Abstract
The fine structural characteristics of the antennal sensory organs of a female millipede Oxidus gracilis (Polydesmida: Paradoxomatidae) were observed with both field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). We could identify four apical cones and three basic types of antennal sensillae in O. gracilis as follows: chaetiform sensilla (CS), trichoid sensilla (TS) and basiconic sensilla (BS). Of these, both types of CS and TS can be observed throughout all antennal segments except the terminal 8th article, whereas the BS are observed within the cuticular depressed regions of the articles from the 5th to the 7th segment. According to their relative size, microstructure and location, the BS are divided further into three subtypes: large (BS1), small (BS2) and spiniform (BS3). The BS1 can be seen on the 5th article only, while BS2 can be seen on the 5th and 6th articles. The BS3 is characteristically seen within the depressive region of the 7th article. Both the CS and TS of O. gracilis are similar in structure, and they are related to the function of mechanical reception; however, four large apical cones (AP) and three subtypes of BS are likely to function in gustatory and olfactory reception.
Keywords:
Introduction
It has been estimated that there are approximately 10,000 described species of millipedes in the world. The true flat-backed millipede, Polydesmida, is the largest order of millipedes, containing more than 2700 species worldwide (Hopkins and Read Citation1992). They are blind and have completely fused sclerites, and most of them have 20 segments (James and Michael Citation2003; Marek and Bond Citation2006).
Millipedes are usually found under foliage or stones on the surface of the soil and play an important role in the decomposition processes of decaying plant (Hopkins and Read Citation1992). The antenna is one of major channels for sensory reception, including receptors for volatile odors and pheromones, contact chemoreception, water vapor, carbon dioxide, sound, proprioception and touch (Hashimoto Citation1991; Steinbrecht Citation1997; Kleineidam et al. Citation2000).
The antennae of the millipede are covered in a variety of sensory organs with mechanical and chemical receptors (Hopkins and Read Citation1992). Previous work has shown that male millipedes do not mate successfully with females if their antennae are removed, presumably because they do not detect the stimuli produced by the female pheromones (Haacker Citation1974; Carey and Bull Citation1986; Diehl et al. Citation2003).
Although the sensory receptors and neurosecretory systems of the antennal sensilla in various insects (Zacharuk Citation1980; Okada et al. Citation1992; Saïd et al. Citation2003) were well studied, little was known of the fine structure and function of the nerves of millipedes (Nguyen Duy-Jacquemin 1989Duy-Jacquemin 1997; Chung and Moon Citation2006a, Citationb, Citation2007), and more precise research is required in this area. The purpose of the present research was to identify the fine structural characteristics of various sensory organs on the antenna of the millipede Oxidus gracilis to better understand their possible behavioral and ecophysiological roles.
Materials and methods
Adult female millipedes of Oxidus gracilis (Polydesmida: Paradoxosomatidae) were collected at a dumping site for dredging soil in the Busan New Port (Yongwon-dong, Jinhae City, Gyungnam Province, Korea). All individuals were maintained under ambient conditions with natural lighting in enclosures with a wooden frame. Specimens were anesthetized with CO2 and fixed in a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde buffered with 0.1 mole/L phosphate buffer at pH 7.4. Postfixation was performed with 1% osmium tetroxide in the same buffer.
For scanning electron microscopic examination, the specimens were washed in 0.1 mole/L phosphate buffer following fixation, and put through an ethanol series from 30 to l00% (30 minutes at each concentration, with one repeat with absolute ethanol). The specimens were then either critical-point-dried or transferred to hexamethyldisilazane (HMDS) for air-drying. All samples were coated with gold-palladium alloy using a sputter coater to a thickness of approximately 20 nm. The coated specimens were examined on a Hitachi S-4300 field emission scanning electron microscope (Hitachi Co., Tokyo, Japan) operated with accelerating voltages of 5–20 kV.
