Abstract
This study evaluated ecological health, using various biomarkers and bioindicators, of pale chub (Zacco platypus) as a sentinel species, in Daejeon Stream, South Korea, during April–May 2011. The biomarkers and bioindicators were compared among three sites of control: Reference (Cz), transition (Tz), and the urban zones (Uz); and the 7-Ethoxyresorufin-O-deethylase (EROD) activity, DNA damage, acetylcholinesterase (AChE) activity, and vitellogenin (VTG) concentrations were more significantly increased in the Uz than in the Cz. Also, physiological markers such as condition factor, liver somatic index, visceral somatic index, and gonad somatic index were significantly increased in the Uz than in the Cz. For the health assessments, three categorized parameters of blood chemistry, molecular biomarkers, and physiological bioindicators were standardized and calculated as a star-plot, representing values of Integrated Health Response (IHR). Values of IHR had more significant (P<0.05) increases in the Uz than any other zones, indicating an impairment of ecological health by organic matter, nutrients (N, P), and toxic chemicals. This study is based on low levels of biological organization approach of molecular and physiological biomarkers and bioindicators, so further study of high-levels of biological organization approach such as community and population is required for overall range of health assessments. The approach of IHR values, however, may be useful in providing early warning of future impacts on ecological health.
Introduction
The ecological health of urban streams and rivers are rapidly impaired by industrialization, urbanization, and high population density within the watershed (Finkenbine et al. Citation2000; Miserendino et al. Citation2008). Especially, urban stream ecosystems are more frequently disturbed by point-sources such as wastewater disposal plants as well as non-point sources (Kim and Yeom Citation2009; Yeom et al. Citation2009), and also by habitat modifications of channel structures and dredging by massive urban developments (Finkenbine et al. Citation2000). This phenomenon will increase toxic pollutions and eutrophication in urban streams. Such rapid urbanization may result in channelization of natural streams, which cause decreases of habitat diversity (Nolan and Guthrie Citation1998), increases of organic matters and nutrients (Meyer et al. Citation2005), and rapid sedimentations of heavy metal with organic matters from the water column (Finkenbine et al. Citation2000), resulting in modifications of biological functions by energy flow, material cycling, and trophic competition (Karr and Chu Citation2000).
Numerous studies of health assessments in lotic ecosystems (Barbour et al. Citation1999; Miserendino et al. Citation2008) pointed out that ecosystem health analysis and diagnosis were mainly based on high-levels of biological organization (H-LBO) approach, such as population to community studies. This phenomenon is well demonstrated in ecological health assessments, based on multi-metric fish models in North American, European waterbodies (Barbour et al. Citation1999; Karr and Chu Citation2000). The representative model is Rapid Bioassessment Protocol (RBP), which was developed by Barbour et al. (Citation1999) and based on the H-LBO approach of fish community. This approach has been widely applied in many developed countries of USA, Canada, France, United Kingdom, Australia, and Japan in assessing modifications of physical habitat, biological health, and chemical impacts. This approach, however, could not detect the potential impacts on physiological, cellular, and molecular levels of individuals (organisms), and the assessments diagnosed just community or population levels. Adams and Greeley (Citation2000) pointed out such disadvantages in conventional health assessments of aquatic ecosystems. In fact, inputs of industrial and domestic wastewater to urban streams influence wide-range biological responses from low-levels of biological organization (L-LBO) such as cells, tissues, organs, and individuals to H-LBO such as populations and communities (Adams and Greeley Citation2000). Thus, biological responses of cellular, physiological, and molecular levels may not directly influence the health of population and community, but have still potential impacts on chemical pollutants and disturbance (USGS Citation2000; Triebskorn et al. Citation2001). These problems were solved by studies of cellular, physiological and molecular biomarkers, and bioindicators (Peakall and Walker Citation1994).
The use of biomarkers and bioindicators provides valuable information that cannot be obtained from chemical analysis of pollutants and reflects effects of mixtures of chemicals over long exposure periods (Lam and Gray Citation2003). In addition, this approach detects pollutant effects early in time, e.g. shortly after emission has started, and responds to concentrations below those causing irreversible effects (Gestel and Brummelen Citation1996). For this reason, the L-LBO approach has been frequently evaluated as a pre-warning (or early-warning) tool for environmental surveillance in aquatic ecosystems and thus is applied widely to polluted urban streams in developed countries (Lam and Gray Citation2003).
