Abstract
Stress urinary incontinence (SUI) is a common condition that primarily affects women. Here, we investigate the effects of human adipose-derived stem cells (ADSCs) in a rodent model of SUI. Female Sprague-Dawley rats at 7 weeks of age were randomly divided into three groups (n=8 per group): sham-operation, SUI-induction by transabdominal urethrolysis, and SUI-induction followed by transplantation of human ADSCs into the urethra. The abdominal leak point pressure at 8 weeks after the operation was markedly decreased by transabdominal urethrolysis, confirming successful induction of SUI. Interestingly, transplantation of human ADSCs into the urethra significantly blunted the decrease of abdominal leak point pressure in SUI-induced rats. Accordingly, we observed expression of α-smooth muscle actin in a significant proportion of transplanted ADSCs, indicating differentiation of ADSCs into smooth muscle cells in the urethra. Moreover, the SUI-induced elevations of c-Fos immunoreactivities in the pontine micturition center (PMC) and in the ventrolateral periaqueductal gray (vlPAG) were clearly suppressed by transplantation of human ADSCs. These results imply that human ADSCs can be an effective therapeutic modality to ameliorate the symptoms of SUI.
Introduction
Stress urinary incontinence (SUI), an involuntary leakage of urine during activities, such as exertion, sneezing and coughing, is a common condition that primarily affects women (Jack et al. Citation2005; Miller Citation2005). While possible causes of SUI can be diverse and precise mechanisms of SUI are still poorly understood, the anatomic changes in urethral support and dysfunction of the intrinsic urethral sphincter seem to contribute to the involuntary urine loss during activities that cause abdominal straining (Rodríguez et al. Citation2005). Many therapeutic interventions, including pharmacologic treatments and bulking agents, have been attempted for the treatment of SUI, but with some adverse effects (Radley et al. Citation2001).
Recently, stem cell therapy has emerged as a potential cure for a deficient sphincter in SUI (Lee et al. Citation2003). Indeed, various stem cell therapies in general have been attempted to replace, repair or enhance biological functions of damaged tissues in lots of pathological conditions. While practical uses of embryonic stem cells are quite limited by ethical considerations, uses of adult stem cells are relatively free from these issues (Furuta et al. Citation2007). Consequently, adult adipose-derived stem cells (ADSCs) are considered as an ideal source of autologous progenitor cells because adipose tissue can be obtained in relatively large quantities without severe affliction. Moreover, ADSCs have been demonstrated to be stable in long-term cultures: they maintain a consistent population doubling rate, and also exhibit low levels of senescence (Zuk et al. Citation2002). ADSCs were found to differentiate into various cell types including adipogenic and osteogenic cells in the presence of lineage-specific induction factors (Rodríguez et al. Citation2006). Nevertheless, studies on the therapeutic effectiveness of ADSCs in SUI are still scarce and they have not been fully assessed in animal studies.
One of the crucial areas lacking researchers’ attention is the micturition centers of the brain. Actually, the voiding reflex involving activation of the parasympathetic input to the bladder and inhibition of the sympathetic and somatic inputs to the urethra is known to be coordinated in the pontine micturition center (PMC) of the brainstem (Kavia et al. Citation2005; Miller Citation2005). The PMC, located in the dorsomedial pons, is the first brain structure related to micturition based on animal studies: an electrical or chemical stimulation of the PMC produces bladder contractions (Mallory et al. Citation1991). Along with the PMC, the periaqueductal gray (PAG) of the midbrain is also known to play an important role in the control of micturition (Kavia et al. Citation2005). The PAG is an important site in ascending pain transmission, and is involved in many other functions, including fear, anxiety and cardiovascular control (Behbehani Citation1995). Recent works indicate that this midbrain region is activated during the micturition (Nour et al. Citation2000) as well as storage phases (Athwal et al. Citation2001).
In the present study, we transplant human ADSCs into the urethra of SUI-induced female rats and examine the changes of urethral resistance to verify the effectiveness of human ADSCs in the amelioration of SUI. In addition, we determined c-Fos immunoreactivities in the micturition centers of the brain as a marker for stimuli-induced metabolic changes of neurons.