For transmission electron microscopic examination, specimens were gently removed and fixed in a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde buffered with 0.1 M phosphate buffer at pH 7.4. Postfixation was performed with 1% osmium tetroxide in the same buffer and samples were washed several times in 0.1 M phosphate buffer following fixation. The specimens were then dehydrated in ascending concentrations of ethanol and embedded in Poly/Bed 812-Araldite medium (Polysciences Inc., USA) via propylene oxide.
Semi-thin sections stained with 1% toluidine blue (dissolved in 1% borax) were used to study the gross morphology. Ultrathin sections were obtained from an LKB Ultratome V (LKB, Stockholm, Sweden) and were double stained with uranyl acetate followed by lead citrate. After these treatments, the sections were examined with a JEM 100 CX-II transmission electron microscope (JEOL) at 80 kV.
Results
The antenna of adult female millipede has eight distinct articles, and each article is roughly cylindrical but increases in diameter towards the terminal section. The average length of the antenna of adult female millipede is less than that of the male; length is 2.0 (± 0.18, n = 5, range 1.8–2.2) mm. The serrate antenna of this millipede consists of the scape (1st article), pedicel (2nd article) and six flagellomeres (3rd to 8th articles) (, A–C).
Figure 1. (A–C) The antenna of adults of the female millipede Oxidus gracilis has eight distinct articles: a scape (S), a pedicel (P) and 6 flagellomeres from the 3rd to 8th articles. Each article is roughly cylindrical but increases in diameter towards the terminal article. (D–G) On the 3rd to 8th articles, four subtypes of sensillum can be seen: chaetiform (CS), trichoid (TS) and two subtypes of basiconic sensilla. The trichoid sensilla are straight hairs, and the chaetiform sensilla are long, sickle-shaped strong bristles with deep longitudinal grooves. Scale bars indicate 500 μm (A, B, D, E), 100 μm (C) and 50 μm (F, G).
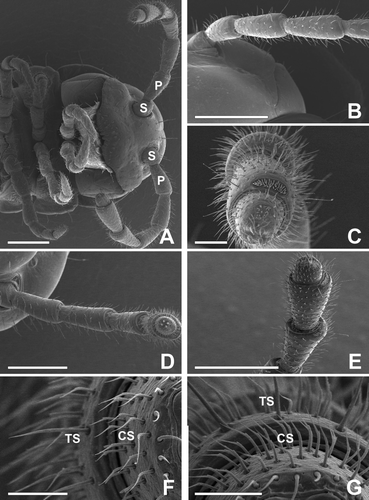
Table 1. Mean length (m) of antenna in the Oxidus gracilis.
On the surface of the antenna, there are a variety of sensory receptors, including olfactory and mechanical receptors. According to their morphological and fine structural characteristics, three basic types of antennal sensillum are identified: chaetiform sensilla (CS), trichoid sensilla (TS) and basiconic sensilla (BS) (D–G). Of these, the BS are divided further into three subtypes: large basiconic sensilla (BS1) on the 5th and 6th articles, small basiconic sensilla (BS2) on the 5th article, and a distinct type of the basiconic spiniform sensilla (BS3) on the 7th article (A–F and 3A–C). The most prominent sensory structure is four large apical cones on the distal tip of the 8th segment. The 8th article of the antenna bears four characteristic sensory apical cones (, D–F).
Figure 2. Scanning electron micrographs of the antenna in female of Oxidus gracilis. (A–C) On the 5th article, two subtypes of the basiconic sensillum, large basiconic sensilla (Bs1) and small basiconic sensilla (Bs2), can be seen. These two subtypes are scattered within cuticular depressions of this article, and they have a straight, finger-like appearance, commonly. (D–F) On the surface of the 6th article, a subtype of small basiconic sensilla (BS2), and both types of chatiform sensilla (CS) and trichoid sensilla (TS) can be seen. Each has one or two sensory pores (arrows) around the basal surface. Scale bars indicate 50 μm (A, B, D), 25 μm (C, E) and 10 μm (F).