In this study, we evaluated ecological health using physiological and molecular biomarkers and bioindicators in Daejeon Stream as an example of case study and determined Integrated Health Response (IHR) values using star-plot approach by multi-parameters. The L-LBO approach applied here may be used as a pre-warning tool for predicting and diagnosing the ecological health in urban streams, which are largely impacted by toxic chemicals, nutrients, and habitat disturbances.
Materials and methods
Sampling sites
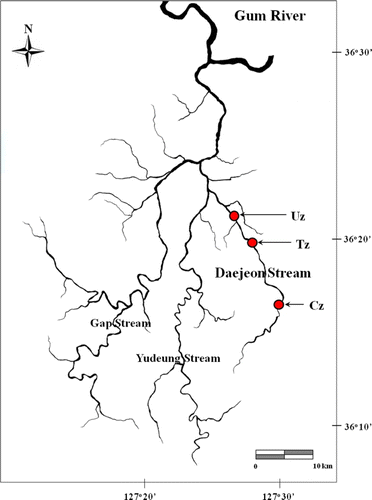
For ecological health assessments of an urban stream, we selected Daejeon Stream, which is largely influenced by metropolitan urban. We designated three sampling sites of the control zone (Cz), impacted urban zones (Uz), and the transition zone (Tz) located between Cz and Uz for the experimental tests (). We used a satellite image from the website geographic service (www.daum.net) to measure the rate of land-use type by calculating the area of land-use type (forest, agriculture, and urban) for the sampling site selection. The Cz is a second-order stream, which is surrounded by forests (>70%), agriculture (<20%), and urban (<10%) land-use. In contrast, Uz is a third-order stream, which is dominated by urban geography (100%) and largely influenced by domestic wastewater and high-population density. The Tz is a third-order stream between the control and impacted zone, and the land-use pattern is intermediate condition (forests<50%<urban area) of the Cz and Uz.
Selection of sentinel species and the sample collections
We selected Zacco platypus as a sentinel species, which is a representative common species in nationwide lotic ecosystems and has easiness of sample collection in the fields and highly efficient sample preparation of bloods and tissues in the laboratory. The sampling was conducted hydrologically stable and fish spawning period of April–May.
Fish samples were collected from various habitats including riffle, run, and pool in the upstream direction using casting nets (mesh-size: 5×5 mm) and hand nets (mesh-size: 4×4 mm). The distance and time sampled were at least 200 m and 60 minutes, respectively, at each zone. After the collection of fish, we selected 10 individuals of female (5) and male (5) fish with >10 cm in total length in each sampling site and then transferred the fish to the laboratory for analysis of various biomarkers and bioindicators.
Biomarker and bioindicator analysis
Sample preparation
After the fishes were fainted in ice, total length and total weight were measured and then blood samples for various analyses of blood chemistry, comet assay, and VTG were prepared. Also, we prepared liver samples for 7-Ethoxyresorufin-O-deethylase (EROD) analysis and brain samples for AChE analysis, and measured the total weight of liver, gonad, viscera, and spleen for physiological indicators.
Blood chemistry analysis
The blood samples were analyzed by ARKRAY blood analyzer (Model SP-4430, Japan), and parameters analyzed were albumin (Alb), alanine aminotransferase (ALT), total protein (T-Pro), blood urea nitrogen (BUN), triglyceride (TG), and total cholesterol (T-Cho).
Molecular biomarkers
The analysis of EROD activity using liver samples followed after the approach of Kim et al. (Citation2010). The samples were thawed at room temperature, phosphate buffer added, the samples grinded, and then centrifuged (7300×g, 4°C) for 30 minutes. After, the supernatant was isolated from the centrifuged samples, centrifugation was conducted for 1 hr at the 16,000×g, and then the pellets (microsomes) were mixed with phosphate buffer. The EROD activities in the microsomes were measured in the reaction product (resorufin) using a fluorescence analyzer (Fluoroskan Ascent, Thermo Labsystems, Finland), at 530 and 590 nm, respectively. The protein assays were analyzed by a fluorescamine assay of Kennedy and Jones (Citation1994).