Materials and methods
Animals
Virgin Sprague-Dawley rats weighing 210±10 g (7 weeks of age, n=24) were used in this experiment. To minimize inadvertent c-Fos protein expression induced by environmental stimuli, the animals were gently and carefully handled during the experimental process. The animals were housed under controlled temperature (20±2°C) and lighting (08:00 to 20:00) conditions and were supplied with food and water available ad libitum before and after surgery. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The animals were randomly divided into the following three groups (n=8 per group): sham-operation, SUI-induction, and SUI-induction followed by human ADSCs injection.
Surgical induction of SUI
To induce SUI, transabdominal urethrolysis was performed, as previously described (Phull et al. Citation2007; Chung et al. Citation2008). Briefly, the rats were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Virbac Laboratories, Carros, France). After a lower abdominal midline incision, the bladder and ureters were detached from surrounding tissues, and the urethra was detached from the anterior vaginal wall and pubic bone by sharp dissection. A cotton-tip swab was put into the vagina to aid with the dissection. All rats were allowed to recover for 2 weeks after surgery. The rats in the sham-operation group underwent only a lower abdominal midline incision, and then were sutured.
Cell labeling
Human ADSCs were generously provided by Kangbuk Samsung Hospital (Seoul, Republic of Korea) according to a previously described method (Cho et al. Citation2009) after approval by the Institution Review Board of Kangbuk Samsung Hospital. They were then labeled. Briefly, human ADSCs were rinsed with phosphate-buffered saline (PBS) and incubated in Hank's balanced salt solution (HBSS; Sigma Chemical Co., St. Louis, MO, USA) containing 1 µg/ml chloromethyl-1,1-dioactadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate dye (CM-Dil; Invitrogen, Carlsbad, CA, USA) at 37°C for 5 min and at 4°C for 15 min with occasional mixing. Labeled human ADSCs were washed, centrifuged, and resuspended in HBSS.
Cell injection
Human ADSCs were injected into both sides of the urethra at 2 weeks after transabdominal urethrolysis according to the previously described method (Jack et al. Citation2005). The SUI-induced rats were anesthetized with Zoletil 50®. After an abdominal incision was made, 10 µl of human ADSCs (1×106 cells) was injected into both sides of the urethra using a 10 µl Hamilton microsyringe with a 26 gauge Hamilton needle (Fisher Hamilton, Pittsburgh, PA, USA). Localization of cells within the urethra was confirmed by visualization of a raised wheal.
Measurement of the abdominal leak point pressure
Eight weeks after injection of human ADSCs, the abdominal leak point pressure was measured to assess the urethral resistance according to the previously described method (Hsieh et al. Citation2001; Chung et al. Citation2008). After induction of anesthesia, all rats underwent a T9 spinal cord transection to block the reflex of bladder contractions. After an abdominal incision was made, an intravesical catheter connected to a pressure transducer (Harvard Apparatus Inc., Holliston, MA, USA) was inserted into the dome of the bladder, and the abdominal leak point pressure was measured using a labscribe (iWork/CB Science, Inc., Dover, NH, USA). All rats were mounted on a tilt table in a vertical position. Saline at room temperature was infused through the catheter and the maximal bladder capacity was determined when the first drop of urine appeared at the urethral meatus. The bladder was manually emptied by squeezing and then filled to one-third capacity with saline. Then, the abdominal leak point pressure was determined as the peak bladder pressure inducing leakage of urine at the urethral meatus using manual abdominal compression. In this way, the abdominal leak point pressure was determined 10 times for each rat.
Tissue preparation
Four weeks after injection of human ADSCs, two rats were sacrificed to confirm the localization of human ADSCs within the bladder and urethra. The remaining rats were sacrificed at 8 weeks after injection of human ADSCs to examine the expression of α-smooth muscle actin. Bladder and urethra tissue preparations were procured at 4 weeks and 8 weeks after injection of human ADSCs according to the previously described method (Jack et al. Citation2005). After the rats were fully anesthetized with Zoletil 50®, the entire bladder and urethra were harvested by removing the symphysis pubis and preserving the entire urethral segment. The specimens were fixed in optimal cutting temperature (OCT) compound (Sakura Finetek, Tokyo, Japan) and quickly frozen at -70°C until analysis. The specimens were transversely step-sectioned in 10 µm thicknesses using a freezing microtome (Leica, Nussloch, Germany) to include the urethral injection sites. Brain tissue was prepared at 8 weeks after the injection of human ADSCs as previously described (Sim et al. Citation2008). Briefly, the rats were perfused transcardially with 50 mM PBS, and then fixed with a freshly prepared solution consisting of 4% paraformaldehyde (PFA) in 100 mM phosphate buffer (PB; pH 7.4) after harvest of the bladder and urethra. The brains were then removed, post-fixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections, 40 µm in thickness, were made using a freezing microtome (Leica).
