Abstract
To examine variation in trophic pathways and the characteristics of food webs from organic matters to aquatic insects, we used stable isotopes to study an intermittent stream system of the Inukami River, Japan. The aquatic insects, including Glossosoma spp., Chironominae spp., Stenelmis spp., Rhyacophilla nigrocephala, and Hexatoma spp., were characterized by different feeding strategies. The δ13C values for these species indicated that Glossosoma spp. graze upon periphyton; Chironominae and Stenelmis spp. mainly feed on benthic particulate organic matter, and R. nigrocephala and Hexatoma spp., which were identified as predators, feed upon Glossosoma, Stenelmis, and/or Chironominae spp. This suggests that the trophic position of consumers at each station may be determined by the trophic position of basal food sources in situ. For trophic pathways, the δ13C values for both organic matter and aquatic insects tended to gradually decrease, whilst the δ15N values increased from the upper reach to the lower reaches, relative to the physicochemical and geographical conditions. These parameters indirectly influence the flow of energy from organic matter to consumers within food web in an intermittent stream system.
Introduction
Rivers and streams are spatially and temporally variable in their biological communities, physical characteristics, and ecosystem processes (Winemiller et al. Citation2010). Lotic ecosystems and intermittent stream system environments involve a dynamic hydrological system, characterized by a period of water flow and followed by drought, leading to environmental heterogeneity (Simões et al. Citation2008). The dynamics of these rivers are related to local and regional influences of seasonality, such as infiltration, evaporation, precipitation, runoff, hydroperiod, and exchanges with groundwater (Boulton and Lake Citation1992; Schwartz and Jenkins Citation2000).
River food webs typically depend on two main carbon energy sources: inorganic carbon fixed by algae or other aquatic plants, and particulate organic matter derived from terrestrial plant matter (e.g. Vannote et al. Citation1980; Thorp and Delong Citation1994; Doucett et al. Citation2007; Ishikawa et al. Citation2010). In view of these major energy sources, recent studies based on intermittent stream systems have reported the origin of allochthonous or autochthonous organic matter as food sources within stream ecosystems (Bunn et al. Citation2003; Reid et al. Citation2008; Dekar et al. Citation2009). In order to understand the carbon dynamics of stream ecosystems, many studies have used carbon and nitrogen stable isotope ratios to examine trophic pathways (e.g. Finlay Citation2001; Finlay et al. Citation2002; Delong and Thorp Citation2006; Doi et al. Citation2006; Ishikawa et al. Citation2010). The carbon stable isotope ratio (δ13C) is slightly enriched in the route of consumption and assimilation, whilst the nitrogen stable isotope ratio (δ15N) in the tissues of consumers is enriched relative to their diet (Vander Zanden and Rasmussen Citation2001; Post Citation2002). Information regarding how factors such as climate, land use, and physical and hydrological properties interact with other abiotic processes to influence the structure of, and energy flow within, aquatic communities is required (Reid et al. Citation2008). Understanding the trophic basis of stream food webs is crucial for conserving ecosystems exposed to harsh conditions, including drought and intermittent stream drying (Dekar et al. Citation2009).
In this study, we aimed to identify trophic pathways, from organic matter to aquatic insects (including primary consumers and predators), using stable isotopes (carbon and nitrogen), and to characterize food webs in an intermittent stream system of the Inukami River in Japan. To compare relationships between aquatic insects and their food sources, we classified functional feeding groups (FFGs) based on the origins of the food items and the mechanisms of food acquisition (Merritt and Cummins Citation1996).