Comet assays were conducted by the approach of Singh et al. (Citation1988). After the blood samples were centrifuged for 5 minutes at 3000 rpm, the samples were smeared on the coated-slide glass using 1% agarose, and then treated by lysis buffer over 3 hrs. The sample slides treated by the lysis conducted electrophoresis (25 volt, 300 mA) in the buffer solution for 25 minutes. After electrophoresis, the sample slides were rinsed in a neutral buffer and fixed using ethanol. The DNA in the sample slide was stained with ethidium bromide (Etbr). The DNA damage was calculated as the tail extent moment ([TEM] tail length×tail DNA%/100) using the Komet 5 (Kinetic Imaging Ltd., UK).
We followed the method of Jung et al. (Citation2008) to analyze the AChE activity. The brain samples were thawed at room temperature, phosphate buffer added, the samples grinded, and then centrifuged for 10 min (10,000g, 4°C). After, the supernatant was isolated from the centrifuged samples, AChE activities were determined using the bicinchoninic acid (BCA) protein kit (Pierce, Rockford, USA) with bovine serum Alb as a standard. The microplate reader method was used based on the absorbance measurements (415 nm).
The plasma VTG concentrations were measured with a VTG enzyme-linked immunoassay kit (Biosense Lab., Norway). We used Zacco platypus antibody (monoclonal, polyclonal) and Vn standard which was produced by Dongeui University, South Korea. The ELISA analysis was conducted in accordance with the protocol of a monoclonal-based sandwich ELISA method (Jung et al. Citation2004).
Physiological bioindicators
Five physiological markers, such as condition factor (CF), liver somatic index (LSI), gonad somatic index (GSI), visceral somatic index (VSI), and spleen somatic index (SSI), were analyzed, and the equations used here are as follows: CF=total weight×100/total length3; LSI=liver weight×100/total weight; GSI=gonad weight×100/total weight; VSI=viscera weight×100/total weight; SSI=spleen weight×100/total weight.
Integrated health response analysis
For the overall health assessments, we calculated values of IHR, which was originally developed by Beliaeff and Burgeot (Citation2002). In this study, three parameters of molecular biomarkers, blood chemistry, and physiological bioindicators were categorized for ecological health assessments and expressed as a sum of each area by star-plot, representing values of IHR.
Statistical analysis
We conducted normal distribution tests and equal variance tests on the biomarkers and bioindicators. After two tests were satisfied in the initial analysis, we tested one-way analysis of variance (ANOVA) and non-parametric tests along with log-transformation of data. Statistical differences of IHR values among control zone (Cz), impacted urban zone (Uz), and the transition zone (Tz) were compared with the significance level of 95% confidence interval (P<0.05) using statistical package of SYSTAT 11.0K (SYSTAT software Inc. Citation2004).
Results and discussions
Molecular biomarkers
The analyses of four molecular biomarkers such as EROD activity, comet assay, AChE activity, and VTG concentrations () in Daejeon Stream indicated that ecological health impacts on Zacco platypus were significantly (P<0.05) greater in the Uz than in the Cz and Tz.
Figure 2. Measurements of various molecular biomarkers of EROD activity (a), DNA tail moment by comet assay (b), acetylcholinesterase (AChE) activity (c), and vitellogenin (VTG) concentrations (d) in the male and female pale chubs (Zacco platypus) of the control zone (Cz), transition zone (Tz), and urban zone (Uz) of Daejeon Stream, respectively. Each value was expressed as mean±standard deviation, and the asterisk indicates the meaning when the value in each zone was significantly (Cz, P<0.05) different from the controls.
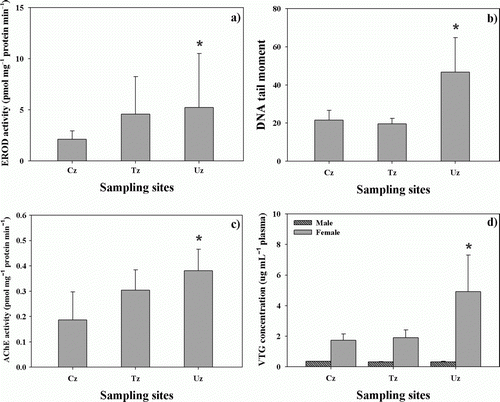
The EROD activity was 2.12, 4.59, and 5.24 in the Cz, Tz, and Uz, respectively. Statistical analysis showed that health impacts on sentinel species were 2.5-fold greater (P<0.05) in the urban than the control, but there was no statistical difference between the transition and urban zone (a). Previous studies of fish (Adams et al. Citation1996; USGS Citation2000) suggested that EROD activity increased through the proteins induced from the liver when exogenic toxicants were exposed to the fish in aquatic environment. Especially, EROD activity was high in point-sources of industrial wastewater, oil refineries, and pulp industries, which produce polycyclic aromatic hydrocarbons (PAHs) and heavy metals (USGS Citation2000), and was also high in urban streams influenced by point-source and non-point sources (Yeom et al. Citation2009). These results support higher EROD activity in Uz zone than in any other zones of Daejeon Stream. In the mean time, Adams et al. (Citation1996) showed some influences of EROD activity by pH, water temperature, habitat quality, and endocrine disruptors, so further studies are needed.