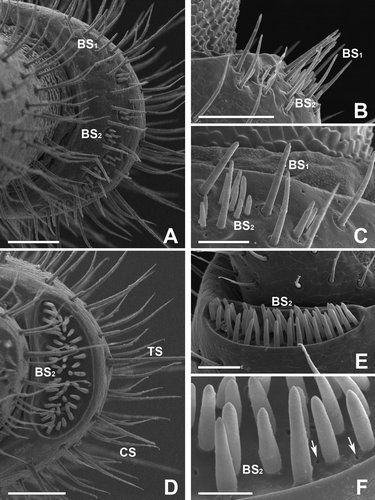
Figure 3. Scanning electron micrographs of the antenna in female of Oxidus gracilis. (A,B) On the cuticular depressions of the 7th article, another subtype of basiconic sensilla can be seen. The spiniform basiconic sensilla (BS3) are spine-like sensilla with a smooth surface and a sharp, narrow tip. (C–F) The 8th article bears four apical cone-shaped sensilla (AC) distributed in a rectangular arrangement. The apical cones on this article are the largest sensilla. Each sensillum has an apical pore (arrow) at the pointed end. Scale bars indicate 50 μm (C), 25 μm (D) and 10 μm (A, B, E, F).
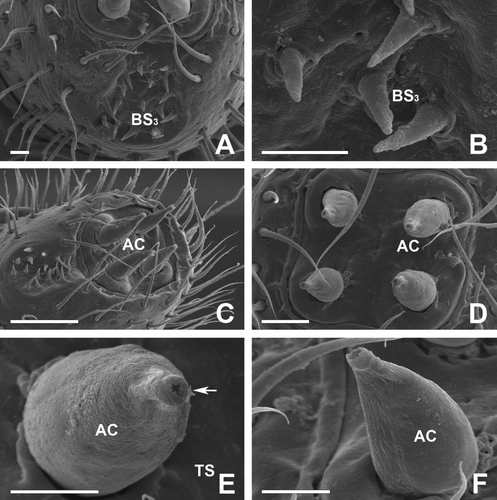
Table 2. Features of antennal sensilla in Oxidus gracilis female.
The CS were observed on the surfaces of whole segments of the antenna, with the exception of the 8th article. The CS on the articles are structurally similar to the TS, but are shorter and more abundant. They are long, sickle-shaped, strong bristles with longitudinal grooves acuminating toward the tip. They are distributed encircling at each segment. The TS were also identified on the surfaces of whole segments of the antenna, with the exception of the 8th article. They are distributed encircling at each segment together with the CS (D,E). The external structures of the TS are blunt-tipped, almost straight hairs with deep longitudinal grooves in their lower parts. TS length is mostly 60–170 µm (F,G).
The 5th to 7th articles are also the most prominent antennal segments in this millipede. On the surfaces of the 5th to 7th antennae, the three subtypes of the BS are distinguished within the cuticular depressive regions of the articles. They are the large basiconic sensilla (BS1), small basiconic sensilla (BS2) and a distinct type of the spiniform basiconic sensilla (BS3). The BS1 can be seen on the 5th article only, while BS2 can be seen on the 5th and 6th articles. The BS3 is characteristically seen within the depressive region of the 7th article. However, there were no BS on the 1st to 4th articles because these articles contain no locally depressed areas on their surfaces.
Each of the BS1 is a straight, finger-like sensillum with a smooth surface and a dull tip. These sensilla are scattered in the vicinity of the cuticular depressive area of the 5th article. The average size of these sensilla is 30 µm, and the average number is 18 (A–C). The BS2 are also straight, finger-like sensilla with a smooth surface, similar to the BS1. However, they are shorter than the BS1, with considerably smaller dimensions. The average size of these sensilla is 10 µm and the average number is 50 (A–C).
There is a most distinct and conspicuous cuticular depression on the 6th article, which contains sensilla of type BS2. The crescent-shaped cuticular depression is located on the dorsal surface of this article. The total number of BS2 found here is approximately 50 in the female millipede. They are compactly arranged over the whole surface of the cuticular depressive region. Each BS2 of this article is a straight and finger-like sensillum with a smooth surface. The length of these sensilla is mostly 18 µm and the average diameter of the base is 3.0 µm (D–F).
