Abstract
Thymoquinone (TQ), a drug extracted from the black seeds of Nigella sativa, has been shown to exhibit anti-inflammatory, anti-oxidant, and anti-neoplastic effects in numerous cancer cells. The effects of TQ on cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production in MDA-MB-231, however, remain poorly understood. Western blot analysis and immunofluorescence staining were performed to study the expression levels of inflammation regulatory proteins in MDA-MB-231. PGE2 assay was conducted to explore the TQ-induced production of PGE2. In this study, we investigated the effects of TQ on COX-2 expression and PGE2 production in MDA-MB-231. TQ significantly induced COX-2 expression and increased PGE2 production in a dose-dependent manner, as determined by a Western blot analysis and PGE2 assay. Furthermore, the activation of Akt and p38 kinase, respectively, was up-regulated in TQ treated cells. Inhibition of p38 kinase with SB203580 and PI3kinase (PI3K) with LY294002 abolished TQ-caused COX-2 expression and decreased PGE2 production. These results collectively demonstrate that TQ effectively modulates COX-2 expression and PGE2 production via PI3K and p38 kinase pathways in the human breast cancer cell line MDA-MB-231.
Introduction
Breast cancer is one of the most common cancers observed in women, and its incidence rate continues to rise rapidly throughout the world in young women (Jemal et al. Citation2010). Because Asian women generally ingest more natural plant products, such as fruits and vegetables, than Western women, the incidence of breast cancer in Asian women is lower than that in Western women (Ziegler et al. Citation1993). These results suggest the possibility of using plant products as alternative medicines for breast cancer.
Cyclooxygenase (COX) enzymes catalyze the conversion of arachidonic acid to prostaglandins (PGs) (Smith et al. Citation2000). COXs mediate numerous physiological and pathophysiological effects, including homeostasis, pain, fever, and inflammation (O'Banion Citation1999). Two forms of COX have been identified: COX-1 and COX-2 (Smith et al. Citation1996). COX-1 is a stable protein that is constitutively expressed in resting cells of many tissues. In contrast, COX-2 is a stimulus-inducible protein whose expression is short-lived in epithelial, endothelial, smooth muscle, and fibroblast cells (Smith et al. Citation1996). Numerous studies have also confirmed that COX-2 plays an important role in tumorigenesis in significant premalignant and malignant tumors (Stasinopoulos et al. Citation2008). Its over-expression in tumors was found to stimulate angiogenesis by PGs and increases the resistance to apoptosis, and it can also cause local immune suppression (Costa et al. Citation2002). As a result, some tumors expressing COX-2 are reported to inhibit clinical behavior. In breast cells, in particular, COX-2 expression was only shown in tumor breast cells and was not detected in normal breast cells (Costa et al. Citation2002). The over-expression of COX-2 has been found to adversely affect the prognosis in breast cancer (Park et al. Citation2006).
Previous studies have shown that the seeds of the medial plant Nigella sativa and its oil extract, thymoquinone (TQ), possess anti-tumor, anti-oxidant, and anti-inflammatory activities in conjunction with a number of diseases (Ali and Blunden Citation2003; El Mezayen et al. Citation2006). However, there are no data concerning the underlying mechanisms of TQ on COX-2 expression and prostaglandin E2 (PGE2) production in MDA-MB-231 cells.
The expression of COX-2 was reported to be modulated through the signaling pathway of mitogen-activated protein kinases (MAPKs), such as p38, ERK and JNK, and PI3kinase(PI3K) in a wide variety of cancer cells (Yoon et al. Citation2011).
However, the effects and mechanisms of TQ on COX-2 expression and PGE2 production are not clearly understood in MDA-MB-231 cells. So, we analyzed the effects of TQ on COX-2 expression and PGE2 production with a focus on the PI3kinase and p38 kinase pathways in human breast cancer cell lines, MDA-MB-231 cells.