Comet assay analysis, based on TEM of DNA, is determined by the magnitude of DNA damage by environmental factors such as toxic chemicals, nutrients, and habitat quality (Richard and Steinert Citation2003). In Daejeon Stream, TEM was 2-fold greater in the Uz than the Cz and Tz, and the differences were statistically significant (P<0.05; b), as shown in our EROD activity. Previous studies of comet assay (Richard and Steinert Citation2003; Kim et al. Citation2010) showed that DNA damages are frequently induced by PAHs, and agricultural pesticides as well as environmental conditions of stream habitat. Especially, Daejeon Stream might have been damaged by the combined effects of irradiation by direct natural UV because of no forests in the landuse and urban wastewater pollutants (Kim et al. Citation2000).
In aquatic ecosystems, AChE is an enzyme that degrades (through its hydrolytic activity) the neurotransmitter acetylcholine, producing choline and an acetate group. It is mainly found at neuromuscular junctions and cholinergic nervous system of fish, where its activity serves to terminate synaptic transmission. The AChE activity was 0.19 and 0.38 in the Cz and Uz, respectively (c); thus, significant statistical differences (P<0.05) between the two zones were found. The higher activity of AChE in the urban zone may be related to resuspension of bottom sediments by previous intensive agricultural activities, even if the agricultural activity is not present any more (Sturm et al. Citation2007). Such phenomenon is partially supported by the fact that biomarkers of AChE in fish samples are directly damaged by agricultural pesticides of organic phosphorus and carbamate compounds (Sturm et al. Citation2007).
The concentrations of VTG in the male samples did not show any statistical differences, whereas the VTG concentrations in the female samples were significantly greater in the Uz than the Cz (d). In general, VTG concentrations, as an indicator of endocrine disrupting chemicals (EDCs), increase in female during sexual developmental period and are low in male samples, and the major toxicants inducing high VTG concentrations are known as EDCs, such as PAHs and heavy metals in aquatic systems (Jung et al. Citation2004). Our results, however, indicated that EDCs was not influential in the three zones, but high nutrients (TN > 2.0 mg L–1; TP > 300 ug L–1), based on total nitrogen (TN) and total phosphorus (TP), might have resulted in sexual maturation in the urban zone (Uz).
Blood chemistry bioindicators
For blood chemistry analysis, six physiological parameters such as liver-damage (ALT, Alb, and T-Pro), gill and spleen damage (BUN), and nutrients-metabolic indicators (TG and T-Cho) were analyzed as shown in . According to the blood sample analysis of pale chub, Alb as an indicator of Alb protein produced by liver cells was 2-fold greater (P<0.05) in the Uz (mean=1.1±0.3, range=0.8–1.6) than the Cz (mean=0.5±0.3, range = 0.0–0.8). Also, the value of T-Pro as total protein contained in the bloods was 4.8 g/dL in the Uz, and this was 1.6-fold greater (P<0.05) in the Cz (). In the mean time, mean values of ALT in the Tz and Uz were 270 IU/L and 219 IU/L, respectively and these values were 4.7- and 3.8-fold greater than the control.
Table 1. Summary of the blood chemistry parameters of the pale chub (Zacco platypus) collected from the Daejeon Stream, April 2011, South Korea.
