine heart mitochondria. II. Effect of mitochondrial metabolism . J Biol Chem. , 247 : 4604 – 4614 .
- Chance , B , Sies , H and Boveris , A. 1979 . Hydroperoxide metabolism in mammalian organs . Physiol Rev. , 59 : 527 – 605 .
- Chuang , HY , Patek , DR and Hellerman , L. 1974 . Mitochondrial monoamine oxidase . J Biol Chem. , 249 : 2381 – 2384 .
- De Pinto , VD and Palmieri , F. 1992 . Transmembrane arrangement of mitochondrial porin or voltage-dependent anion channel (VDAC) . J Bioenerg Biomembr. , 24 : 21 – 26 .
- Garrick , MD and Garrick , LM. 2009 . Cellular iron transport . Biochim Biophys Acta. , 1790 : 309 – 325 .
- Hackenbrock , CR and Chazotte , B. 1986 . Lipid enrichment and fusion of mitochondrial inner membranes . Methods Enzymol. , 125 : 35 – 43 .
- Halliwell , B and Gutteridge , JM. 1984 . Role of iron in oxygen radical reactions . Methods Enzymol. , 105 : 47 – 56 .
- Harman , D. 1956 . Aging: a theory based on free radical and radiation chemistry . J Gerontol. , 11 : 298 – 300 .
- Harman , D. 1972 . A biologic clock: the mitochondria? . J Am Geriat Soc. , 20 : 145 – 147 .
- Haworth , RA and Hunter , DR. 2000 . Control of the mitochondrial permeability transition pore by high-affinity ADP binding at the ADP/ATP translocase in permeabilized mitochondria . J Bioenerg Biomembr. , 32 : 91 – 96 .
- Jennings , RB , Hawkins , HK , Lowe , JE , Hill , ML , Klotman , S and Reimer , KA. 1978 . Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog . Am J Pathol. , 92 : 187 – 214 .
- Jennings RB Steenbergen C Jr 1985 Nucleotide metabolism and cellular damage in myocardial ischemia Annu Rev Physiol 47 727 749
- Kim , M , Kim , J , Cheon , C-I , Cho , DH , Park , JH , Kim , KI , Lee , K-H and Song , E. 2008 . Increased expression of the F1F0 ATP synthase in response to iron in heart mitochondria . BMB Rep. , 2 : 153 – 157 .
- Kim , M and Song , E. 2010 . Iron transport by proteolipoosomes containing mitochondrial F1Fo ATP synthase isolated from rat heart . Biochimie , 92 : 333 – 342 .
- Klingenberg , M. 2008 . The ADP and ATP transport in mitochondria and its carrier . Biochim Biophys Acta. , 1778 : 1978 – 2021 .
- Ko , YH , Delannoy , M , Hullihen , J , Chiu , W and Pedersen , PL. 2003 . Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP . J Biol Chem. , 278 : 12305 – 12309 .
- Lange , H , Kispal , G and Lill , R. 1999 . Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria . J Biol Chem. , 274 : 18989 – 18996 .
- Levi , S and Rovida , E. 2009 . The role of iron in mitochondrial function . Biochim Biophys Acta. , 1790 : 629 – 636 .
- Magnus , G and Keizer , J. 1997 . Minimal model of beta-cell mitochondrial Ca2+ handling . Am J Physiol. , 273 : C717 – C733 .
- Minta , A , Kao , JPY and Tsien , RY. 1989 . Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores . J Biol Chem. , 264 : 8171 – 8178 .
- Moreau , B and Parekh , AB. 2008 . Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase . Curr Biol. , 18 : 855 – 859 .
- Mullinax , TR , Mock , JN , McEvily , AJ and Harrison , JH. 1982 . Regulation of mitochondrial malate dehydrogenase. Evidence for an allosteric citrate-binding site . J Biol Chem. , 257 : 13233 – 13239 .
- Napier , I , Ponka , P and Richardson , DR. 2005 . Iron trafficking in the mitochondrion: novel pathways revealed by disease . Blood. , 105 : 1867 – 1874 .
- Pande , SV and Blanchaer , MC. 1971 . Reversible inhibition of mitochondrial adenosine diphosphate phosphorylation by long chain acyl coenzyme A esters . J Biol Chem. , 246 : 402 – 411 .
- Pierrel , F , Cobine , PA and Winge , DR. 2007 . Metal Ion availability in mitochondria . Biometals. , 20 : 675 – 682 .
- Regitz , V , Paulson , DJ , Hodach , RJ , Little , SE , Schaper , W and Shug , AL. 1984 . Mitochondrial damage during myocardial ischemia . Basic Res Cardiol. , 79 : 207 – 217 .
- Romslo , I. 1975 . Energy-dependent accumulation of iron by isolated rat liver mitochondria. IV. Relationship to the energy state of the mitochondria . Biochim Biophys Acta. , 387 : 69 – 79 .
- Romslo , I and Flatmark , T. 1973 . Energy-dependent accumulation of iron by isolated rat liver mitochondria. II. Relationship to the active transport of Ca2+ . Biochim Biophys Acta , 325 : 38 – 46 .
- Romslo , I and Flatmark , T. 1974 . Energy-dependent accumulation of iron by isolated rat liver mitochondria. 3. Submitochondrial localization of the iron accumulated . Biochim Biophys Acta. , 347 : 160 – 167 .
- Russell PJ Jr Horenstein JM Goins L Jones D Laver M 1974 Adenylate kinase in human tissue. I. Organ specificity of adenylate kinase isoenzymes J Biol Chem 249 1874 1879
- Salnikov , V , Lukyánenko , YO , Frederick , CA , Lederer , WJ and Lukyánenko , V. 2007 . Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles . Biophys J. , 92 : 1058 – 1071 .
- Samson , FE and Nelson , SR. 2000 . The aging brain, metals and oxygen free radicals . Cell Mol Biol (Noisy-le-grand) , 46 : 699 – 707 .
- Saris , NE and Carafoli , E. 2005 . A historical review of cellular calcium handling with emphasis on mitochondria . Biochemistry (Mosc) , 70 : 187 – 194 .
- Vile , GF , Winterbourn , CC and Sutton , HC. 1987 . Radical-driven Fenton reactions: studies with paraquat, adriamycin, and anthraquinone 6-sulfonate and citrate, ATP, ADP, and pyrophosphate iron chelates . Arch Biochem Biophys. , 259 : 616 – 626 .
- Whereat , AF , Orishino , MW and Nelson , J. 1969 . The location of different synthetic systems for fatty acids in inner and outer mitochondrial membranes from rabbit heart . J Biol Chem. , 244 : 6498 – 6506 .