Abstract
We investigated the in vitro effects of nonylphenol (NP) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) on steroidogenesis in redlip mullet, Chelon haematocheilus, oocytes. In experiment 1, we investigated the effects of NP and PCB126 on steroid production from exogenous steroid precursors. Vitellogenic oocytes (0.75 mm in diameter) were incubated with 10 and 100 ng/ml NP or PCB126 with [3H]17α-hydroxyprogesterone as a precursor. The major metabolites produced were androstenedione, testosterone (T), estrone, and estradiol-17β (E2). Both NP and PCB126 increased T production and decreased E2 production, except for 100 ng/ml PCB126. In experiment 2, oocytes (0.65–0.75 mm in diameter) were exposed to NP and PCB126 at different concentrations (0.01, 0.1, 1, 10, and 100 ng/mL). After the incubation, T and E2 production was measured by radioimmunoassay. NP inhibited E2 production at concentrations of 0.01 and 0.1 ng/ml in 0.75-mm-diameter oocytes. NP at 1 and 100 ng/mL stimulated T production, but had no observable effect on E2 production. PCB126 treatment did not affect E2 production at any of the concentrations tested. NP alone at 0.1 ng/mL resulted in a significant decrease in E2 production in 0.65-mm-diameter oocytes. PCB126 did not show any significant effects on either T or E2 production at all concentrations tested. These results suggest that NP acts like an antiestrogen at lower concentrations (0.01–0.1 ng/ml) in vitellogenic oocytes of redlip mullet.
Introduction
Environmental chemicals are suspected of causing various reproductive effects reported in aquatic wildlife populations. The most commonly observed reproductive effects are reduced gonad size or feminization of genetically male fish, skewed sex ratios, impaired gametogenesis, altered adult sexual maturity, delayed ovulation and spawning, and modified hormone levels in fish (Arcand-Hoy and Benson Citation1998; Jobling et al. Citation2003).
Many studies have focused on the reproductive disturbances in fish caused by chemicals with estrogenic or anti-estrogenic activity (Jobling et al. Citation1995; Kime, Citation1998; Segner et al. Citation2003). Estrogens play an important role in controlling fish reproductive processes. The induction of vitellogenesis, which is controlled by estrogens, is a widely used endpoint for detecting the effects of estrogenic activity (Navas and Segner Citation2006; Scholz and Mayer Citation2008). Furthermore, estrogens inhibit gonadotropin-induced oocyte maturation and ovulation of intact follicles in vitro (Kime 1998). Some studies have shown that estradiol-17β (E2) is generally not effective for inducing fish oocyte maturation (Young et al. Citation1982; Trant and Thomas Citation1988).
4-Nonylphenol (NP) is a degradation product of nonylphenol ethoxylates, which are major non-ionic surfactants used in plastics, pesticides, and industrial detergents (Maguire Citation1999; Servos Citation1999; Ying et al. Citation2002). Several studies have reported that NP has weak estrogenic potency in fish. NP elevates plasma concentrations of E2, vitellogenin, and the zona radiata protein in both male and female fish and causes gonadal abnormalities, such as the induction of ovotestes in males (Jobling et al. Citation1996; Gray and Metcalfe Citation1997; Arukwe et al. Citation1998; Ashfield et al. Citation1998; Kinnberg et al. Citation2000). An opposite effect was found in a study using Atlantic salmon (Salmo salar), in which exposure to NP caused a 24–43% decrease in plasma E2 levels (Arukwe et al. Citation1997). Recently, Cionna et al. (Citation2006) reported that in grey mullet (Liza aurata), NP exerts estrogenic effects only at the highest dose injected. However, the mechanism by which NP exerts estrogenic and other endocrine disrupting effects remains unclear.