In general, the ALT, known as an enzyme in the mitochondria of liver cells, increases as the liver toxicity increases. In the Uz of Daejeon Stream, maximum ALT value attained up to 472 IU/L, suggesting a large damage of liver cells in the urban zone (Adams et al. Citation1996). Previous researches (Folmar et al. Citation1993) showed that as the toxicants, such as PAHs and heavy metals, increase in the ambient water, the levels of ALT increases in the fish, but the values of Alb and T-Pro decreases. In Daejeon Streams, the indicators of liver damage showed highest values in the urban zone. Our results are supported by previous researches of Daejeon Stream by Kim and Lee (Citation1996) and Cho et al. (Citation2000) who showed higher trends of heavy metals and aromatic compounds toward the urban zone (Uz) along with organic matter and nutrients (N, P; Miserendino et al. Citation2008). These results suggest that high toxic chemicals increased ALT concentrations through damages of fish liver, and high nutrient enrichments (N, P) also increased proteins of Alb and T-Pro in the bloods as shown in other polluted streams (Adams et al. Citation1996).
The analysis of T-Cho and TG, as a bioindicator of fish trophic state, showed that the values of T-Cho were significantly greater (P<0.01) in the Uz than Cz, but values of TG did not show significant statistical differences (P>0.05) between the two zones (). According to previous studies, Folmar et al. (Citation1993) suggested that bioindicators of TG and T-Cho are known as a health diagnosis of fat contents in the bloods and increase as the uptake of nutrients and metabolic activity increase. These results in our urban streams suggest that the food availability and metabolic activity increased due to inputs of high nutrient enrichments (N, P) and organic matter contents in the urban (Meyer et al. Citation2005). Meanwhile, the analysis of BUN as a bioindicator of kidney function did not show significant differences (P>0.05) compared with the control (reference site).
Physiological bioindicators
The analyses of physiological bioindicators such as CF, LSI, GSI, VSI, and SSI in Daejeon Stream indicated that fish health condition on organism-level response was significantly (P<0.05) greater in the Uz than the Cz (). The CF, which reflects nutritional status of the overall fish health and effects of environmental pollution such as toxic chemicals, heavy metal, and eutrophication, was significantly higher (P<0.05) in the Uz than the Cz. The higher CF in the urban has been frequently found for tolerant fishes in the nutrient enrichment drainage exposed to pulp mill effluents, urban effluents, and intensive agricultural runoffs (Adams et al. Citation1996). And the VSI, which reflects lipid stores in the viscera, was also significantly higher (P<0.05) in the Uz than the Cz. The greater values of CF and VSI indicate that Uz had higher nitrogen and phosphorus in the ambient water, compared to the control site (Cz). Also, values of LSI and GSI were significantly increased in the Uz compared with the Cz, and these conditions might have resulted in trophic state of the fishes (feeding) and/or greater toxic contaminant exposures to the fishes. The LSI, known as an bioindicator reflecting the metabolism, may increase in response to toxic contaminant exposure, potentially due to the increased metabolic disturbances and increased production of proteins which function in the detoxification process of the liver (Adams and McLean Citation1985; USGS Citation2000). These high physiological responses in the various parameters have been frequently reported in the nutrients (N, P) pollution area such as urban runoffs, domestic and industrial wastewater discharges, and agricultural runoffs (Adams et al. Citation1996). Under the nutrient enrichment and increased food availability, fishes could get over body-weight compared to its total length (CF), resulting in health problems for fishes (Busacker et al. Citation1990).
Table 2. Measurements of various physiological bioindicators of pale chub (Zacco platypus) collected from the Daejeon Stream, April 2011, South Korea.
The nutrient enrichments of nitrogen and phosphorus also resulted in overdevelopment of gonads and are also detected by increases in the GSI (Kim and Yeom Citation2009). Our data suggest that the nutrients and toxic chemicals in the urban could affect these physiological indices by modifying the diet of fish and/or disturbing metabolism (Adams and McLean Citation1985).