Materials and methods
Study stations
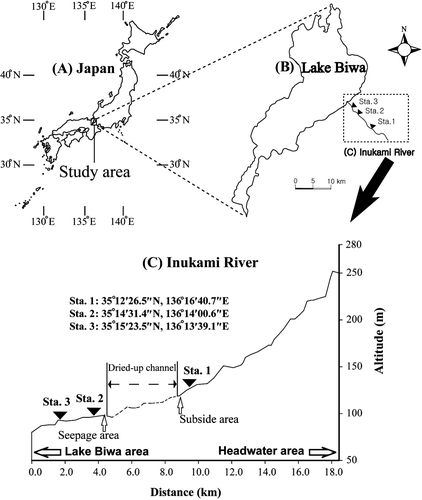
This study was conducted in the Inukami River (35°0′N, 136°0′E), located in the north–eastern region of Shiga prefecture. The Inukami River runs through farmlands, villages, and a town, and flows into the eastern Lake Biwa region of Japan (). The Inukami River is 27 km long and has a catchment area of approximately 105 km2. It has a temperate monsoon climate, and the mean annual precipitation in this watershed is 1571 mm (Hikone Regional Meteorological Office). In addition, the Inukami River has typical limestone geological features and habitat structures such as riffles, runs, and pools. The stream bed of the Inukami River is mainly composed of cobbles and pebbles. Although the river runs through an agricultural area and a town, it is relatively well preserved in its natural state. However, during the dry season (August and November–December), the Inukami River has a dried-up channel approximately 4–9 km from the river mouth (C). We expected this to alter the quality of the stream water. Therefore, we established one station upstream (Station 1) and two stations downstream (Stations 2 and 3) of this channel area.
Figure 1. (A) Map of the study area in Japan. (B) Map of the study stations along the Inukami River and in Lake Biwa. (C) A longitudinal section of the Inukami River, including Station (Sta.) 1 in the upper reach, and Stations 2 and 3 in the lower reaches of the dried-up channel. The arrows indicate the locations and characteristics of stations along the Inukami River.
Sample collection and preparation
Benthic macroinvertebrates were collected at the three stations in July 2008. We conducted sampling with three replicates, using a square Surber sampler (250-µm mesh) 0.25 m2 in size. All biota were separated from other material and were then sorted in the laboratory. Identification was mostly performed using a stereoscopic microscope. Aquatic insects were identified to the lowest feasible classification level (mostly to the genus level). Overall, 94 species were identified from the various samples. Among the collections from sampling stations were aquatic insects from different feeding groups (scrapers: Glossosoma spp.; scrapers or collectors: Stenelmis spp. and Chironominae spp.; predators: Rhyacophilla nigrocephala and Hexatoma spp). Based on their relatively high abundance, these insects were selected for following analyses.
Analysis of environmental factors
Current velocity was measured at all sampling stations, with a digital current meter (Model–3631, Yokogawa Co.), at 1-m intervals from the right to the left bank. Water temperature (WT) and electric conductivity (EC) were measured using a multiple water quality sensor (U–22, Horiba Co.), and pH was measured by colorimetry. The concentration of dissolved oxygen (DO) was determined using the methods described by Winkler (1888). The water samples to be used for the measurement of major cations (Na+, K+, Mg2 +, and Ca2+) and anions (Cl− and SO4 2−) were filtered through filter paper (Toyo No. 5C; pore size 1.0 µm), and were stored in a refrigerator. The concentrations of major ionic elements and nitrate were analyzed using an ion chromatographic analyzer (Dionex DX–120). Water samples to be used for the analyses of nutrients and dissolved organic carbon concentrations were filtered through a glass-fiber filter (Whatman GF/F; pore size 0.7 µm), after combustion at 420°C for 3 h. The ammonium concentration was determined using the methods reported by Sagi (Citation1966); nitrite concentration was determined using the methods reported by Bendschneider and Robinson (Citation1952); phosphate, using the methods reported by Mullin and Riley (Citation1962); and silicate, using the methods reported by Mullin and Riley (Citation1955). The dissolved organic carbon (DOC) concentration was measured by a high-temperature oxidation technique using a total organic carbon analyzer (Shimadzu Model 5000). We collected the matter attached to the surface of stones in a quadrant (5 cm×5 cm) by scouring the surface of the stones with a brush, and then measured its dry weight. We considered that most of the attached matter consisted of periphyton, and therefore the term ‘attached matter’ is used to represent periphyton. The chlorophyll a (chl. a) concentration was determined using a fluorometer (Turner Designs 10–AU).