The BS3 are spine-like sensilla with a sharp, narrow tip and a smooth surface. They are found in the depressive region on the 7th article (A,C). They are markedly different in size and shape from the other types of basiconic sensillum (BS1 and BS2). The total number of BS3 is approximately 40 in female millipede and average length is 9 µm. A variety of sensory pits are observed on the surface of the cuticular depression. There are one or two sensory pores around the base of each individual BS2 (A-C).
The most prominent sensory structure is four large apical sensory cones on the distal tip of the 8th segment. The last segment of this millipede is very small and somewhat difficult to observe without viewing from the front. In the top view of the article, four of the apical cones (AP) are distributed in an exact rectangular arrangement (A–D). They have several longitudinal grooves in their external surfaces. These AP are 33 µm in length with a basal diameter of 15 µi. Each cone has its apical pore at the pointed end, and the average diameter of the pore is approximately 1 µm (E,F).
Transmission electron micrography analyses of the base of AP in Oxidus gracilis showed that the sensillar lumen is filled by a variable number of dendritic processes that show a certain degree of branching within the shaft. The dendrites are embedded by an electron-dense sensillar lymph (A,B). Within the electron-lucent luminal spaces of AP, bundles of electron-dense dendritic branches are visible. In addition, ciliary bundles for constriction of the cone sensillium are also distributed near the dendritic branches. The lamellated membrane which ensheathes these bundles is deeply folded. At the ciliary constriction level, the sensory neurons are surrounded by the thecogen and trichogen cells (C,D).
Figure 4. Transmission electron micrographs of apical cone sensillum in female of Oxidus gracilis. (A,B) The lumen of antennal sensillum is filled by a variable number of dendritic processes (D). The dendrites are embedded by an electron-dense sensillar lymph. (C,D) Bundles of dendritic branches (DB) and ciliary bundles (CB) are surrounded by the thecogen (TH) and trichogen (TR) cells. (E,F) Each cilia (C) within the ciliary bundle has a microtubular complex which composed of two central singlets and nine pairs of pheripheral doublets. (G,H) The cuticular wall (CU) of the apical cone is externally smooth, and about 50–55 dendritic processes are observed. Cross section through the tip of antenna showing dendritic bundles ensheathed by the dendritic sheath (DS). Scale bars indicate 5 μm (A), 1 μm (B) and 0.5 μm (C–H).
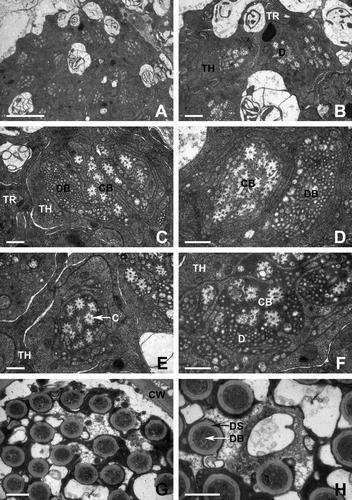
High-magnification electron microscopic observation reveals that each cilia within the ciliary bundle has a characteristic microtubular complex substructure which is composed of two central singlets and nine pairs of pheripheral doublets (E,F).
A cross-section taken at the tip of the AP shows a double-walled organization for this sensillum. Two distinct cavities are defined by two cuticular walls with different thickness ranging from 0.1 µm (inner wall) to 0.4 µm (outer wall). The cuticular wall of the AP is externally smooth, and approximately 50–55 dendritic processes extend along the longitudinal direction. Approximately eight to 13 sensory neurons innervating each dendritic process are wrapped by a thick dendritic sheath (G,H).
Discussion
Previous work has shown that the external morphology of the antenna is similar to those in other insects (Lavoie and McNeil Citation1987; Lopes et al. Citation2002). Most antennae of the millipede have been reported to have eight segments (Nguyen Duy-Jacquemin 1989Duy-Jacquemin 1997), and each article is roughly cylindrical but increases in diameter towards the terminal segment (Chung and Moon Citation2006a, Citationb).