Polychlorinated biphenyls (PCBs) are synthetic chemicals that may disrupt follicular steroidogenesis either by mimicking natural hormones as agonists or antagonists or by altering the pattern of hormone synthesis (Gregoraszczuk et al. Citation2003a, Citation2003b). Among PCB congeners, the coplanar non-ortho 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) is the most toxic PCB congener (Safe Citation1994), with dioxin-like properties. PCB126 has known antiestrogen effects in rat uterus, bone, and ovary (Astroff and Safe Citation1990; Lind et al. Citation1999; Muto et al. Citation2002), as well as in porcine ovarian tissue (Wojtowicz et al. 2000; Gregoraszczuk et al. 2003b). In experiments with fish, PCB126 has been shown to possess potent anti-estrogenic activity in the sea bass in vivo, as it inhibits E2-induced vitellogenesis (Vaccaro et al. 2005). Recently, however, Mortensen and Arukwe (2008) demonstrated that PCB126 produced significantly higher vitellogenin and zona radiata protein levels in a salmon in vitro system. Additionally, Liu et al. (Citation2006) reported similar effects in mammalian systems. Thus, PCB126 can act as an estrogen or antiestrogen depending on the exposure time and concentration and the stage of follicular development.
We conducted a direct, in vitro study to assess whether NP and PCB126 have estrogen agonistic or antagonistic effects on redlip mullet (Chelon haematocheilus) steroid production in vitellogenic oocytes with average diameter 0.65 and 0.75 mm, collected during spawning season. Mullet species have been used for many years in coastal pollution monitoring programs (UNEP Citation1997) due to their widespread distribution where toxic compounds reach the sea and bioaccumulate in sediment (Cionna et al. 2006; Hong et al. Citation2009).
Materials and methods
1. Chemicals
Both NP and PCB126 (Aldrich Chemical, Milwaukee, WI, USA) were prepared as stock solutions (mg/mL) by dilution in ethanol. The ethanol concentration in the incubation medium was maintained at less than 0.1%. Testosterone (T) and E2 were purchased from Sigma Chemical (St. Louis, MO, USA) or Steraloids, Inc. (Wilton, NH, USA). Antiserum for T was purchased from Sigma Chemical, and that for E2 was donated by Dr. Alexis Fostier (INRA, Rennes, France). Radioactive [3H]17α-hydroxyprogesterone ([3H]17α-OHP), [3H]T, and [3H]E2 were obtained from Amersham Life Science (London, England).
2. Experimental fish and ovarian development histology
The redlip mullet used in this study were captured in coastal waters off Ganghwado, South Korea, during the spawning season (May–June). Oocytes were separated into groups using fine forceps. Oocytes with average diameters of 0.75 and 0.65 mm were used for the in vitro studies. Several ovarian pieces were fixed in Bouin's solution for 24 hours for histological observations of oocytes. The fixed samples were washed, dehydrated, and embedded in paraffin. Serial sections of 4–6-µm thickness were prepared; the slides were stained in Mayer's hematoxylin and 0.5% eosin and mounted with malinol. Histological samples were observed through a light microscope (BX50, Olympus, Tokyo, Japan).
3. In vitro steroidogenesis
We performed two separate experiments. In experiment 1, we incubated ovarian fragments with a radiolabeled steroid precursor. Ovaries were separated into small pieces in ice-cold balanced salt solution (132.96 mM NaCl, 3.09 mM KCl, 0.28 mM MgSO4·7H2O, 0.98 mM MgCl2·6H2O, 3.40 mM CaCl2·6H2O, and 3.65 mM HEPES), and approximately 20 0.75-mm follicle-enclosed oocytes were incubated in each well of 24-well culture plates containing 1 ml of Leibovitz L15 medium (Gibco, Grand Island, NY, USA). Incubations were initiated by adding 55 kBq of [3H]17αP as the radiolabeled precursor with 10 or 100 ng/mL of E2, NP, or PCB126. The pH and osmolarity of the media were adjusted to 7.2 and 460 mOsm, respectively. The plates were incubated for 30 h at 18°C with constant gentle shaking.
In experiment 2, 0.75-mm and 0.65-mm oocytes were incubated with 0.01–100 ng/mL NP, PCB126, or E2. The pH and osmolarity of the media were adjusted as described above. The plates were incubated for 38 h at 18°C with constant gentle shaking.