Materials and methods
Cell cultures
MDA-MB-231 cell line was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). MDA-MB-231 cells were maintained in RPMI-1640 medium (Invitrogen, Burlington, ON, Canada) containing 10% fetal bovine serum (FBS), 50 µg/ml streptomycin, and 50 units/ml penicillin. Fetal calf serum was heat inactivated for 30 min in a 56 °C water bath before use. Cell cultures were grown at 37 °C, in a humidified atmosphere of 5% CO2 in a SANYO CO2 incubator.
Western blot analysis
Proteins were isolated in cold radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% NP-40, and 0.1% sodium dodecyl sulfate (SDS), supplemented with protease inhibitors and phosphatase inhibitors] and equal amounts of total cellular proteins were resolved by SDS-PAGE and transferred to nitrocellulose (NC) membranes (Whatman Schleicher and Schuell, Dachen, Germany). The NC sheet was blocked with 5% nonfat dry milk in Tris-buffered saline. Antibodies to COX-2 (Cayman Chemical, Ann Arbor, MI, USA), pp38 (Cell Signaling Technology, Denvers, MA, USA), pAkt (Cell Signaling Technology), and β-actin (Santa Cruz Biotechnology, CA, USA) were used for probing corresponding NC blots overnight at 4 °C. Membranes were then washed three times with Tris-buffered saline/Tween-20 and incubated with horseradish peroxidase-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) for 2 h followed by exposure in an LAS-3000 imager (Fuji Film Co., Tokyo, Japan) according to the manufacturer's instructions.
PGE2 assay
MDA-MB-231 cells were seeded in standard 96-well microtiter plates at 2×104 cells/well. Day 2 cultures were treated with various reagents, such as TQ (thymoquinone; Sigma-Aldrich), for 1 h prior to treatment with LY (LY294002; Calbiochem, San Diego, CA, USA) or SB (SB203580; Calbiochem). After incubating the cells for 24 h, the culture medium was collected, and the amount of PGE2 released by the cells was determined using enzyme immunoassay kits for PGE2 (Assay Design Inc., Ann Arbor, MI, USA) according to the manufacturer's instructions.
Immunofluorescence staining
MDA-MB-231 cells were fixed with 3.5% paraformaldehyde in PBS for 15 min at room temperature. The cells were permeabilized in PBS containing 0.1% Triton X-100 for 15 min. The fixed MDA-MB-231 cells were washed with PBS and incubated for 2 h with the antibody against COX-2 (Cayman Chemical, Ann Arbor, MI, USA). Next, the cells were washed and incubated with secondary antibodies for 1 h. The cells were washed three times with PBS and observed under a fluorescence microscope.
Statistics
The values given are means±SEM. The significance of difference between the experimental groups and controls was assessed by a one-way analysis of variance (ANOVA) test. The difference is significant if the p value is <0.05.
Results
TQ induces COX-2 expression and increases PGE2 production in MDA-MB-231 cells
The cytotoxicity of TQ on MDA-MB-231 cells was initially determined by the MTT assay (data not shown). The results showed that the TQ at various concentrations ranging from 10 to 50 µM exhibited no cytotoxicity on MDA-MB-231 cells, which were used for subsequent experiments (data not shown). The first aim of this study was to determine whether TQ regulates the expression of COX-2 and the production of PGE2 in MDA-MB-231 cells. Cells were treated with various concentrations of TQ for 24 h (A, C) or with TQ at 30 µM for the indicated time period (B, D). As a result, stimulation of cells with TQ showed a striking increase in COX-2 expression and PGE2 production, which was apparent within 3 h after treatment of TQ. COX-2 expression and PGE2 production, respectively, showed a maximum at 3 h, and the increases remained detectable for up to 24 h (B, D). Concentration-dependent increases in COX-2 expression and PGE2 production were measured by Western blot analysis and PGE2 assay (A, C).
Figure 1. Thymoquinone (TQ) induces COX-2 expression and increases PGE2 production in MDA-MB-231 cells. (A–D) MDA-MB-231 cells were untreated or treated with specific concentrations of TQ for 24 h or 30 µM TQ for the indicated time periods. (A, B) Expressions of COX-2, pp38, p38, pAkt, and Akt were detected by a Western blot analysis. Expressions of β-actin were used as loading controls. (C, D) Secreted PGE2 was determined by an assay kit. The data represent a typical experiment, and similar results were obtained from four independent experiments (A, B) and as mean values with standard deviation (C, D) (n = 4). *P<0.05, compared with untreated cells.