Several types of sensory organs have been reported on the antenna of arthropod animals, and various authors have suggested the subdivisions of the particular types into subtypes (Albert and Seabrook Citation1973; Wirth and Navai Citation1978; George and Nagy Citation1984; Saïd et al. Citation2003). Although these terms were developed using light microscopic observation, they are still in use, and refer to the appearance of an antenna sensilla (Altner et al. Citation1983; Altner and Loftus Citation1985).
Based on the morphological characteristics of the antenna of Oxidus gracilis, we could identify eight distinct articles: scape (1st article), pedicel (2nd article) and six flagellomeres (3rd–8th articles). In addition, three basic types of antennal sensillum are also identified in the female O. gracilis: the chaetiform sensilla (CS), the trichoid sensilla (TS) and the basiconic sensilla (BS).
The CS are generally referred to as long, sickle-shaped, strong bristles with longitudinal grooves, whereas the TS are generally referred to as sharp-tipped or blunt-tipped sense organs in this millipede. These two types of sensilla in O. gracilis can be observed throughout all antennal segments except the terminal 8th article. Based on the morphological and microstructural characteristics, both the CS and TS of O. gracilis are similar in structure to those reported in other millipedes (Nguyen Duy-Jacquemin 1989; Chung and Moon Citation2006a, Citationb) and other insects (Jefferson et al. Citation1970; Liu and Liu 1984; Lopes et al. Citation2002).
Although some variations have been identified in some insects according to their microstructural differences (Wall Citation1978; Lavoie and McNeil Citation1987; Renthal et al. Citation2003; Saïd et al. Citation2003), our observation was that both of CS and TS of most articles in the millipede were morphologically identical, but with variation in size and location.
The function of these sensilla has been suggested to be contact chemoreceptors in moth (Jefferson et al. Citation1970; Albert and Seabrook Citation1973), but to have a mechanoreceptive function in moth (Van der Pers and Den Otter Citation1978) and mosquito (Davis and Sokolove Citation1975). Previous work has shown sharp-tipped hairs in mosquitoes that respond to a variety of odors (McIver Citation1982). A similar type of sensilla with an olfactory reception function has also been reported in the silk moth (Popov et al. Citation1994). Therefore, it is likely that these sensilla of this millipede also respond to various olfactory stimulations (Shanbhag et al. Citation1999; Sen and Mitchell Citation2001; Hummel et al. Citation2006).
Previous research has shown that the simple sense organs of the millipede can be categorized as mechanoreceptors, olfactory receptors and gustatory receptors (Haupt Citation1979). Among them, the gustatory receptors have an articulation membrane and a mecheno-receptive dendrite at the base (Hopkins and Read Citation1992). They respond to direct contact with chemicals, in contrast to olfactory receptors, which respond to molecules in the air. Thus, Nguyen Duy-Jacquemin (1989) has demonstrated that the sensilla of this type may have a dual function, mechanoreceptive and chemoreceptive.
Following the study by Sakwa (Citation1974), it has been reported that there were two kinds of gustatory receptors on the antennae of the millipede: large basiconic cones at the apex of the terminal article and smaller BS at the distal ends of articles 5, 6 and 7. We also observe that the apical cone on the 8th article is the largest sensory structure in O. gracilis regardless of age or sex. In addition, the BS are also observed within the cuticular depressed regions of the articles from the 5th to the 7th segment.
They are divided further into three subtypes according to their relative size, microstructure and location: large basiconic sensilla (BS1) on the 5th and 6th articles; small basiconic sensilla (BS2) on the 5th article, and a distinct type of the basiconic spiniform sensilla (BS3) on the 7th article. The fine structural aspects of these sensilla have been reported in millipedes of P. lagurus (Nguyen Duy-Jacquemin Citation1997) and O. pekuensis (Chung and Moon Citation2006a, Citationb) and some moths (Cuperus Citation1983; George and Nagy Citation1984).