4. Steroid extraction and analysis of steroid metabolism
At the end of the incubation, steroids were extracted three times from the media and oocytes using 4 mL dichloromethane. The extracts were concentrated and applied to a thin-layer chromatography (TLC) plate (60F254; Merck, Darmstadt, Germany) with non-radioactive standard steroids as carrier steroids, and developed in a mixture of benzene:acetone (4:1) and benzene:ethyl acetate (4:1). Radioactive steroid metabolites were analyzed using a BAS 1500 bio-imaging analyzer (Fuji Film, Tokyo, Japan), and estrone (E1) and E2 standards were visualized by exposure to iodine vapor. Other standard steroids were detected by UV absorption at 254 nm. The migration zones corresponding to the four carrier steroids, androstenedione (A4), T, E2, and E1, were eluted twice from the silica plate bands with 5 ml of dichloromethane:methanol (90:10). Following centrifugation at 1000×g for 10 min, the supernatants were vacuum dried before finally being dissolved in 20 µl of acetonitrile. Extracts were then analyzed by reverse-phase high-performance liquid chromatography (HPLC). Briefly, a Waters Alliance HPLC system (Waters, Milford, MA, USA) equipped with a binary pump (515 HPLC pump, Waters) and UV detector (2487 multi-wavelength absorbance detector, Waters) was used. Chromatographic separation was performed on a Sunfire C18 analytical column (4.6×150 mm I.D., 3.5 µm particle size; Waters Sunfire). The flow rate and mobile phase were 0.6 mL/min with 20% methanol and 0.4 mL/min with absolute acetonitrile. The injection volume for the samples was 20 µl, and the ending time for each sample was 15 min. After identifying the metabolites, the radioactivity of each fraction was counted on a liquid scintillation counter (Packard, Peoria, IL, USA).
5. Radioimmunoassay
After the incubations, steroids were extracted twice from aliquots of medium using five volumes of ethyl acetate:cyclohexane (1:1). Then, the T and E2 levels were measured by RIA following Kobayashi et al. (Citation1987). The intra-assay coefficients of variance were 2.3% (n=3) and 3.4% (n=3) for the T and E2 assays, respectively, and the respective inter-assay coefficients of variance were 12.5% (n=5) and 11.5% (n=5). The minimum detectable limits were 10 and 12.5 pg/mL for T and E2, respectively.
6. Statistics
All data are expressed as means with the standard error of the mean (SEM). SPSS 11.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for the Kruskal–Wallis test followed by the Bonferroni adjustment. A P-value <0.05 was considered statistically significant.
Results
1. Oocyte histology
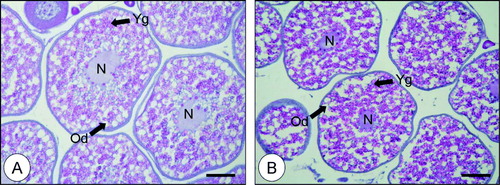
In both the 0.75- and 0.65-mm-average-diameter oocytes, yolk granules were spread throughout the ooplasm, oil droplets were distributed over the ooplasm (), and the nuclei remained in the middle of the ooplasm.
2. Effects of NP and PCB126 on [3H]17αOHP metabolism
When vitellogenic oocytes (0.75 mm in diameter) were incubated with [3H]17α-OHP, the four major metabolites identified were A4, T, E2, and E1 (). Steroid metabolites produced from [3H]17αOHP in the presence of NP and PCB126 were compared with the control ().
Figure 2. Autoradiograms of steroid metabolites produced in 0.75 mm redlip mullet oocytes incubated with [3H]17α-hydroxyprogesterone. Steroids were extracted from oocytes and incubation media with dichloromethane. Four metabolites from the extracts were separated by thin-layer chromatography and developed with benzene:acetone (4:1) or benzene:ethyl acetate (4:1).
