Abstract
Information about the mating behaviors of an endangered species is critical for the understanding of the natural history of the species as well as in situ and ex situ breeding programs designed to rehabilitate field populations. We describe the mating behaviors of the Mongolian racerunner (Eremias argus), an endangered species in South Korea. The mating of this species consists of precopulatory, copulatory, and postcopulatory stages and is composed of 12 different mating behaviors. During the postcopulatory stage, other males or females not involved in mating show more interference behaviors than during the precopulatory and copulatory stages. The male E. argus has an extraordinarily long postcopulatory bite, which may function as a type of mate-guarding behavior. This study is the first report on the mating behavior of a South Korean reptile.
Introduction
The study of behavior, such as the mating behavior of an endangered species, is critical for both the understanding of the natural history of the species and for in situ and ex situ breeding programs designed to rehabilitate field populations (Lemm et al. Citation2004; He et al. Citation2010). Although the captive propagation of an endangered population is often considered if population sizes in the field are small, a lack of information about the ethological aspects of endangered species has resulted in the failure of captive breeding programs (Burghardt and Milostan Citation1995). Therefore, studies on the mating and related behaviors of various endangered species (Rodríguez-Domínguez and Molina-Borja Citation1998; Schofield et al. Citation2006; Kim et al. Citation2009; Lee et al. Citation2010) have been successfully applied to captive breeding programs (Gillingham and Miller Citation1991; Mesa-Avila and Molina-Borja Citation2007). However, the mating behaviors of endangered reptiles are rarely studied in Asia (Qi et al. Citation2011).
The mating behavior of lizards has been studied in various species, including the Northern grass lizard (Takydromus septentrionalis) (Du and Yao Citation2007), Lacerta kulzeri (In den Bosch and Zandee Citation2001), and the Sand lizard (Lacerta agilis) (Fearnley Citation2002), in the field or in the laboratory. In most lizard species, mating starts when a male encounters a female (Carpenter and Ferguson Citation1977). During the initial contact, the male lizards recognize the female's sex and reproductive condition using visual and chemical cues (Carpenter and Ferguson Citation1977). During copulation, the male lizards often bite the female's body parts (reviewed in Du and Yao Citation2007). In each species, various courtship and mating behaviors are reported, such as chasing, biting, copulation, guarding, and head bobbing (In den Bosch and Zandee Citation2001; Fearnley Citation2002). In Lacertidae, mating processes can be divided into the stages of introduction, copulation, postcopulatory bite, and the period immediately after physical separation (In den Bosch and Zandee Citation2001). Detailed descriptions of the mating behaviors of a lizard species have not been reported from South Korea.
The Mongolian racerunner (Eremias argus) is a small lizard found in Mongolia, China, and Korea. In South Korea, these lizards primarily inhabit coastal sand dunes. The habitats of E. argus in coastal sand dunes are characterized by approximately 75% plant coverage and medium-sized sand grains (Kim et al. Citation2010b). E. argus males and females are sexually mature at an age of 2–3 years. The females oviposit approximately six eggs in at least two separate clutches from late May through early July (Kim et al. Citation2010a). Thermal conditions such as body temperature affect the activity, foraging behaviors, and sprint speed of E. argus (Luo et al. Citation2006; Zhao et al. Citation2008). Due to the steady decline of natural populations from the alteration of coastal sand dune areas in South Korea, E. argus has been designated an endangered species since 2005 by the Korean Ministry of Environment. Information about the natural history and biology of this species is essential for any type of conservation program and is lacking for E. argus. In this study, we describe the mating behavior of E. argus by analyzing 13 successful matings in the laboratory.
Materials and methods
This study was conducted from April through May/June of 2010 and 2011. During each year of the study, 10 males and 10 females (the maximum number allowed by the law protecting endangered species in South Korea) were collected from sand dunes on the west coast of Korea (36° 24′ 34.9′′ N, 126° 23′ 01.4′′ E) and transported to the laboratory located in Chuncheon, Kangwon Province. The male and female lizards were kept separately in manufactured lizard aquaria (110 cm long, 72 cm wide, and 35 cm high). Fewer than four lizards were housed per aquarium. Approximately 18 cm of sand (range: 5–20 cm), collected from the natural habitat of the lizards, was placed at the bottom of each aquarium, along with paper towels and paper egg crates as hiding places. Aquaria were placed on the rooftop of a four-story building so that the temperature and photoperiod followed the local conditions. The lizards were fed daily with crickets and mealworms (approximately two individuals per lizard) and supplied with water in a plastic dish (7 cm diameter, 1 cm depth). The body size of the lizards used in this study and the oviposition period of this species indicated that the lizards were adults in breeding condition (Kim et al. 2010a).