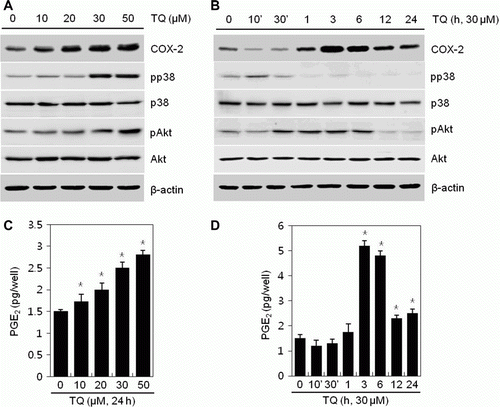
The expression of COX-2 is known to regulate a number of cellular processes through PI3K and p38 kinase signaling pathways (Cao et al. Citation2007). Therefore, the activation of PI3K and p38 kinase has the possibility to TQ-induced COX-2 expression and PGE2 production. Consequently, activation of PI3K and p38 kinase, determined by assessing the phosphorylation of pAkt and p38, increased in TQ treated cells (). Phosphorylation of p38 and Akt was up-regulated by TQ in a dose-dependent manner (A) and showed a maximum at 10 min and at 6 h, respectively (B).
These results show that TQ induces the expression of COX-2 and the production of PGE2 and increases phosphorylation of pAkt and p38 in MDA-MB-231 cells.
TQ induces COX-2 expression and increases PGE2 production via PI3K and p38 kinase signaling pathways in MDA-MB-231 cells
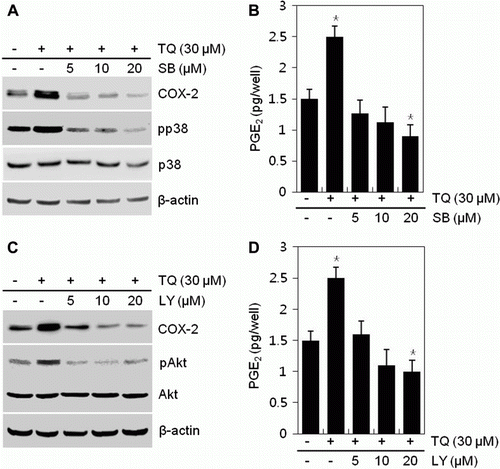
To determine the molecular mechanisms underlying the COX-2 expression and the PGE2 production of MDA-MB-231 cells, we applied various concentrations of inhibitors, LY294002 or SB203580, in the presence of 30 µM TQ and determined the expression of COX-2 and production of PGE2 via a Western blot analysis and PGE2 assay (). TQ-induced COX-2 expression and PGE2 production was abolished by the addition of inhibitors, LY294002 or SB203580, respectively, in a concentration-dependent manner. LY294002 and SB203580 at a concentration of 20 µM inhibited COX-2 expression and PGE2 production by approximately 1.5-fold, respectively, compared to TQ-treated cells ().
Figure 2. Thymoquinone (TQ) modulates COX-2 expression and PGE2 production through PI3K/p38 kinase signaling in MDA-MB-231 cells. (A, B) MDA-MB-231 cells were untreated or treated with 30 µM TQ for 24 h in the absence or presence of different concentrations of SB203580 (SB). (A) Expressions of COX-2, pp38, and p38 were detected by a Western blot analysis. Expression of β-actin was used as a loading control. (B) Production of PGE2 was determined by an assay kit. (C, D) MDA-MB-231 cells were untreated or treated with 30 µM TQ for 24 h in the absence or presence of different concentrations of LY294002 (LY). (C) Expressions of COX-2, pAkt, and Akt were detected by a Western blot analysis. Expression of β-actin was used as a loading control. (D) Production of PGE2 was determined by an assay kit. The data are typical results from four independent experiments with similar results (A, C) and as mean values with standard deviation (B, D) (n = 4). *P<0.05, compared with untreated cells.