Cell tracking
Excised tissues were analyzed for the presence of viable human ADSCs according to the previously described method (Jack et al. Citation2005). The CM-DiI tracking method was used to localize the cells and to determine cell survival. The excised sections were mounted with fluorescent mount medium (DakoCytomation, Carpinteria, CA, USA), and then detected with a confocal microscope (Axiovert LSM 510 META; Zeiss, Obërkochen, Germany).
Immunofluorescence for α-smooth muscle actin
To examine α-smooth muscle actin expression from injected human ADSCs, immunofluorescence for α-smooth muscle actin was performed according to the previously described method (Jack et al. Citation2005). Briefly, frozen-tissue sections on slides were permeabilized with 0.5% Nonidet P-40 (NP-40) in PBS for 5 min, and blocked with 5% horse serum for 1 h at room temperature. Tissue sections were washed two times with PBS, and then incubated overnight at 4°C with primary antibody (Sigma Chemical Co.) at a dilution of 1:400. After washing three times with PBS, the sections were incubated for 1 h with fluorescein (FITC)-conjugated goat anti-mouse secondary antibody (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). The sections were washed three times with PBS, and then mounted with fluorescent mount medium (DakoCytomation). The sections were detected and photographed using a confocal microscope (Zeiss).
Immunohistochemistry for c-Fos
To detect c-Fos expression, immunohistochemistry was performed as previously described (Sim et al. Citation2008). On average, 10 sections were selected from each brain in the region spanning from Bregma -9.68 to -9.80 mm for the PMC and from Bregma -7.64 to -8.00 mm for the vlPAG. Tissue sections were incubated overnight with rabbit anti-c-Fos antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at a dilution of 1:1000. The sections were then incubated for 1 h with anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were subsequently incubated with an avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1 h at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma Chemical Co.) and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.6) for approximately 5 min. The sections were then washed three times with PBS and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount®. To assess c-Fos expression, cell counting was performed using the Image-Pro® Plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). The number of c-Fos-positive neurons was counted hemilaterally, and the results were expressed as the number of cells/section of the selected brain region.
Statistical analysis
All data were analyzed using the statistical software SPSS (version 12.0; SPSS, Inc., Chicago, IL, USA). The data are expressed as the mean±standard error of the mean (SEM). For the comparison between groups, one-way ANOVA and Scheffé's post-hoc test were performed and the differences among the groups were considered statistically significant at P<0.05.
Results
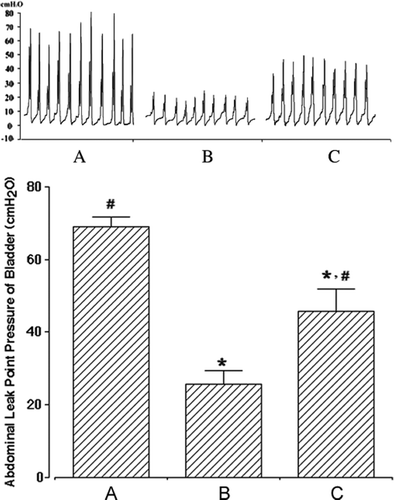
Transplantation of human ADSCs into the urethra significantly blunts the transabdominal urethrolysis-induced decrease of abdominal leak point pressure
Various animal models of SUI, such as simulating birth trauma with intravaginal balloon inflation (Sievert et al. Citation2001) and pudendal nerve crush (Kerns et al. Citation2000), have been developed to evaluate the impact of experimental interventions for the treatment of SUI. However, these models have limitations due to low rates of incontinence, difficulties of reproducibility, high rates of bladder dysfunction, and relatively short durability. In contrast, transabdominal urethrolysis seems to be a reliable and long-lasting animal model compared to other models of SUI (Rodríguez et al. Citation2005). Therefore, we conducted transabdominal urethrolysis to induce SUI in female rats. Following transabdominal urethrolysis, the abdominal leak point pressure was measured to assess the induction of SUI. At 8 weeks after operation, the abdominal leak point pressures were 68.94±2.67 cmH2O with sham operation, 25.73±3.57 cmH2O with SUI induction, and 45.78±6.12 cmH2O with SUI induction followed by human ADSC injection (). A clear and significant decline in the abdominal leak point pressure after transabdominal urethrolysis indicates successful induction of SUI in female rats. Interestingly, transplantation of human ADSCs into the urethra of SUI-induced female rats significantly recovered the abdominal leak point pressure. These results indicate that transplanted human ADSCs can ameliorate transabdominal urethrolysis-induced SUI in female rats.