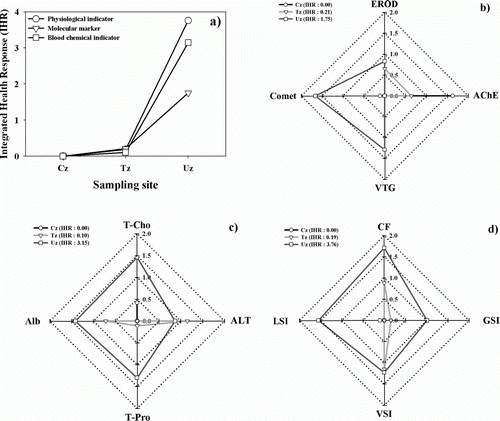
Integrated stream health response
The integration of various biomarkers and indicators was evaluated in the low-LBO of stream ecological health as shown in . The IHR in the stream was categorized as three key parameter components of molecular biomarker, blood chemistry, and physiological indicators, and these components were standardized and integrated as star plots (). The IHR in the Uz was completely different from those in the Cz and Tz. Values of IHR tended to more increase in the Uz than the Cz (a). As shown in , it is evident that the urban zone (Uz) was more affected by some toxic chemicals and high nutrients (N, P), which resulted in impaired molecular biomarkers, blood chemistries, and physiological symptoms in fish. Thus, urbanized zone appeared to be more stressful to fishes than the control and transition zones. Especially, integrated values of IHR suggested that the blood chemistry (IHR value: 3.15, c) and physiological indicators (IHR value: 3.76, d) reflecting the high nutrient enrichment had greater impairment than the molecular-based biomarker (IHR value: 1.75, b) reflecting the toxic chemicals.
Figure 3. The values of Integrated Health Response (IHR), based on the integration of physiological indicators, molecular biomarkers, and blood chemical markers in the control (Cz), transition (Tz), and urban zones (Uz) along with three star-plots of (EROD-comet-AChE-VTG), (Alb-T-Cho-ALT-T-Pro), and (CF-LSI-GSI-VSI) in the three zones.
Overall, these approaches may be highly effective in the pre-assessing of stream ecosystem health prior to extensive field studies of population or community level, providing the key methodology to diagnose the stream health impairment in the low-LBO surveys. By analyzing each biomarker and bioindicator of the sentinel fish and integrating their parameters into one, it is expected to increase the probability to determine the magnitude of ecological impacts by toxic chemicals and environmental stressors. For these reasons, the new approach of IHRs may be used as a key tool for early warning in stream health assessments and may also provide some important clues in identifying the major factors influencing integrated stream health.
Acknowledgements
This subject is supported by Korea Ministry of Environment as “The Eco-technopia 21 project” ID: 091-071-056.
References
- Adams , SM and Greeley , MS. 2000 . Ecotoxicological indicators of water quality: using multi-response indicators to assess the health of aquatic ecosystems . Water Air Soil Pollut , 123 : 103 – 115 .
- Adams , SM , Ham , KD , Greeley , MS , LeHew , RF , Hinton , DE and Saylor , CF. 1996 . Downstream gradients in bioindicator response: point source contaminant effects on fish health . Can J Fish Aquat Sci , 53 : 2177 – 2187 .
- Adams , SM and McLean , RB. 1985 . Estimation of largemouth bass, Micropterus salmoides Lacepede, growth using the liver somatic index and physiological variables . J Fish Biol , 26 : 111 – 126 .
- Barbour MT , Gerritsen J , Snyder BD , Stribling JB . 1999 . Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish , 2nd ed . EPA 841-B-99-002 . Washington , DC : U.S. Environmental Protection Agency, Office of Water .
- Beliaeff , B and Burgeot , T. 2002 . Integrated biomarker response: a useful tool for ecological risk assessment . Environ Toxicol Chem , 21 : 1316 – 1322 .
- Busacker GP , Adelman IR , Goolish EM . 1990 . Growth . In : Schreck CB , Moyle PB , Methods for fish biology . Bethesda , MD : American Fisheries Society , Chapter 11 , p. 363 – 387 .
- Cho , HJ , Lee , BH and Lee , KB. 2000 . A study on the characteristics of benthic sediments in an urban river . Korean J Civil Engineers , 20 : 607 – 618 .
- Finkenbine , JK , Atwater , JW and Mavinic , DS. 2000 . Stream health after urbanization . Am Water Res Assoc , 36 : 1149 – 1160 .
- Folmar LC , Gardner GR , Hinckey J , Bonomelli S , Moody T . 1993 . Serum chemistry and histopathological evaluations of brown bullheads (Ameiurus nebulosus) from the Buffalo and Niagara Rivers , New York. Arch Environ Contam Toxicol . 25 : 298 – 303 .
- Gestel , CAM and Brummelen , TC. 1996 . Incorporation of the biomarkers concept in ecotoxicology calls for a redefinition of terms . Ecotoxicology , 5 : 217 – 225 .