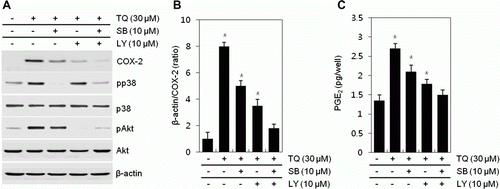
To obtain supporting data, we also studied the effects of inhibiting the PI3K and p38 kinase inhibitors LY294002 or SB20358, alone or in combination, on TQ-induced COX-2 expression and PGE2 production (A). When translational levels of protein were quantified, expression of COX-2 was decreased by approximately 1.6-fold by inhibiting PI3K, whereas it was reduced by 2.3-fold by preventing p38 compared with TQ-treated cells (B). Inhibition of PI3K and p38 kinase with LY294002 or SB203580 reduced up-regulation of PGE2 production by TQ treatment (C). PGE2 production was reduced by 1.6-fold by treatment of LY294002 and by 2.2-fold by treatment of SB203580 (C). These effects suggested that TQ-regulated COX-2 expression and PGE2 production was inhibited by inhibiting PI3K than p38 kinase (). Also, phosphorylation of p38 was not changed by treating of LY294002. Also, treatment of SB203580 showed any effect on expression of pAkt. These results indicated that COX-2 expression and PGE2 production by TQ was regulated by independent pathway, PI3K and p38 kinase, respectively. Co-treatment with LY294002 and SB203580 in the presence of TQ synergistically showed a decrease of COX-2 expression and PGE2 production (A, B).
Figure 3. Thymoquinone (TQ) regulates COX-2 expression and PGE2 production via PI3K/p38 kinase pathway in MDA-MB-231 cells. (A–C) MDA-MB-231 cells were untreated or treated with different concentrations of TQ for 24 h or 30 µM TQ for the specified time periods. (A) Expressions of COX-2, pp38, p38, pAkt, and Akt were detected by a Western blot analysis. Expressions of β-actin were used as loading controls. (B) The relative amounts of COX-2 were quantified by a densitometric analysis (Image J program). (C) Secreted PGE2 was determined by an assay kit. The data represent a typical experiment, and similar results were obtained from four independent experiments (A, B) and as mean values with standard deviation (B) (n = 4). P <0.05. Compared with untreated cells.
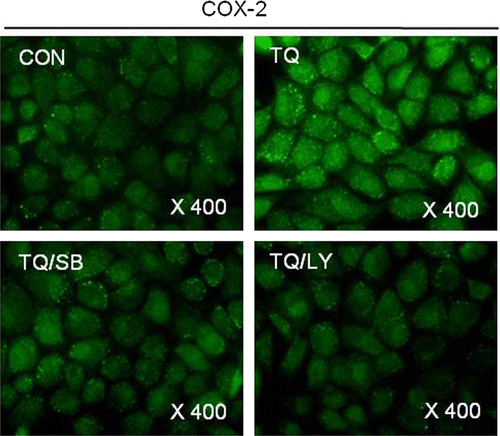
Consistent with the Western blot analysis data, immunofluorescence staining showed a decrease of TQ-induced expression of COX-2 by the addition of LY294002 or SB203580, respectively ().
Figure 4. Thymoquinone (TQ)-caused COX-2 expression is inhibited by inhibiting PI3K/p38 kinase in MDA-MB-231 cells. MDA-MB-231 cells were untreated or treated with 30 µM TQ for 24 h in the absence or presence of SB203580 (SB) or LY294002 (LY). Expressions of COX-2 were detected by immunofluorescence staining. The data are typical results from four independent experiments with similar results.
Taken together, these results suggest that TQ-induced expression of COX-2 and production of PGE2 was modulated by PI3K and p38 kinase in MDA-MB-231 cells.