The mating experiments were conducted in the laboratory in experimental aquaria (45 cm long, 30 cm wide, and 25 cm high) with 2 cm of sand at the bottom. Lighting was provided by a 100-watt bulb approximately 50 cm above the aquarium. For the experiments, two females and three males were randomly selected. The three males were first placed in an aquarium followed 10 minutes later by the two females and we began recording mating behaviors with a camcorder (DCR-SR65, Sony Korea, Seoul, Korea) located approximately 1 m above the aquarium. One or two mating trials were conducted daily between 0900 and 1600 (as in Du and Yao Citation2007). If a successful mating occurred, we measured the snout-vent length (SVL), head length, head width, and body weight of the mated lizards using a Vernier caliper (CD-15 CPX, Mitutoyo Korea, Seoul, Korea) and a field balance (TERN TMB 120-1, Tern, Germany). We reused unmated males and females because of the limited number of available lizards; we did not measure the physical condition of unmated males and females. After a lizard was used in a mating experiment, it was given at least one day of rest in outdoor aquaria. No mated males and females were reused. We conducted a total of 57 mating trials from April 7 to June 1, 2010 and 42 mating trials from April 17 to May 31, 2011.
The video recordings were analyzed from the beginning of each mating to 2 hr after the completion of copulation. Based on a previous study (In den Bosch and Zandee Citation2001), we divided each mating of E. argus into precopulatory, copulatory, and postcopulatory stages and described all mating behaviors observed during each stage. In addition, we recorded any interference behaviors directed at the mating pair by males and females that were not involved in the mating. These data were analyzed statistically by calculating Spearman correlation coefficients to evaluate the possible associations of male and female physical conditions with mating behaviors. The analyses were performed using SPSS (v. 17.0, SPSS Inc., Chicago, IL, USA). The data are presented as the mean±standard error throughout the article.
Results
In a total of 108 trials, we observed 13 successful matings (12%). Most unsuccessful mating trials resulted because the male did not show any interest in the female or, in several cases, because the male could not hold the female during his first bite. The physical characteristics of the male and female in a mated pair showed no significant relationships (P > 0.05 for all cases). Based on the 13 matings, we described 12 mating behaviors and three interference behaviors (). The successful matings were initiated by the contact of the male's nostril with the female's cloacal region (touching cloaca; , A) an average of 399.8 sec (range: 23–1978 sec, n=12) after the females were placed into the aquarium. The males’ first mating bite, the first bite of the female's body by the male (), was directed at the female's trunk region (n=4), cloacal region (n=4), tail (n=3), or limbs (n=2). The females actively shook their bodies for a period of time during the first mating bite, but no males released their bites during the successful matings. Approximately 25 sec (range: 8–41 sec, n=13) after the first mating bite, the males achieved a precopulatory bite, a single or series of bites of the female's cloacal region performed by the male (, B). The time interval from the first mating bite to the precopulatory bite showed negative correlations with the female's SVL (r= − 0.65, P=0.017), body weight (r= − 0.65, P=0.016), and head length (r= − 0.76, P=0.003). The precopulatory bite of most males (10 of 13 males) was accomplished from the left side of the female.
Figure 1. Photographs of mating behaviors of the Mongolian racerunner (Eremias argus): (A) touching cloaca, (B) precopulatory bite, (C) copulatory bite, (D) copulation, (E) release bite, (F) postcopulatory bite.
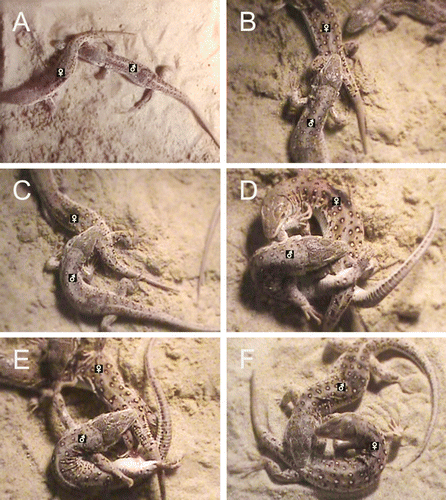
Table 1. Definitions of observed mating behaviors of the Mongolian racerunner (Eremias argus). Except for mating interference behaviors, the behaviors are presented in the linear order observed during a mating. Terminology is based on Carpenter and Ferguson (Citation1977) and In den Bosch and Zandee (Citation2001).