![Figure 2. Autoradiograms of steroid metabolites produced in 0.75 mm redlip mullet oocytes incubated with [3H]17α-hydroxyprogesterone. Steroids were extracted from oocytes and incubation media with dichloromethane. Four metabolites from the extracts were separated by thin-layer chromatography and developed with benzene:acetone (4:1) or benzene:ethyl acetate (4:1).](/cms/asset/3f9532b4-2fe5-4573-8f2d-13174a57f4b3/tacs_a_604938_o_f0002g.gif)
Figure 3. Effects of NP and PCB126 on steroid metabolites from [3H]17α-hydroxyprogesterone in 0.75-mm redlip mullet oocytes. The percentage of radioactivity associated with each isolated steroid was calculated based on the percentage of total steroid recovered from the initial thin-layer chromatography. Values are mean±SE (in duplicate wells, 20 oocytes/well). A4, androstenedione; T, testosterone: E2, esradiol-17β; E1, estrone.
![Figure 3. Effects of NP and PCB126 on steroid metabolites from [3H]17α-hydroxyprogesterone in 0.75-mm redlip mullet oocytes. The percentage of radioactivity associated with each isolated steroid was calculated based on the percentage of total steroid recovered from the initial thin-layer chromatography. Values are mean±SE (in duplicate wells, 20 oocytes/well). A4, androstenedione; T, testosterone: E2, esradiol-17β; E1, estrone.](/cms/asset/c50adef1-0eb6-41df-8cb6-fbae44e61972/tacs_a_604938_o_f0003g.gif)
In the NP-treatment group, 10 and 100 ng/ml NP increased T production (73.84±0.34 and 85.55±0.85%, respectively) compared with controls (68.24±1.37%), whereas E2 production decreased at both NP concentrations (0.79±0.09 and 0.44±0.02%, respectively) compared with controls (0.91±0.11%).
In the PCB126-treatment group, 10 ng/ml PCB126 increased T production (82.18±0.04%), but decreased E2 production (0.57±0.11%), whereas the opposite occurred in the 100 ng/ml PCB126 treatment.
3. Effects of NP and PCB126 on endogenous steroid production
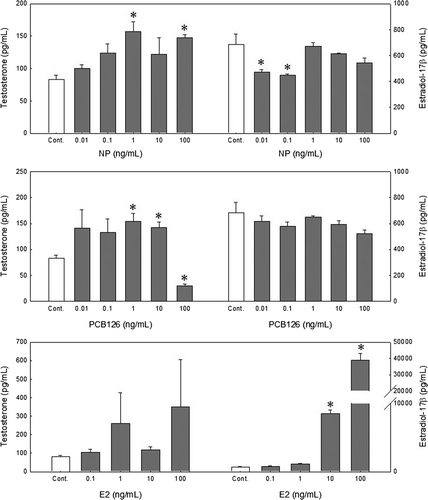
We examined the effects of NP and PCB126 (0.01–100 ng/ml) on endogenous T and E2 production in 0.65- and 0.75-mm-diameter oocytes. E2 treatment served as a positive control.
NP inhibited endogenous E2 production in the 0.75-mm oocytes at concentrations of 0.01 and 0.1 ng/ml (474.92±15.04 and 450.88±9.00 pg/mL, respectively) compared with controls (684.83±80.07 pg/mL) (). NP at 1 and 100 ng/mL stimulated endogenous T production (122.44±30.40 and 147.26±5.81 pg/mL, respectively) compared with controls (82.79±6.67, P<0.05). Concentrations of 1 and 10 ng/mL PCB126 stimulated T production (153.73±16.54 and 142.01±10.75 pg/mL, respectively) compared with controls (82.79±6.67 pg/mL), but PCB126 at 100 ng/mL inhibited T production (30.26±2.79 pg/mL).
Figure 4. Effects of nonylphenol (NP) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) on in vitro steroidogenesis in 0.75-mm redlip mullet oocytes after a 38 h incubation. Values are the mean±SE of the ratio of each steroid in three replicate wells with 20 oocytes/well. Data were analyzed using the Kruskal–Wallis test followed by the Bonferroni adjustment. Asterisks show significant differences from controls (P<0.05).