Although various authors have suggested a possible role of the sensillum in the sensory stimuli for prey or mate detection, its exact function is not clear yet and more work is required. Cuperus (Citation1983) and Faucheux (Citation1991) observed pores in the BS of a Lepidoptera (Y. vigintipunctatus) and a moth (H. nebulella), and concluded that these pores have an olfactory function, perhaps for the reception of the volatile odors of plants (Van der Pers Citation1981; Cuperus Citation1983; Faucheux Citation1991; Saïd et al. Citation2003).
The cone-shaped AS on the 8th article was the largest sensillum in O. gracilis and other species of millipedes (Chung and Moon Citation2006a, Citationb, Citation2007) regardless of age or sex. It has been thought that the main function of these sensilla is likely to be olfactory reception of the volatile odors of plants (Van der Pers Citation1981; Cuperus Citation1983; Faucheux Citation1991; Hopkins and Read Citation1992). In our present study using both of SEM and TEM, we could observe that each apical cone sensillum has its apical pore and bundles of its dendritic processes that make them suitable for a double mechano-chemosensory function.
Recently, Isidoro et al. (1996) and Romani and Stacconi (Citation2009) have also described this type of sensilla as the uniporous gustatory sensilla which act as both mechanoreceptors and contact chemosensilla. This type of sensillum has been described also in other heteropteran species by Brezot et al. (Citation1996) and Chinta et al. (Citation1997) with the same functional hypothesis. This supposed gustatory function finds a good correspondence with their location at the distal end of the last segment of antenna. However, its exact function in the life of the millipede is still not clear and more work is required, even though various authors have suggested a possible role of the sensillum in the sensory stimuli for prey or mate detection.
References
- Albert , PJ and Seabrook , D. 1973 . Morphology and histology of the antenna of the male eastern spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) . Can J Zool. , 4 : 443 – 448 .
- Altner , H and Loftus , R. 1985 . Ultrastructure and function of insect thermo- and hygroreceptors . Ann Rev Entomol. , 30 : 273 – 295 .
- Altner , H , Schaller-Seizer , L , Stetter , H and Wohlrab , I. 1983 . Poreless sensilla with inflexible sockets. A comparative study of a fundamental type of insect sensilla probably comprising thermo- and hygro-receptors . Cell Tissue Res. , 234 : 279 – 307 .
- Brezot , P , Tauban , D and Renou , M. 1996 . Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): Sensillum types and numerical growth during the post-embryonic development . Int J Insect Morphol Embryol , 25 : 427 – 441 .
- Carey , CJ and Bull , CM. 1986 . Recognition of matesin the Portugese millipede Ommatoiulus moreleti . Aust J Zool. , 34 : 837 – 842 .
- Chinta , S , Dickens , CJ and Baker , GT. 1997 . Morphology and distribution of antennal sensilla of the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) . Int J Insect Morphol Embryol. , 26 : 21 – 26 .
- Chung , KH and Moon , MJ. 2006a . Fine structure of the antennal sensilla of the millipede, Orthomorphella pekuensis (Polydesmida: Paradoxosomatidae) . Entomol Res. , 36 : 172 – 178 .
- Chung , KH and Moon , MJ. 2006b . Antennal sensory organs in the female millipede Orthomorphella pekuensis (Polydesmida: Paradoxosomatidae) . Integr Biosci. , 10 : 183 – 189 .
- Chung , KH and Moon , MJ. 2007 . Microstructure of the Antennal Sensory Organs in the Millipede Cawjeekkelia pyongana (Polydesmida: Paradoxosomatidae) Korean J Electr Microsc , 37 : 73 – 82 .
- Cuperus , PL. 1983 . Distribution of antennal sense organs in male and female ermine moth, Yponomeuta vigintipunctatus (Retzius) (Lepidoptera: Yponomeutidae) . Int J Insect Morphol Embryol. , 12 : 59 – 66 .
- Davis , EE and Sokolove , PG. 1975 . Temperature responses of antennal receptors of the mosquito Aedes aegypti . J Comp Physiol. , 6 : 223 – 236 .