Discussion
In the present study, we investigated the effects and the regulatory mechanisms of TQ on the expression of COX-2 in a breast cancer cell line, MDA-MB-231. Although COX-2 is important for tumorigenesis, the underlying regulatory mechanism of COX-2 in MDA-MB-231 it not yet understood. TQ has been reported to exert pro-apoptosis and anti-metastasis of various human cancers both in vitro and in vivo including skin, prostate, colorectal, ovarian, pancreatic carcinoma, and leukemia (Velho-Pereira et al. Citation2011). TQ has been shown to inhibit growth, increase apoptosis in cancer cells, whereas it induces minimal toxicity in normal cells (Shoieb et al. Citation2003; Gali-Muhtasib et al. Citation2006). It has been suggested that, owing to these pro-apoptotic effects, TQ can be exploited as a therapeutic agent. Increasing evidence suggests that the inhibition of proliferation by TQ is associated with diminished COX-2 expression.
However, our results showed a different pattern compared with most previously reported data (data not shown). There are no effects on cell growth in response to TQ in MDA-MB-231.
Another breast cancer cell line, MCF-7, has been shown to affect cell viability. Treatment of TQ at 10 µM induced inhibition of cell growth by 15% compared with the control (Ravindran et al. Citation2010). In contrast to MDA-MB-231, MCF-7 has an estrogen receptor and expresses wild-type p53, a tumor-suppressor protein. These differences between MDA-MB-231 and MCF-7 may account for the observed results. Also, several studies have reported that resveratrol, a pharmacological inducer of COX-2 in cancer cells, resveratrol exerted COX-2 expression and implicated p53-dependent apoptosis in breast, thyroid, head, and neck squamous cancer cells (Lin et al. Citation2008). COX-2 has both positive and negative functions on a case-by-case basis in cancer cells.
Interestingly, our results revealed that TQ increases COX-2 expression and PGE2 production in MDA-MB-231 cells, which was accompanied by the regulation of the PI3K and p38 kinase pathways. MDA-MB-231 cells have been shown to express COX-2 in a dose-dependent manner, and the highest expression of COX-2 was observed at 3 h after treatment of TQ, as analyzed by a Western blot analysis.
Because the regulatory mechanisms of COX-2 by TQ are not clearly understood, we treated MDA-MB-231 with TQ, and the expression of COX-2 and the production of PGE2 were detected. Although TQ is known to regulate the expression of COX-2 in various cell types, this is the first report that TQ caused the expression of COX-2 in MDA-MB-231.
Cyclooxygenase-2 is regulated through a cell type specific- and stimulator dependent-pathway, and its regulation is associated with multiple signaling pathways (Schneider and Stahl Citation1998). Among several pathways involved in the regulation of COX-2 expression, pathways of MAPKs (Yang et al. Citation2000) and PI3K (St-Germain et al. Citation2004; Takeda et al. Citation2004) appear to play crucial roles such as controlling cellular physiological and pathophysiological processes, including cell differentiation, inflammation, and cell growth. Also, recent reports suggest that the MAPK and PI3K pathways are related with PG synthesis (Takeda et al. Citation2004).
Consequently, we have evaluated the roles of MAPK and PI3K in the regulation of COX-2 by TQ in MDA-MB-231. In this study, we determined the activation of p38 and Akt. However, ERK and JNK did not have any effects in response to TQ. Our findings demonstrate that inhibition of p38 and Akt with pharmacological reagents abolished TQ-mediated expression of COX-2 and synthesis of PGE2. All data suggest that TQ regulates the COX-2 expression and PGE2 production via the p38 and PI3Kinase signaling pathways.
Further studies are required to explore the underlying effects of TQ on the expression of COX-2 and the production of PGE2 in MDA-MB-231 cells.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (2010-0003239 & 2009-0084569).
References
- Ali , BH and Blunden , G. 2003 . Pharmacological and toxicological properties of Nigella sativa . Phytother Res , 17 : 299 – 305 .
- Cao , Z , Liu , LZ , Dixon , DA , Zheng , JZ , Chandran , B and Jiang , BH. 2007 . Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells . Cell Signal , 19 : 1542 – 1553 .
- Costa , C , Soares , R , Reis-Filho , JS , Leitao , D , Amendoeira , I and Schmitt , FC. 2002 . Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer . J Clin Pathol , 55 : 429 – 434 .