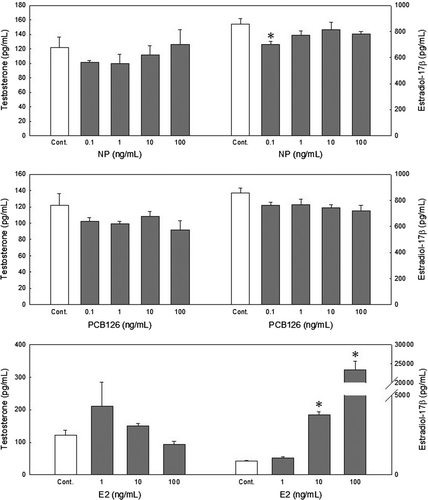
NP alone at 0.1 ng/mL resulted in a significant decrease in E2 production (700.02±21.66 pg/mL) in the 0.65-mm oocytes compared with controls (858.42±38.36 pg/mL) (). PCB126 showed no significant effects on either T or E2 production at all concentrations tested.
Figure 5. Effects of nonylphenol (NP) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) on in vitro steroidogenesis in 0.65-mm redlip mullet oocytes after a 38 h incubation. Values are the mean±SE of the ratio of each steroid in three replicate wells with 20 oocytes/well. Data were analyzed using the Kruskal–Wallis test followed by the Bonferroni adjustment. Asterisks show significant differences from controls (P<0.05).
Discussion
Many chemicals in surface waters and sediments have estrogenic or antiestrogenic activities. Among these chemicals, NP has been suggested as an environmental estrogen or xenoestrogen that can mimic the effects of E2 in various aquatic animals (Billsson et al. Citation1998; Arukwe et al. Citation2000; Sheahan et al. Citation2002). Experiments with fish have shown that NP increases endogenous E2, vitellogenin, and the zona radiata protein in both males and females (Jobling et al. 1996; Gray and Metcalfe 1997; Arukwe et al. 1998; Ashfield et al. 1998; Kinnberg et al. 2000). In female fish, E2 is the main steroid produced by vitellogenic oocytes and is associated with vitellogenesis and ovarian development (Arukwe and Goksoyr Citation2003).
In our previous studies, NP stimulated in vitro estrogen synthesis using fully vitellogenic oocytes of the longchin goby, Chasmichthys dolichognathus (Baek et al. Citation2003). Also, NP had shown estrogenic potency in vitro on vitellogenic and fully matured oocytes by increasing E2/T, E2/17α,20β-dihydroxy-4-pregnen-3-one in the greenling, Hexagrammos otakii (Hwang et al. Citation2008). However, an opposite effect was found in a study using Atlantic salmon in which exposure to NP caused a 24–43% decrease in plasma E2 levels (Arukwe et al. 1997). Using grey mullet (Liza aurata), Cionna et al. (2006) reported that the estrogenic effect of NP was elicited only by the highest dose (250 mg/kg body weight); a lower dose of NP (0.25 mg/kg body weight) did not induce vitellogenesis or increase plasma vitellogenin levels. In the present study, we found that NP inhibited endogenous E2 production by vitellogenic oocytes (0.65–0.75-mm diameter) at lower concentrations (0.01 and 0.1 ng/ml). Additionally, Baek et al. (Citation2009) demonstrated that NP decreased the E2/T ratio in fully vitellogenic oocytes of the yellowfin goby, Acanthogobius flavimanus. These different effects of NP on steroid production might be dependent on the different fish species used, exposure concentrations, or ovarian developmental stage.