Analysis of stable isotope ratios
Aquatic insects and their potential food sources were collected from all of the sampling stations. The aquatic insects were maintained in filtered river water at 5°C for 24 h to allow them to eliminate their gut contents, and then the samples were freeze-dried. The potential food sources included suspended particulate organic matter (SPOM), benthic particulate organic matter (BPOM), and periphyton.
For the analysis of stable isotopes, SPOM was collected by filtering the river water through GF/F glass-fiber filters, BPOM was collected from the streambed using sieves with mesh sizes<250 µm, and attached matter (periphyton and chl. a) was collected by scouring stones in a quadrant (5 cm×5 cm) using a brush. SPOM, BPOM, and periphyton samples were acidified with 1 mol·L−1 HCl to remove carbonates. All of the samples were freeze-dried and stored in a freezer at −20°C until the stable isotope ratios were analyzed.
Generally 6 to 140 individuals of the same species were compiled in each sample. The value of all species were measured as an average of compiled individuals. Three replicate measurements of the carbon and nitrogen isotope ratios were taken for each sample, using an elemental analyzer EA1108 (Fisons), which was connected to a mass spectrometer (Delta S, Finnigan MAT), with an interface (Conflo II, Finnigan MAT). The results were reported using the delta notation and have been calculated as follows: δ13C or δ15N (‰)=(Rsample/Rstandard –1)×1000, where R is the 13C/12C ratio for δ13C and the 15N/14N ratio for δ15N. The standards used for δ13C and δ15N were Pee Dee Belemnite (PDB) and atmospheric nitrogen, respectively. The analysis errors for δ13C and δ15N were within ±0.2‰.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to test for significant differences in the δ13C and δ15N values of aquatic insects and their food sources. We used SPSS (SPSS Inc.) and Sigma Plot (Systat Software Inc.) to compare the δ13C and δ15N values of aquatic insects between stations. For descriptive purposes, the mean± standard deviations (SDs) are displayed.
Results
River environmental parameters
The environmental parameters recorded at the sampling stations are displayed in . These included river width, water depth, current velocity, discharge, WT, EC, pH, and the concentrations of DO and the major ions (Na+, K+, Mg2+, Ca2+, Cl−, and SO4 2−). The water temperature at Station 1, characterized by stagnant water formed as a result of the sub-flow process, was 33.2°C±0.3°C and was higher than that at the other stations. The pH value at Station 1, which was located in the upper reach of the dried-up channel, was 8.4 and was also higher than those of the lower reaches (Stations 2 and 3). The DO concentrations (8.3–9.9 mgL−1) and EC (185–214 µScm−1) did not differ among the stations. The chloride, sulfate, and sodium ion concentrations differed between Station 1 and Stations 2 and 3. However, the concentrations of the other major ions were relatively similar for all the stations.
Table 1. Physicochemical parameters and stream water quality at each sampling station (mean (SD), n=3).
The dissolved inorganic nitrogenous compounds, phosphate, silicate, chl. a, and periphyton biomass concentrations are shown in . Most of the dissolved inorganic nitrogen (DIN: sum of nitrate, nitrite, and ammonium nitrogen) in the river was composed of nitrate. Although the DIN concentrations were variable, they did not differ between stations. The nitrate concentration at Station 2 (43.8±2.4 µM) and the phosphate concentration at Station 1 (2.3 µM) were relatively higher than those at the other stations. The phosphate concentration at Station 2 (1.3 µM) and the silicate concentration at Station 1 (176 µM) were also comparatively higher than those at the other stations. The DOC concentrations slightly declined from the upper reach (1.5 mgL−1) to the lower reaches (0.8–0.9 mgL−1).
Table 2. Concentrations of nitrogenous compounds, phosphate, silicate, dissolved organic carbon (DOC), periphyton, and chlorophyll a, at the sampling stations. Mean (SD) values of chlorophyll a, and periphyton are displayed with the range values in parentheses.