- Diehl , PA , Guerenstein , VP and Guerin , PM. 2003 . Ultrastructure and receptor cell responses of the antennal grooved peg sensilla of Triatoma infestans (Hemiptera: Reduviidae) . Arthropod Struct Dev. , 31 : 271 – 285 .
- Faucheux , MJ. 1991 . Morphology and distribution of sensilla on the cephalic appendages, tarsi and ovipositor of the European sunflower moth, Homoeosoma nebulella Den. and Schiff. (Lepidoptera: Pyralidae) . Int J Insect Morphol Embryol. , 20 : 291 – 307 .
- George , JA and Nagy , BAL. 1984 . Morphology, distribution, and ultrastructural differences of sensilla trichodea and basiconica on the antennae of the oriental fruit moth, Grapholitha molesta (Busck) (Lepidoptera: Tortricidae) . Int J Insect Morphol Embryol. , 13 : 157 – 170 .
- Haacker , U. 1974 . Patterns of communication in courtship and mating behaviour of millipedes (Diplopoda) . Symp Zool Soc London , 32 : 317 – 328 .
- Hashimoto , Y. 1991 . Phylogenetic study of the family formicidae based on the sensillium structures on the antennae and labial palpi (Hymenoptera: Aculeata) . Japan J Entomol. , 59 : 125 – 140 .
- Haupt , J. 1979 . “ Phylogenetic aspects of recent studies on myriapod sense organs ” . In Myriapod biology , Edited by: Camatini , M . 391 – 406 . London : Academic Press .
- Hopkins , SP and Read , HJ. 1992 . The biology of millipedes , New York : Oxford University Press .
- Hummel , NA , Zalom , FG and Peng , CYS. 2006 . Structure of female genitalia of glassy-winged sharpshooter, Homalodisca coagulata (Say) (Hemiptera: Cicadellidae) . Arthropod Struct Dev. , 35 : 111 – 125 .
- James , ML and Michael , JS. 2003 . Litter breakdown by the Seychelles giant millipede and the conservation of soil processes on Cousine Island, Seychelles . Bio Conserv. , 113 : 125 – 132 .
- Jefferson , RN , Rubin , RE , Mcfarland , SU and Shorey , HH. 1970 . Sex pheromones of noctuid moths, XXII. The external morphology of the antennae of Trichoplusiani, Heliothiszea, Prodeniaornithogalli and Spodopteraexigua . Ann Entomol Soc Am. , 63 : 1227 – 1238 .
- Kleineidam , C , Romani , R , Tautz , J and Isidoro , N. 2000 . Ulrastructure and physiology of the CO2 sensillum ampullaceum in the leaf-cutting ant Atta sexdens . Arthropod Struct Dev. , 29 : 43 – 55 .
- Isidoro , N , Bin , F , Colazza , S and Vinson , SB. 1996 . Morphology of antennal gustatory sensilla and glands in some parasitioid Hymenoptera with hypothesis on their role in sex and host recognition . J Hymenoptera Res. , 5 : 206 – 239 .
- Lavoie , DJ and McNeil , JN. 1987 . Sensilla of the antennal flagellum in Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae) . Int J Insect Morphol Embryol. , 16 : 153 – 167 .
- Liu , HJ and Liu , TP. 1984 . Sensilla on the antennal flagellum of the bertha army worm, Mamestra configurata Walker (Lepidoptera: Noctuidae): A scanning electron microscope study . Ann Entomol Soc America. , 77 : 236 – 245 .
- Lopes , O , Barata , EN , Mustaparta , H and Araújo , J. 2002 . Fine structure of antennal sensilla basiconica and their detection of plant volatiles in the eucalyptus woodborer, Phoracantha semipunctata Fabricius (Coleoptera: Cerambycidae) . Arthropod Struct Dev. , 31 : 1 – 13 .