PCB126 is one of the most toxic dioxin-like contaminants (Safe 1994) and has known antiestrogen activity in mammalian cell-based systems (Astroff and Safe 1990; Lind et al. 1999; Wojtowicz et al. Citation2000; Gregoraszczuk et al. 2003a, Citation2008), but relatively few studies have been performed in fish. Using an in vitro system, Gregoraszczuk et al. (2003a) reported that PCB126 inhibited E2 secretion by porcine ovarian follicular cells. PCB126 has been shown to have potent anti-estrogenic activity in the sea bass in vivo, as it inhibits E2-induced vitellogenesis (Vaccaro et al. Citation2005). However, opposite results were shown by Mortensen and Arukwe (Citation2008), who found that PCB126 produced increases in estrogen receptor-α, vitellogenin, and Zr-protein mRNA levels in a primary salmon hepatocyte cultures. In our study, PCB126 had no significant effect on either T or E2 production in 0.65-mm-diameter oocytes, whereas PCB126 increased T production at lower concentrations (1 and 10 ng/ml) but decreased T production at higher concentration (100 ng/ml) in 0.75-mm-diameter oocytes. No marked differences in E2 production were observed. The sensitivity of oocytes to chemicals varies depending on oocyte size. Oocytes of 0.75 mm diameter appeared to be more sensitive than those that were 0.65 mm in diameter, even though they were in the same ovarian developmental stage (vitellogenic phase).
Based on these results, we suspect that NP acts as an antiestrogen at lower concentrations (0.01–0.1 ng/ml) in the vitellogenic oocytes of redlip mullet.
Acknowledgements
This work was supported by Pukyong National University Research Abroad Fund in 2006 (PS-2006-028).
References
- Arcand-Hoy , LD and Benson , WH. 1998 . Fish reproduction: an ecologically relevant indicator of endocrine disruption . Environ Toxicol Chem , 17 : 49 – 57 .
- Arukwe , A and Goksøyr , A. 2003 . Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation-oogenetic, population, and evolutionary implications of endocrine disruption . Comp Hepatol , 2 : 4
- Arukwe , A , Knudsen , FR and Goksoyr , A. 1997 . Fish zona radiata (eggshell) protein: a sensibile biomarker for enviromental estrogens . Environ Health Perspect , 105 : 418 – 422 .
- Arukwe , A , Celius , T , Walther , BT and Goksoyr , A. 1998 . Plasma levels of vitellogenin and eggshell zona radiate proteins in 4-nonylphenol and o, p’-DDT treated juvenile atlantic salmon (Salmo salar) . Mar Environ Res , 46 : 133 – 136 .
- Arukwe , A , Celius , T , Walther , BT and Goksoyr , A. 2000 . Effects of xenoestrogen treatment on zona radiata protein and vitellogenin expression in Atlantic salmon (Salmo salar) . Aquat Toxicol , 49 : 159 – 170 .
- Astroff , B and Safe , S. 1990 . 2,3,7,8-tetrachlorodibenzo-p-dioxin as an antiestrogen: effect on rat uterine peroxidase activity . Biochem Pharmacol , 39 : 485 – 488 .
- Ashfield , LA , Pottinger , TG and Sumpter , JP. 1998 . Exposure of female juvenile rainbow trout to alkylphenolic compounds results in modifications to growth and ovosomatic index . Environ Toxicol Chem , 3 : 679 – 686 .
- Baek , HJ , Park , MH , Lee , YD and Kim , HB. 2003 . Effect of in vitro xenoestrogens on steroidogenesis in mature female fish, Chasmichthys dolichognathus . Fish Physiol Biochem , 28 : 413 – 414 .
- Baek , HJ , Hwang , IJ , Park , MH and Kim , HB. 2009 . Effects of nonylphenol and 2,2′,4,6,6′-pentachlorobiphenyl on in vitro sex steroid production in maturing oocytes of the yellowfin goby, Acanthogobius flavimanus . Fish Aqua Sci , 12 : 293 – 298 .
- Billsson , K , Westerlund , L , Tysklind , M and Olsson , P. 1998 . Developmental disturbances caused by polychlorina-ted biphenyls in zebrafish (Brachydanio rerio) . Mar Environ Res , 46 : 461 – 464 .