The mean values of periphyton biomass (26–53 gm−2) were not significantly different among stations (one-way ANOVA: df=4, F=0.8, P>0.05). However, the mean chl. a concentrations were significantly different between Station 1 and Stations 2 and 3 (one-way ANOVA: df=4, F=4.4, P<0.04).
Isotopic signatures of periphyton, SPOM, and BPOM
The δ13C and δ15N (‰) values of aquatic insects and their potential food sources at each study station are displayed in and . The mean δ13C values of periphyton (−21.6 to −19.1‰) were different among stations (one-way ANOVA: df=3, F=293.6, P<0.01; Tukey multiple comparison, P<0.01 for all values). The mean δ13C values of SPOM (−25.4 to −21.4‰) and BPOM (−24.2 to −22.2‰) were significantly different among stations (one-way ANOVA: df=3, F=4.4 and 22.9, respectively; P<0.01). However, these values did not differ between Stations 2 and 3, which were located in the lower reaches (Tukey multiple comparison, P>0.05). The mean δ15N values of periphyton (1.8–3.7‰) were also considerably different among stations (one-way ANOVA: df=3, F=114.1, P<0.01; Tukey multiple comparison, P<0.02 for all values). The mean δ15N values of SPOM (1.3–3.7‰) and BPOM (2.3–4.4‰) were relatively different among the stations (one-way ANOVA: df=3, F=58.5 and 76.3, respectively, P<0.01). However, again these values did not differ between Stations 2 and 3 in the lower reaches (Tukey multiple comparison, P>0.05). The δ13C and δ15N values of SPOM and BPOM varied in a similar pattern from Station 1 to 3 (). Although Stations 2 and 3 had similar values, the δ13C values of SPOM and BPOM tended to gradually decrease from Stations 1 to 3, whilst the δ15N values of SPOM and BPOM tended to increase from Stations 1 to 3.
Figure 2. Carbon and nitrogen (mean±SD) stable isotope plots of aquatic insects and their potential food sources in the Inukami River. The triangles indicate Station 1, gray circles indicate Station 2, and black squares indicate Station 3. SPOM, suspended particulate organic matter; BPOM, benthic particulate organic matter; 1, Glossosoma spp., 2, Stenelmis spp., 3, Chironominae spp., 4, Rhyacophila nigrocephala; 5, Hexatoma spp.
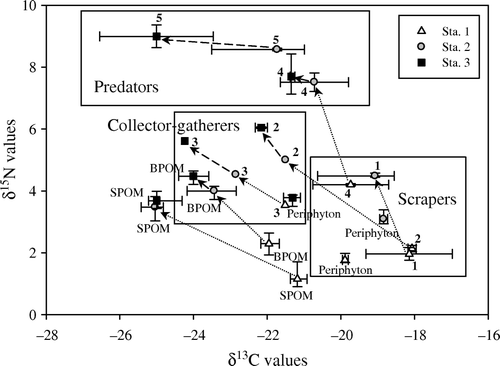
Table 3. δ13C and δ15N (‰) values of aquatic insects and their potential food sources at each station in the Inukami River.
Isotopic signatures of aquatic insects
The mean δ13C and δ15N values of aquatic insects (ranging from −25.0 to −18.1‰ and 2.0 to 9.0‰ respectively) differed among stations and species (). The δ13C values of Glossosoma spp. ranged between −19.0 and −18.1‰, whilst the δ15N values ranged between 2.0 and 4.5‰. The δ13C values at Station 1 and the δ15N values at Station 2 were higher than those at the other stations. The δ13C and δ15N values of Chironominae spp. (ranging from −24.2 to −21.5‰ and 3.5 to 5.6‰, respectively) were slightly different among stations. The δ13C values of Stenelmis spp. ranged from −22.2 to −18.1‰, while the δ15N values ranged from 2.1 to 6.0‰. The δ13C and δ15N values of Chironominae and Stenelmis spp. at Station 1 were higher than those at Station 3. Although the δ13C values of Hexatoma spp. and R. nigrocephala at Station 2 were higher than those at Station 3, the δ15N values of these species appeared to be similar among stations.