Figure 1. Effect of human adipose-derived stem cells (ADSCs) on the abdominal leak point pressure after induction of stress urinary incontinence (SUI). Upper panel: photomicrographs of the abdominal leak point pressure. Lower panel: the mean±SEM of the abdominal leak point pressure in sham-operation (A), SUI-induction (B) and SUI-induction followed by human ADSC injection (C) groups. * represents P<0.05 compared with the sham-operation group and # represents P<0.05 compared with the SUI-induction group.
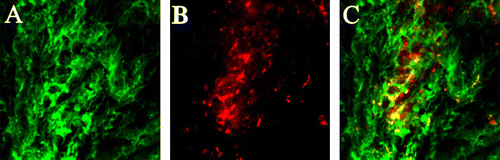
Transplanted human ADSCs are viable and express α-smooth muscle actin in the urethra
Four weeks after injection of human ADSCs, localization of human ADSCs within the bladder and urethra was confirmed by the DiI tracking method (Jack et al. Citation2005). Injected human ADSCs in both sides of the urethra were detected as viable in the neighboring injection sites (data not shown). At 8 weeks after transplantation, double immunofluorescence of tissue sections for ADSCs (DiI) and α-smooth muscle actin (fluorescein-5-isothiocyanate) was performed to see whether transplanted human ADSCs can express α-smooth muscle actin. As clearly seen in , transplanted human ADSCs were successfully incorporated into the urethra and a large proportion of ADSCs expressed α-smooth muscle actin (see yellow areas in ). This indicates that transplanted human ADSCs are viable and can differentiate into smooth muscles in the damaged urethra.
Figure 2. Expression of α-smooth muscle actin in human adipose-derived stem cells (ADSCs) at 8 weeks after transplantation. Representative fluorescent staining for α-smooth muscle actin (A, green), human ADSCs stained with DiI (B, red) and superimposed images are shown here (C). Original magnification:×100.
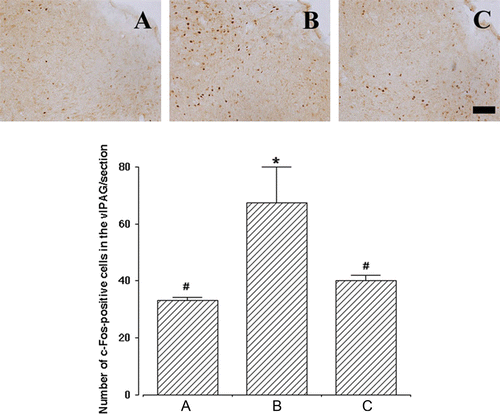
Transplantation of human ADSCs into the urethra suppresses SUI-induced c-Fos expression in the PMC and vlPAG
Next, we determined c-Fos immunoreactivities in the PMC and vlPAG, the micturition centers of the brain, as a marker for stimuli-induced metabolic changes of neurons (Morgan and Curran Citation1991; Rodella et al. Citation1998). Representative photomicrographs of c-Fos-positive cells in the PMC and vlPAG are presented in and , respectively. The number of c-Fos-positive cells in the PMC was 6.81±0.53/section with sham operation, 10.55±0.71/section with SUI induction, and 7.61±0.50/section with SUI induction followed by human ADSC injection (). In the vlPAG, the number of c-Fos-positive cells was 33.05±1.09/section with sham operation, 67.30±12.57/section with SUI induction, and 40.00±2.09/section with SUI induction followed by human ADSC injection (). These results clearly indicate that the induction of SUI by transabdominal urethrolysis significantly enhances c-Fos expression in the vlPAG as well as in the PMC, while injection of human ADSCs into the urethra can efficiently block c-Fos expression in these brain areas near to the level of the sham-operation group.