- Cionna , C , Maradonna , F , Olivotto , I , Pizzonia , G and Carnevali , O. 2006 . Effects of nonylphenol on juveniles and adults in the grey mullet, Liza aurata . Reprod Toxicol , 22 : 449 – 454 .
- Gray , MA and Metcalfe , CD. 1997 . Induction of testis-ova in Japanese medaka (Oryzias latipes) exposed to p-nonylphenol . Environ Toxicol Chem , 16 : 1082 – 1086 .
- Gregoraszczuk , EL , Grochowalski , A , Chrzaszcz , R and Wegiel , M . 2003a . Congener-specific accumulation of polychlorinated biphenyls in ovarian follicular wall follows repeated exposure to PCB126 and PCB153. Comparison of tissue levels of PCB and biological changes . Chemosphere , 50 : 481 – 488 .
- Gregoraszczuk , EL , Sowa , M , Kajta , M , Ptak , A and Wojtowicz , A . 2003b . Effect of PCB 126 and PCB 153 on incidence of apoptosis in cultured theca and granulosa cells collected from small, medium and large preovulatory follicles . Reprod Toxicol , 17 : 465 – 471 .
- Gregoraszczuk , EL , Rak , A , Ludewig , G and Gasin′ska , A. 2008 . Effects of estradiol, PCB3 and their hydroxylated metabolites on proliferation, cell cycle, and apoptosis of human breast cancer cells . Environ Toxicol Pharmacol , 25 : 227 – 233 .
- Hong , L , Fujita , T , Wada , T , Amano , H , Hiramatsu , N , Zhang , X , Todo , T and Hara , A. 2009 . Choriogenin and vitellogenin in red lip mullet (Chelon haematocheilus): purification, haracterization, and evaluation as potential biomarkers for detecting estrogenic activity . Comp Biochem Physiol , 149C : 9 – 17 .
- Hwang , IJ , Lee , YD , Kim , HB and Baek , HJ. 2008 . Estrogenic activity of nonylphenol in marine fish, Hexagrammos otakii, during oocyte development by evaluating sex steroid levels . Cybium , 32 ( 2 ) : 251 – 252 .
- Jobling , S , Reynolds , T , White , R , Parker , MG and Sumpter , JP. 1995 . A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic . Environ Health Perspect , 103 : 582 – 587 .
- Jobling , S , Sheahan , D , Osborne , JA , Matthiessen , P and Sumpter , JP. 1996 . Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic chemicals . Environ Toxicol Chem , 15 : 194 – 202 .
- Jobling , S , Casey , D , Rodgers-Gray , T , Oehlmann , J , Pawlowski , S , Baunbcck , T , Turner , AP and Tyler , CR. 2003 . Comparative responses of molluscs and fish to environmental oestrogens and an oestrogenic effluent . Aquat Toxicol , 65 : 205 – 220 .
- Kime , DE. 1998 . Endocrine Disruption in Fish , 240 – 245 . Boston , MA : Kluwer Academic .
- Kinnberg , K , Korsgaard , B , Bjerregaard , P and Jespersen , A. 2000 . Effects of nonylphenol and 17ß-estradiol on vitellogenin synthesis and testis morphology in male platyfish, Xiphophorus maculates . J Exp Biol , 203 : 171 – 181 .
- Kobayashi , M , Aida , K , Sakai , H , Kaneko , T , Asahina , K , Hanyu , I and Ishii , S. 1987 . Radioimmunoassay for salmon gonadotropin . Nippon Suisan Gakk , 53 : 995 – 1003 .
- Lind , PM , Eriksen , EF , Sahlin , L , Edlund , M and Orberg , J. 1999 . Effects of the antiestrogenic environmental pollutant 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) in rat bone and uterus: diverging effects in ovariectomized and intact animals . Toxicol Appl Pharmacol , 154 : 236 – 244 .
- Liu , S , Abdelrahim , M , Khan , S , Ariazi , E , Jordan , VC and Safe , S. 2006 . Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells . Biol Chem , 387 : 1209 – 1213 .