Discussion
Physicochemical features of the upper and lower reaches of the dried-up channel
Mulholland and Hill (Citation1997) used calcium and sulfate as a means to separate stream discharge into three catchment flow paths. In the present study, the concentrations of a conservative solute (silicate) and some major ions (chloride, sulfate, and sodium ions) differed among the upper reach (Station 1) and lower reaches (Stations 2 and 3) ( and ). These levels may be influenced by water seepage from underground layers in the lower reaches. Furthermore, the pH values in the lower reaches were lower than those in the upper reach (Table 2). These results were likely to be influenced by the water that seeped from Station 2 to 3.
Allan (Citation1995) reported that primary production was relatively high in small spring streams, which are exposed to sunlight, are algae-rich and contain adequate concentrations of nitrogen and phosphate. The chl. a, nitrogen, and phosphate concentrations in the lower reaches were higher than those in the upper reach (). These results were probably influenced not only by water seepage, but also by contaminated groundwater with possible inflow of ions, such as chloride, sulfate, and sodium ions, which may be associated with anthropogenic factors.
Trophic pathway of aquatic insects and their food sources
Potential food sources for herbivores include periphyton, SPOM, and BPOM. The δ13C and δ15N values of SPOM and BPOM differed across the sampling stations. Moreover, the δ13C values of SPOM and BPOM were not close to those recorded for C3 terrestrial plant litter (−29 to −26‰) (Wada et al. 1987). The δ13C values of SPOM and BPOM were higher than those of C3 terrestrial plant litter, perhaps because SPOM and BPOM contained a greater number of benthic diatoms.
Analysis of trophic relationships using stable isotope ratios is generally based on the premise that δ13C enrichment during trophic transfer is slight (0.8±1.1‰, mean±1 SD), whilst that of δ15N is substantial (3.4±1.1‰) (Deniro and Epstein 1978; Minagawa and Wada Citation1984; Post Citation2002). The δ13C values of Glossosoma spp. were close to those of periphyton in situ, indicating that they generally feed upon periphyton. This is consistent with the findings of Merritt and Cummins (Citation1996), who reported that Glossosoma spp. belongs to the scraper group. The δ13C values of Chironominae and Stenelmis spp. were similar to those of BPOM at each station (, ), indicating that BPOM constitutes the major food source for those species. Merritt and Cummins (Citation1996) reported that Chironominae spp. belong to the collector-gatherer group, whilst Stenelmis spp. belong to either the scraper or the collector-gatherer group. However, our results indicate that Stenelmis spp. may be closer to the collector-gatherer group than the scraper group. Moreover, analysis of their stable isotope ratios revealed that the δ13C values were gradually depleted and that the δ15N values were enriched from Station 1 to 3 (). The δ13C values of the predator group, which included Hexatoma spp. and R. nigrocephala, were lower at Station 3 than at Station 2. The δ13C values of R. nigrocephala were similar to, and particularly appeared to change in parallel with, those of Stenelmis and Glossosoma spp. This indicates that the larvae of R. nigrocephala may feed on Stenelmis and Glossosoma larvae at each station. The δ13C values of Hexatoma spp. were close to those of Chironominae and Stenelmis spp., suggesting that Hexatoma larvae may feed on Chironominae and Stenelmis larvae at each station. Consequently, the predator groups in our study area appeared to have at least one or more food sources, and shifted in the trophic pathways according to their food sources.