- Marek , PE and Bond , JE. 2006 . Phylogenetic systematics of the clorful, cyanide-producing millipedes of Appalachia (Polydesmida, Xystodesmidae, Apheloriini) Using a total evidence Bayesian approach . Mol Phylogenet Evol. , 41 : 704 – 729 .
- McIver , SB. 1982 . Sensilla of mosquitoes (Diptera: Culicidae) . J Med Entomol. , 19 : 489 – 535 .
- Nguyen Duy-Jacquemin , M. 1989 . Ultratructures des sensilles basiconiques bacilliformes des antennes du diplopode cavernicole Typhloblaniulus lorifer Bröl (Myriapode, Diplopode) . Mem Biospe′ol. , 16 : 251 – 256 .
- Nguyen Duy-Jacquemin , M. 1997 . Fine structure and possible functions of antennal sensilla in Polyxenus lagurus (Diplopoda, Penicillate: Polyxenidae) . Ent Scand Suppl. , 51 : 167 – 178 .
- Okada , K , Mori , M , Shimazaki , K and Chuman , T. 1992 . Morphological studies on the antennal sensilla of the cigarette beetle, Lasioderma serricorne (F.) (Coleoptera: Anobiidae) . Appl Entomol Zool. , 27 : 269 – 276 .
- Popov , VI , Nikonov , AA , Agafonova , NK and Fesenko , EE. 1994 . Surface ultrastructure of olfactory receptor sense hairs in the silkmoth Antheraea pernyi . J Microsc. , 174 : 39 – 46 .
- Renthal , R , Velasquez , D , Olmos , D , Hampton , J and Wergin , WP. 2003 . Structure and distribution of antennal sensilla of the red imported fire ant . Micron , 34 : 405 – 413 .
- Romani , R and Stacconi , MVR. 2009 . Mapping and ultrastructure of antennal chemosensilla of the wheat bug Eurygaster maura . Insect Science , 16 : 193 – 203 .
- Saïd , I , Tauban , D , Renou , M , Mori , K and Rochat , D. 2003 . Structure and function of the antennal sensilla of the palm weevil Rhynchophorus palmarum (Coleoptera: Curculionidae) . J Insect Physiol. , 49 : 857 – 872 .
- Sakwa , WN. 1974 . A consideration of the chemical basis of food preference in millipede . Symp Zool Soc Lond. , 32 : 329 – 346 .
- Sen , A and Mitchell , BK. 2001 . Olfaction in the Colorado potato beetle: ultrastructure of antennal sensilla in Leptinotarsa sp . J Biosci. , 26 : 233 – 246 .
- Shanbhag , SR , Müller , B and Steinbrecht , RA. 1999 . Atlas of olfactory organs of Drosophila melanogaster (1) Types, extrenal organization, innervation and distribution of olfactory sensilla . Int J Insect Morphol Embryol. , 28 : 377 – 397 .
- Steinbrecht , RAA. 1997 . Pore structures in Insect olfactory sensilla: a review of data and concepts . Int J Insect Morphol Embryol. , 26 : 229 – 245 .
- Van der Pers , JNC. 1981 . Comparison of electroantennogram response spectra to plant volatiles in seven species of Yponomeuta and in the tortricid Adoxophyes orana . Entomol Exp Appl. , 30 : 181 – 192 .
- Van der Pers , JNC and Den Otter , CJ. 1978 . Single cell responses from olfactory receptors of small ermine moths (Lepidoptera: Yponomeutidae) to sex attractants . J Insect Physiol. , 24 : 337 – 343 .
- Wall , C. 1978 . Morphology and histology of the antenna of Cydia nigricana (F.) (Lepidoptera: Tortricidae) . Int J Insect Morphol Embryol. , 7 : 237 – 250 .
- Wirth , WW and Navai , S. 1978 . Terminology of some antennal sensory organs of Culicoides biting midges (Diptera: Ceratopogonidae) . J Med Entomol. , 15 : 43 – 49 .
- Zacharuk , RY. 1980 . Ultrastructure and function of insect chemosensilla . Annl Rev Entomol. , 25 : 27 – 47 .