Figure 3. Effect of human adipose-derived stem cells (ADSCs) on c-Fos expression in the pontine micturition center (PMC). Upper panels: representative photomicrographs of c-Fos-positive cells in the PMC. The sections were stained for c-Fos-like immunoreactivity (brown) as described in Materials and methods. The scale bar represents 100 µm. Lower panel: the mean number±SEM of c-Fos immunoreactivity in sham-operation (A), SUI-induction (B) and SUI-induction followed by human ADSC injection (C) groups. *represents P<0.05 compared with the sham-operation group and #represents P<0.05 compared with the SUI-induction group.
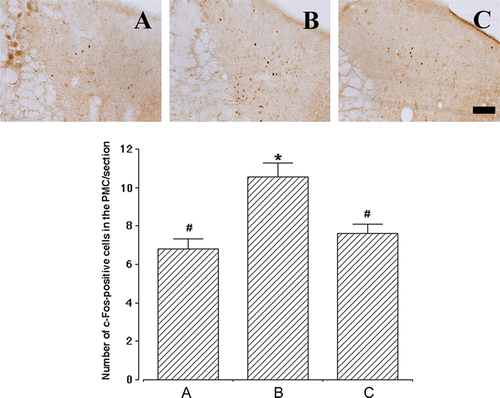
Figure 4. Effect of human adipose-derived stem cells (ADSCs) on c-Fos expression in the ventrolateral periaqueductal gray (vlPAG). Upper panels: representative photomicrographs of c-Fos-positive cells in the vlPAG. The sections were stained for c-Fos-like immunoreactivity (brown) as described in Materials and methods. The scale bar represents 100 µm. Lower panel: the mean number±SEM of c-Fos immunoreactivity in sham-operation (A), SUI-induction (B) and SUI-induction followed by human ADSC injection (C) groups. *represents P<0.05 compared with the sham-operation group and #represents P<0.05 compared with the SUI-induction group.
Discussion
In the present study, we clearly demonstrated that transabdominal urethrolysis can induce SUI in female rats, and human ADSCs transplanted into the urethra can differentiate into smooth muscle cells and ameliorate the symptoms of SUI. Moreover, we found that transabdominal urethrolysis upregulates c-Fos expression in the brain areas related to micturition, and this elevation can be efficiently blocked by human ADSCs injected into the urethra. In the course of our study, two very recent works were published regarding the effects of rodent ADSCs on other animal models of SUI (Fu et al. Citation2010; Lin et al. Citation2010). To our knowledge, however, this work is the first validation of human ADSCs in the amelioration of symptoms of SUI induced by transabodominal urethrolysis in animal models. More importantly, this work extends the effect of transplanted ADSCs to the brain centers related to micturition.
The abdominal leak point pressure has been a useful surrogate for measuring the degree of sphincteric insufficiency (Phull et al. Citation2007). Although less advantageous than measurement of the retrograde leak point pressure in determining intrinsic sphincter function (Rodríguez et al. Citation2005), the abdominal leak point pressure has been widely utilized in many SUI studies (Rodríguez et al. Citation2005; Kwon et al. Citation2006; Phull et al. Citation2007). In this study, transabdominal urethrolysis significantly decreased the abdominal leak point pressure compared with the sham-operation group as expected. Moreover, injection of human ADSCs significantly recovered the abdominal leak point pressure, which is in good agreement with the use of other stem cells (Lee et al. Citation2003; Kwon et al. Citation2006).
Despite the good recovery of abdominal leak point pressure with the transplanted human ADSCs in the urethra, the exact mechanism by which human ADSCs exert their effects is still unclear. Previously, Phull et al. (2007) showed that the smooth muscle content in the urethra was diminished by urethrolysis, and the abdominal leak point pressure was decreased by smooth muscle atrophy. Rodríguez et al. (2005) reported that the decrease in smooth muscle content caused urethral dysfunction. Approximately 50% of urinary incontinence is known to be induced by a loss of urethral support and atrophy of the intrinsic sphincter (Balmforth and Cardozo Citation2003). Interestingly, it was once reported that ADSCs are capable of inducing the expression of a smooth muscle phenotype, and these differentiated cells can contract and relax as the intrinsic smooth muscle cells (Rodríguez et al. Citation2006). Moreover, Jack et al. (2005) reported that human-processed lipoaspirate cells, synonymous to human ADSCs, remain viable up to 12 weeks in the lower urinary tract, and when injected into the urinary tract these cells exhibit morphologic and phenotypic evidence of smooth muscle incorporation and differentiation with time. In this work, human ADSCs were localized at the neighboring injection sites at 4 weeks after injection, and then survived up to 8 weeks after injection when the animals were sacrificed. Moreover, surviving ADSCs were found to express α-smooth muscle actin, which is known to participate in contraction and has been used as a marker of early differentiation of smooth muscle cells (Jack et al. 2005).