Analysis of the δ13C and δ15N values of Glossosoma spp. revealed that the δ15N values tended to get enriched from Station 1 to 2 (). The δ13C values of Chironominae and Stenelmis spp. gradually depleted, whereas the δ15N values were enriched from Station 1 to 3. Moreover, the predator group exhibited depletion in the δ13C values from Station 2 to 3. Thus, our results revealed that δ13C values for the upper reach (Station 1) were high, whereas those for the lower reaches (Stations 2 and 3) were low, based on the absence of a water channel in the Inukami River. Presumably, terrestrial DOC does not flow from the upper reach to the lower reaches due to the dried-up channel. Finlay et al. (Citation2002) suggested that transport of particulate organic carbon from tributary streams to the South Fork Eel River may be reduced because of the absence of summer rainfall. Furthermore, our results suggested that the dried-up channel may also be an important factor in preventing the input of terrestrial DOC. At the same time, the δ15N values tended to increase from the upper to the lower reaches, indicating that the trophic base of organic matter may directly reflect the characteristics of consumers in the upper and lower reaches in terms of habitat, physicochemical conditions (WT, pH, and the concentration of major ions, DOC, chl. a, and periphyton), and geographical conditions (the dried-up channel).
In conclusion, our results revealed that trophic pathways vary considerably according to in situ food sources, and reflect the flow of energy from organic matter to consumers. Furthermore, our results suggested that basal food sources were influenced by riverine characteristics, such as physicochemical and geomorphological conditions. Consequently, these parameters may represent important factors that indirectly influence consumers and the trophic position of basal food sources and consumers in the river system.
Acknowledgements
We thank Prof. I Tayasu and Mr. N Ishikawa from the Center for Ecological Research, Kyoto University, for their assistance with the stable isotope analytical facilities and their incisive suggestions. We also are indebted to Mr. Akatsuka and Mr. Azumi from the Limnological Laboratory, University of Shiga Prefecture, for their generous assistance with the field work. The present study was conducted using the Cooperative Research Facilities (Isotope Ratio Mass Spectrometer) of the Center for Ecological Research, Kyoto University.
References
- Allan , JD. 1995 . Stream ecology , London : Chapman and Hall .
- Bendschneider , K and Robinson , RJ. 1952 . A new spectrophotometric method for the determination of nitrite in sea water . J Mar Res. , 11 : 87 – 96 .
- Boulton , AJ and Lake , PS. 1992 . The ecology two intermittent stream in Victoria, AII, Comparisons of faunal composition between habitats, rivers and years . Feshwater Biol. , 27 : 99 – 121 .
- Bunn , SE , Davies , PM and Winning , M. 2003 . Sources of organic carbon supporting the food web of an arid zone floodplain river . Freshwater Biol. , 48 : 619 – 635 .
- Dekar , MP , Magoulick , DD and Huwel , GR. 2009 . Shifts in the trophic base of intermittent stream food webs . Hydrobiologia. , 635 : 263 – 277 .
- Delong , MD and Thorp , JH. 2006 . Significance of instream autotrophs in trophic dynamics of the upper Mississippi River . Oecologia. , 147 : 76 – 85 .
- Deniro , MJ and Epstein , S. 1978 . Influence of diet on the distribution of carbon isotopes in animals . Geochim Cosmochim Acta. , 42 : 495 – 506 .
- Doi , H , Takai , A , Mizota , C , Okano , J , Nakano , S and Kikuchi , E. 2006 . Contribution of chemoautotrophic productions to the freshwater macroinvertebrates in a headwater stream using multiple stable isotopes . Int Rev Hydrobiol. , 91 : 501 – 508 .
- Doi , H , Takenmon , Y , Ohta , T , Ishida , Y and Kikuchi , E. 2007 . Effects of reach scale canopy cover on trophic pathways of caddisfly larvae in a Japanese mountain stream . Freshwater Res. , 58 : 1 – 7 .
- Doucett , RR , Marks , JC , Blinn , DW , Caron , M and Hungate , BA. 2007 . Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen . Ecology. , 88 : 1587 – 1592 .
- Finlay , JC. 2001 . Stable carbon isotope ratios of river biota: implications for carbon flow in lotic food webs . Ecology. , 82 : 1052 – 1064 .
- Finlay , JC. 2001 . Stable carbon isotope ratios of river biota: implications for carbon flow in lotic food webs . Ecology. , 82 : 1052 – 1064 .