- Maguire , RJ. 1999 . Review of the persistence of nonylphenol and nonylphenol ethoxylates in aquatic environments . Water Qual Res J Can , 34 : 37 – 78 .
- Mortensen , AS and Arukwe , A. 2008 . Activation of estrogen receptor signaling by the dioxin-like aryl hydrocarbon receptor agonist, 3,3′,4,4′,5-Pentachlorobiphenyl (PCB126) in salmon in vitro system . Toxicol Appl Pharmacol , 227 : 313 – 324 .
- Muto , T , Wakui , S , Imano , N , Nakaaki , K , Takahashi , H , Hano , H , Furusato , M and Masaoka , T. 2002 . Mammary gland differentiation in female rats after prenatal exposure to 3,3′,4,4′,5-pentachlorobiphenyl . Toxicology , 177 : 197 – 205 .
- Navas , JM and Segner , H. 2006 . Vitellogenin synthesis in primary cultures of fish liver cells as endpoint for in vitro screening of the (anti)estrogenic activity of chemical substances . Aquat Toxicol , 80 : 1 – 22 .
- Safe , S. 1994 . Polychlorinated biphenyls PCBs: Environmental impact, biochemical and toxic responses, and implications for risk assessment . CRC Crit Rev Toxicol , 24 : 87
- Scholz , S and Mayer , I. 2008 . Molecular biomarkers of endocrine disruption in small model fish . Mol Cell Endocrinol , 293 : 57 – 70 .
- Segner , H , Caroll , K , Fenske , M , Janssen , CR , Maack , G , Pascoe , D , Schafers , C , Vandenbergh , GF , Watts , M and Wenzel , A. 2003 . Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project . Ecotoxicol Environ Safe , 54 : 302 – 314 .
- Servos , MR. 1999 . Review of the aquatic toxicity, estrogenic responses and bioaccumulation of alkylphenols and alkylphenol polyethoxylates . Water Qual Res J Can , 34 : 123 – 177 .
- Sheahan , DA , Brighty , GC , Daniel , M , Jobling , S , Harries , JE , Hurst , MR , Kennedy , J , Kirby , SJ , Morris , S , Routledge , EJ , Sumpter , JP and Waldock , MJ. 2002 . Reduction in the estrogenic activity of a treated sewage effluent discharge to an English river as a result of a decrease in the concentration of industrially-derived surfactants . Environ Toxicol Chem , 21 : 515 – 519 .
- Trant , JM and Thomas , P. 1988 . Structure-activity relationships of steroids in inducing germinal vesicle breakdown of Atlantic croaker oocyte in vitro . Gen Comp Endocrinol , 71 : 307 – 317 .
- UNEP (United Nations Environmental Programme) 1997 Report of the meeting of experts to review the MEDPOL biomonitoring programme . Document UNEP-(OCA)/MED WG. 132/7. UNEP, Athens , Athens , , Greece .
- Vaccaro , E , Meucci , V , Intorre , L , Soldani , G , Di Bello , D and Longo , V. 2005 . Effects of 17beta-estradiol, 4-nonylphenol and PCB126 on the estrogenic activity and phase 1 and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax) . Aquat Toxicol , 75 : 293 – 305 .
- Wojtowicz , AK , Gregoraszczuk , EL , Lyche , JL and Ropstad , E. 2000 . Time dependent and cell-specific action of polychlorinated biphenyls (PCB153 and PCB126) on steroid secretion by porcine theca and granulosa cells in mono- and co-culture . J Physiol Pharmacol , 51 : 555 – 568 .
- Ying , GG , Williams , B and Kookana , R. 2002 . Environmental fate of alkylphenols and alkylphenol ethoxylates-a review . Environ Int , 28 : 215 – 226 .
- Young , G , Kagawa , H and Nagahama , Y. 1982 . Oocyte maturation in the amago salmon (Oncorhynchus rhodurus): in vitro effects of salmon gonadotropin, steroids, and cyanoketon (an inhibitor of 3b-hydroxy steroid dehydrogenase) . J Exp Zool , 224 : 265 – 275 .