One of the interesting findings of this work is that transabdominal urethrolysis enhanced c-Fos expression in the PMC and vlPAG, the brain areas related to micturition (Kavia et al. 2005; Miller 2005). During normal micturition, activation of bladder stretch receptor stimulates the PAG, and then the activated PAG stimulates the PMC. The activated PMC in turn initiates complete synergic micturition responses via excitation of parasympathetic bladder motor neurons in the sacral part of the spinal cord and inhibition of the bladder sphincter motor neurons (Blok and Holstege Citation1997). Mitsui et al. (Citation2003) showed that stimulation of the bladder increased c-Fos expression in the PAG consisting of the dorsomedial, dorsolateral, lateral, and ventolateral regions, and the vlPAG in particular plays a critical role in regulating the micturition reflex. If we assume that c-Fos expression in the brain represents neuronal activation (Guzowski et al. Citation2005), the elevated c-Fos expression in the PMC and vlPAG in SUI-induced rats found in this study indicates stimulation of micturition centers by induction of SUI. Moreover, injection of human ADSCs into the urethra remarkably suppressed c-Fos elevation in the PMC and vlPAG near to a sham-operation level, suggesting that the stimulation of micturition centers calmed down by human ADSCs.
One possible explanation for this phenomenon is that loss of supportive tissues or induction of smooth muscle atrophy by transabdominal urethrolysis might strongly stimulate the micturition-related brain areas (the PMC and vlPAG). Differentiating into smooth muscle cells, human ADSCs might act as a supplement for the loss of supportive tissues or hinder atrophy of the intrinsic sphincter. The supplement of supportive tissues or the delay of smooth muscle atrophy by human ADSCs could increase the abdominal leak point pressure, and then suppress activation of the PMC and vlPAG, resulting in amelioration of SUI symptoms.
Taken together, the results of this work suggest that human ADSCs, an easily accessible cell source for the treatment of SUI, can be an effective therapeutic modality to ameliorate the symptoms of SUI. The effect of transplanted human ADSCs on central micturition centers is an intriguing finding and needs further investigation in future work.
Acknowledgements
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. R11-2008-036-03004-0).
References
- Athwal BS Berkley KJ Hussain I Brennan A Craggs M Sakakibara R Frackowiak RS Fowler CJ 2001 Brain responses to changes in bladder volume and urge to void in healthy men Brain 124 369 377
- Balmforth J Cardozo LD 2003 Trends toward less invasive treatment of female stress urinary incontinence Urology 62 52 60
- Behbehani MM. 1995 Functional characteristics of the midbrain periaqueductal gray Prog Neurobiol 46 575 605
- Blok BF Holstege G. 1997 Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat Neurosci Lett 222 195 198
- Cho JA Park H Kim HK Lim EH Seo SW Choi JS Lee KW. 2009 Hyperthemia-treated mesenchymal stem cells exert antitumor effects on human carcinoma cell line Cancer 115 311 323
- Chung IM Kim YS Sung YH Kim SE Ko IG Shin MS Park HJ Ham DH Lee HJ Kim KJ et al. 2008 Effects of acupuncture on abdominal leak point pressure and c-Fos expression in the brain of rats with stress urinary incontinence Neurosci Lett 439 18 23
- Fu Q Song XF Liao GL Deng CL Cui L. 2010 Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence Urology 75 718 723
- Furuta A Carr LK Yoshimura N Chancellor MB 2007 Advances in the understanding of stress urinary incontinence and the promise of stem-cell therapy Rev Urol 9 106 112
- Guzowski JF Timlin JA Roysam B McNaughton BL Worley PF Barnes CA. 2005 Mapping behaviorally relevant neural circuits with immediate-early gene expression Curr Opin Neurobiol 15 599 606
- Hsieh GC Klutke JJ Kobak WH 2001 Low valsalva leak-point pressure and success of retropubic urethropexy Int Urogynecol J Pelvic Floor Dysfunct 12 46 50
- Jack GS Almeida FG Zhang R Alfonso ZC Zuk PA Rodríguez LV 2005 Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction J Urol 174 2041 2045
- Kavia RB Dasgupta R Fowler CJ 2005 Functional imaging and the central control of the bladder J Comp Neurol 493 27 32
- Kerns JM Damaser MS Kane JM Sakamoto K Benson JT Shott S Brubaker L. 2000 Effects of pudendal nerve injury in the female rat Neurourol Urodyn 19 53 69
- Kwon D Kim Y Pruchnic R Jankowski R Usiene I de Miguel F Huard J Chancellor MB 2006 Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence Urology 68 449 454
- Lee JY Cannon TW Pruchnic R Fraser MO Huard J Chancellor MB. 2003 The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence Int Urogynecol J Pelvic Floor Dysfunct 14 31 37
- Lin , G , Wang , G , Banie , L , Ning , H , Shindel , AW , Fandel , TM , Lue , TF and Lin , CS. 2010 . Treatment of stress urinary incontinence with adipose tissue-derived stem cells . Cytotherapy. , 12 : 88 – 95 .