- Finlay , JC , Khandwala , S and Power , ME. 2002 . Spatial scales of carbon flow in a river food web . Ecology. , 83 : 1845 – 1859 .
- Gregory , SV , Swanson , FJ , McKee , WA and Cummins , KW. 1991 . An ecosystem perspective of riparian zones . BioScience. , 41 : 540 – 551 .
- Ishikawa , NF , Uchida , M , Shibata , Y and Tayasu , I. 2010 . A new application of radiocarbon (14C) concentrations to stream food web analysis . Nucl Instrum Meth B. , 268 : 1175 – 1178 .
- Merritt , WR and Cummins , WK. 1996 . An introduction to the aquatic insects of North America , 3rd ed , Dubugue : Kendall/Hunt .
- Minagawa , M and Wada , E. 1984 . Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age . Geochim Cosmochim Acta. , 48 : 1135 – 1140 .
- Mulholland , PJ and Hill , WR. 1997 . Seasonal patterns in streamwater nutrient and dissolved organic carbon concentrations: separating catchment flow path and in-stream effects . Water Resour Res. , 33 : 1297 – 1306 .
- Mullin , JB and Riley , JP. 1955 . The colorimetric determination of silicate with special reference to sea and natural waters . Anal Chim Acta. , 12 : 162 – 176 .
- Mullin , JB and Riley , JP. 1962 . A modified single solution method for the determination of phosphate in natural waters . Anal Chim Acta. , 27 : 31 – 36 .
- Post , DM. 2002 . Using stable isotopes to estimate trophic position: models, methods, and assumptions . Ecology. , 83 : 703 – 718 .
- Reid , DJ , Quinn , GP , Lake , PS and Reich , P. 2008 . Terrestrial detritus supports the food webs in lowland intermittent streams of south-eastern Australia: a stable isotope study . Freshwater Biol. , 53 : 2036 – 2050 .
- Sagi , T. 1966 . Determination of ammonia in sea water by the indophenol method and its application to coastal and off-shore waters . Oceanogr Mag. , 18 : 43 – 51 .
- Schwartz , SS and Jenkins , DJ. 2000 . Temporary aquatic habitats: constraints and opportunities . Aquat Ecol. , 34 : 3 – 8 .
- Simões , NR , Sonoda , SL and Ribeiro , SMMS. 2008 . Spatial and seasonal variation of microcrustaceans (Cladocera and Copepoda) in intermittent rivers in the Jequiezinho River Hydrographic Basin, in the Neotropical semiarid . Acta Limnol Bras. , 20 : 197 – 204 .
- Thorp JH , Delong MD . 1994 . The riverine productivity model: an heuristic view of carbon sources and organic processing in large river ecosystems . Oikos . 70 : 305 – 308 . doi: 10.23007/3545642 .
- Vander Zanden , MJ and Rasmussen , JB. 2001 . Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies . Limnol Oceanogr. , 46 : 2061 – 2066 .
- Vannote , RL , Minshall , GW , Sedell , JR and Cushing , CE. 1980 . The river continuum concept . Can J Fish Aquat Sci. , 37 : 130 – 137 .
- Wada , E , Terazaki , M , Kabaya , Y and Nemoto , T. 1987 . 15N and 13C abundances in the Antartic Ocean with emphasis on the biogeochemical structure of the food web . Deep-sea Res Part A. , 34 : 829 – 841 .
- Winemiller , KO , Flecker , AS and Hoeinghaus , DJ. 2010 . Patch dynamics and environmental heterogeneity in lotic ecosystems . J N Am Benthol Soc. , 29 : 84 – 99 .
- Winkler , LW. 1888 . The determination of dissolved oxygen in water . Berlin Deut Chem Ges. , 21 : 2843 – 2857 .
- Woodward G , Hildrew AG . 2002 . Food web structures in riverine landscapes . Freshwater Biol . 47 : 777 – 798 . doi: 10.1046/J.1365-2427.2002.00908.X .