- El Mezayen , R , El Gazzar , M , Nicolls , MR , Marecki , JC , Dreskin , SC and Nomiyama , H. 2006 . Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation . Immunol Lett , 106 : 72 – 81 .
- Gali-Muhtasib , H , Roessner , A and Schneider-Stock , R. 2006 . Thymoquinone: A promising anti-cancer drug from natural sources . Int J Biochem Cell Biol , 38 : 1249 – 1253 .
- Jemal , A , Siegel , R , Xu , J and Ward , E. 2010 . Cancer statistics, 2010 . CA Cancer J Clin , 60 : 277 – 300 .
- Lin , HY , Sun , M , Tang , HY , Simone , TM , Wu , YH , Grandis , JR , Cao , HJ , Davis , PJ and Davis , FB. 2008 . Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells . J Cell Biochem , 104 : 2131 – 2142 .
- O'Banion , MK. 1999 . Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology . Crit Rev Neurobiol , 13 : 45 – 82 .
- Park , K , Han , S , Shin , E , Kim , HJ and Kim , JY. 2006 . Cox-2 expression on tissue microarray of breast cancer . Eur J Surg Oncol , 32 : 1093 – 1096 .
- Ravindran , J , Nair , HB , Sung , B , Prasad , S , Tekmal , RR and Aggarwal , BB. 2010 . Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential . Biochem Pharmacol , 79 : 1640 – 1647 .
- Schneider , A and Stahl , RA. 1998 . Cyclooxygenase-2 (COX-2) and the kidney: current status and potential perspectives . Nephrol Dial Transplant , 13 : 10 – 12 .
- Shoieb , AM , Elgayyar , M , Dudrick , PS , Bell , JL and Tithof , PK. 2003 . In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone . Int J Oncol , 22 : 107 – 113 .
- Smith , WL , DeWitt , DL and Garavito , RM. 2000 . Cyclooxygenases: structural, cellular, and molecular biology . Ann Rev Biochem , 69 : 145 – 182 .
- Smith , WL , Garavito , RM and DeWitt , DL. 1996 . Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2 . Critical Rev neurobiol , 271 : 33157 – 33160 .
- Stasinopoulos , I , Mori , N and Bhujwalla , ZM. 2008 . The malignant phenotype of breast cancer cells is reduced by COX-2 silencing . Neoplasia , 10 : 1163 – 1169 .
- St-Germain , ME , Gagnon , V , Mathieu , I , Parent , S and Asselin , E. 2004 . Akt regulates COX-2 mRNA and protein expression in mutated-PTEN human endometrial cancer cells . Int J Oncol , 24 : 1311 – 1324 .
- Takeda , K , Kanekura , T and Kanzaki , T. 2004 . Negative feedback regulation of phosphatidylinositol 3-kinase/Akt pathway by over-expressed cyclooxygenase-2 in human epidermal cancer cells . J Dermatol , 31 : 516 – 523 .
- Velho-Pereira R , Kumar A , Pandey BN , Jagtap AG , Mishra KP. 2011 . Radiosensitization in human breast carcinoma cells by thymoquinone: role of cell cycle and apoptosis . Cell Biol Int . 35 : 1025 – 1029 .
- Yang , T , Huang , Y , Heasley , LE , Berl , T , Schnermann , JB and Briggs , JP. 2000 . MAPK mediation of hypertonicity-stimulated cyclooxygenase-2 expression in renal medullary collecting duct cells . J Biol Chem , 275 : 23281 – 23286 .
- Yoon , JH , Lim , TG , Lee , KM , Jeon , AJ , Kim , SY and Lee , KW. 2011 . Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation . J Agric Food Chem , 59 : 222 – 228 .
- Ziegler , RG , Hoover , RN , Pike , MC , Hildesheim , A , Nomura , AM , West , DW , Wu-Williams , AH , Kolonel , LN , Horn-Ross , PL Rosenthal , JF . 1993 . Migration patterns and breast cancer risk in Asian-American women . J Natl Cancer Inst , 85 : 1819 – 1827 .