- Lin HK Cowan R Moore P Zhang Y Yang Q Peterson JA Jr Tomasek JJ Kropp BP Cheng EY. 2004 Characterization of neuropathic bladder smooth muscle cells in culture J Urol 171 1348 1352
- Mallory , BS , Roppolo , JR and de Groat , WC. 1991 . Pharmacological modulation of the pontine micturition center . Brain Res. , 546 : 310 – 320 .
- Miller , KL. 2005 . Stress urinary incontinence in women: review and update on neurological control . J Womens Health. , 14 : 595 – 608 .
- Mitsui , T , Kakizaki , H , Matsuura , S , Tanaka , H , Yoshioka , M and Koyanagi , T. 2003 . Chemical bladder irritation provokes c-fos expression in the midbrain periaqueductal gray matter of the rat . Brain Res. , 967 : 81 – 88 .
- Morgan , JI and Curran , T. 1991 . Proto-oncogene transcription factors and epilepsy . Trends Pharmacol Sci. , 12 : 343 – 349 .
- Nour , S , Svarer , C , Kristensen , JK , Paulson , OB and Law , I. 2000 . Cerebral activation during micturition in normal men . Brain. , 123 : 781 – 789 .
- Phull , H , Salkini , M , Escobar , C , Purves , T and Comiter , CV. 2007 . The role of angiotensin II in stress urinary incontinence: A rat model . Neurourol Urodyn. , 26 : 81 – 88 .
- Radley , SC , Chapple , CR , Bryan , NP , Clarke , DE and Craig , DA. 2001 . Effect of methoxamine on maximum urethral pressure in women with genuine stress incontinence: a placebo-controlled, double-blind crossover study . Neurourol Urodyn. , 20 : 43 – 52 .
- Rodella , L , Rezzani , R , Gioia , M , Tredici , G and Bianchi , R. 1998 . Expression of Fos immunoreactivity in the rat supraspinal regions following noxious visceral stimulation . Brain Res Bull. , 47 : 357 – 366 .
- Rodríguez , LV , Alfonso , Z , Zhang , R , Leung , J , Wu , B and Ignarro , LJ. 2006 . Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells . Proc Natl Acad Sci USA. , 103 : 12167 – 12172 .
- Rodríguez , LV , Chen , S , Jack , GS , de Almeida , F , Lee , KW and Zhang , R. 2005 . New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency . Am J Physiol Regul Integr Comp Physiol. , 288 : R1332 – R1338 .
- Sievert KD Emre Bakircioglu M Tsai T Dahms SE Nunes L Lue TF. 2001 The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change J Urol 166 311 317
- Sim YJ Kim H Shin MS Chang HK Shin MC Ko IG Kim KJ Kim TS Kim BK Rhim YT et al. 2008 Effect of postnatal treadmill exercise on c-Fos expression in the hippocampus of rat pups born from the alcohol-intoxicated mothers Brain Dev 30 118 125
- Zuk PA Zhu M Ashjian P De Ugarte DA Huang JI Mizuno H Alfonso ZC Fraser JK Benhaim P Hedrick MH. 2002 Human adipose tissue is a source of multipotent stem cells Mol Biol Cell 13 4279 4295