- Jung , JH , Kim , DJ and Han , CH. 2004 . Rockfish (Sebastes schlegeli) vitellogenin purification, characterization and development of sandwich ELISA system . Korean J Fish Sci Technol , 7 : 99 – 108 .
- Jung , JH , Kim , SJ , Lee , TK , Shim , WJ , Woo , SN , Kim , DJ and Han , CH. 2008 . Biomarker responses in caged rockfish (Sebastes schlegeli) from Masan bay and Haegeumgang, South Korea . Mar Pollut Bull , 57 : 599 – 606 .
- Karr , JR and Chu , EW. 2000 . Sustaining living rivers . Hydrobiologia , 422/423 : 1 – 14 .
- Kennedy , SW and Jones , SP. 1994 . Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader . Anal Biochem , 222 : 217 – 223 .
- Kim , KW and Lee , HK. 1996 . Heavy metal contamination of stream water and sediment in the Taejon Area . Korean J Geosys Eng , 33 : 266 – 273 .
- Kim , WK , Lee , SK and Jung , JH. 2010 . Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds . J Hazard Mater , 180 : 395 – 400 .
- Kim , GB , Lee , RF and Mitchell , DL. 2000 . Damage to grass shrimp (Palaemonetes pugio) embryo DNA by summer sunlight followed by DNA repair in the dark . Mar Biol , 137 : 675 – 682 .
- Kim , JH and Yeom , DH. 2009 . Population response of Pale Chub (Zacco platypus) exposed to wastewater effluents in Gap Stream . Toxicol Environ Health Sci , 1 : 169 – 175 .
- Lam , PKS and Gray , JS. 2003 . The use of biomarkers in environmental monitoring programmes . Mar Pollut Bull , 46 : 182 – 186 .
- Meyer , JL , Paul , MJ and Taulbee , WK. 2005 . Stream ecosystem function in urbanizing landscapes . J N Am Benthol Soc , 24 : 602 – 612 .
- Miserendino , ML , Brand , C and Di Prinzio , CY. 2008 . Assessing urban impacts on water quality, benthic communities and fish in streams of the Andes Mountains, Patagonia (Argentina) . Water Air Soil Pollut , 194 : 91 – 110 .
- Nolan , PA and Guthrie , N. 1998 . River rehabilitation in an urban environment: examples from the Mersey basin, North West England . Aqua Conserv Mar Freshw Ecosystem , 8 : 685 – 700 .
- Peakall , DW and Walker , CH. 1994 . The role of biomarkers in environmental assessment . Ecotoxicology , 3 : 173 – 179 .
- Richard , FL and Steinert , S. 2003 . Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals . Mutat Res , 544 : 43 – 64 .
- Singh , NP , McCoy , MT , Tice , RR and Schneider , EL. 1988 . A simple technique for quantification of low levels of DNA damage in individual cells . Exp Cell Res , 175 : 184 – 191 .
- Sturm , A , Radau , TS , Hahn , T and Schulz , R. 2007 . Inhibition of rainbow trout acetylcholinesterase by aqueous and suspended particle-associated organophosphorous insecticides . Chemosphere , 68 : 605 – 612 .
- SYSTAT . 2004 . SYSTAT Software 11.0 K . Richmond , CA : SYSTAT Software .
- Triebskorn , R , Böhmer , J , Braunbeck , T , Honnen , W , Köhler , HR , Lehmann , R , Oberemm , A , Schwaiger , J , Segner , H Schüürmann , G . 2001 . The project VALIMAR (VALIdation of bioMARkers for the assessment of small stream pollution): objectives, experimental design, summary of results, and recommendations for the application of biomarkers in risk assessment . J Aqua Ecosystem Stress Recov , 8 : 161 – 178 .
- USGS (U.S. Geological Survey) . 2000 . Biomonitoring of environmental status and trends (BEST) program: selected methods for monitoring chemical contaminants and their effects in aquatic ecosystems , UITR-2000-0005, Information and Technology Report, Columbia .
- Yeom , DH , Chung , KH , Kim , YH and Adams , SM. 2009 . Ecological health and causal assessment of fish communities experiencing multiple stressors in Gap Stream, South Korea . Toxicol Environ Health Sci , 1 : 97 – 108 .