The males changed the orientation of their bite from the female's cloacal region to her lower trunk region to achieve a copulatory bite (, C). The males immediately bent their lower body and began copulation. At the same time, the male used a hind limb to hold the female's tail in a stationary position (D). At the beginning of copulation, most females directed a female copulatory bite towards the male (). These bites were directed at the male's neck (n=6), trunk (n=5), or front limb (n=1). During copulation, 12 males performed tail thrusts (). The copulation time and the number of tail thrusts were negatively correlated with the male's head width (r= − 0.62, P = 0.023) and the female's head length (r= − 0.70, P=0.012), respectively. Immediately before terminating copulation, the males performed a release bite (, E), by changing the location of the bite from the female's lower trunk region to the upper cloacal region. This behavior resulted in a folded body position with an angle of 21.4±2.2° (range: 12.8–35.9, n=12) between the upper and lower body (E). The males actively unfolded their body during the bite so that the genitalia separated from the female's cloaca, terminating copulation. During this behavior, the male often pushed against the female's body or on the sand using his front limbs.
After terminating copulation, the males performed a postcopulatory bite (, F), by changing the location of the bite from the upper cloacal region to the cloacal region of the female. Approximately 1386 sec (range: 103–4005 sec, n=12) before the termination of the postcopulatory bite, seven females actively displayed a tail undulation, whereas six females did not. Approximately 310 sec (range: 1–910 sec, n = 13) before terminating the postcopulatory bite, the females directed a female release bite (), at the male's head (77.8%, 28 out of 36 bites), forelimbs (19.4%, 7 out of 36 bites), or trunk region (2.8%, 1 out of 36 bites). This biting behavior resulted in the completion of mating in all males.
In the precopulatory stage, interference behaviors were observed at low frequencies. Lying on the body of a mating lizard () occurred a total of three times in two cases of mating, and digging under a mating pair () occurred a total of four times in three cases of mating. During the copulation stage, other males interfered with a copulating pair, in one instance by lying on the body of a copulating lizard and in another instance by digging under a copulating pair. These events occurred during separate matings. During the postcopulatory bite, other males and other females performed interference behaviors. The males were observed to lie on the body of a mating lizard (mean: 4.6 times per individual, range: 0–24) during 10 matings, and they were observed to dig under a mating pair (mean: 2.6 times per individual, range: 0–11) during eight matings. Similarly, the females lay on the body of a mating lizard (mean: 1.5 times per individual, range: 0–6) during six matings and dug under a mating pair (mean: 2.2 times per individual, range: 0–10) during seven matings. Other males also interfered with the mating pair during the postcopulatory bite by biting the mating female. The mean number of these bites was 0.8 per individual male (range: 0–4) for four matings.
Discussion
The ratio of successful matings to mating trials was low (12%), implying that to increase the success rate, only sexually active males and sexually receptive females should be used in mating trials. In mating experiments in reptiles, if the receptivity of females is not evident, mating data can be obtained by conducting repeated mating trials (Duo and Yao 2007; Liu et al. Citation2008). In these cases, the rate of successful matings is usually low. For example, only seven matings (4.2%) of the four-eyed spotted turtle, Sacalia quadriocellata, were observed in 168 trials (Liu et al. Citation2008). When a successful mating of E. argus occurred in this study, the male quickly approached a female when introduced, and each time touched the female's cloaca with his nostril. These results suggest that the occurrence of this nostril-to-cloaca contact behavior (i.e., the male touching the female's cloaca with his nostril) may be used as an indicator of female receptivity in future studies.
To begin mating, male Mongolian racerunners require nostril contact with the female's cloacal region. In several lizard species, such as the Sand lizard (L. agilis), the Northern grass lizard (T. septentrionalis), and the Broad-headed skink (Eumeces laticeps), mating also begins with nostril contact with the female cloaca, and it is hypothesized that chemical cues from the females trigger male mating behaviors (Cooper et al. Citation1986; Fearnley Citation2002; Du and Yao Citation2007). It is well known that male lizards can recognize females and can judge females’ reproductive condition based on female chemical cues (Mason Citation1992). In the successful matings observed in this study, the male touched the female's cloaca with his nostril and subsequently performed a series of mating behaviors. These observations suggest that the E. argus male might initiate mating behaviors in response to chemical cues from the female.
During the precopulatory stage, all 13 E. argus males displayed the precopulatory bite, during which the males bit the females’ cloacal region. The precopulatory bite has also been reported in the Northern grass lizard (T. septentrionalis) and the Sand lizard (L. agilis) (Fearnley Citation2002; Du and Yao Citation2007), but the function of the bite was not suggested. The duration of the bite in these species was 2 min and 30 min, respectively. In our study, all mating males bit the females’ cloacal region during the precopulatory bite, although the sites of the first mating bite included the limb, trunk, tail, or cloacal region of the females. The specificity and conservation of the precopulatory bite across lizard species indicate that this bite might have a specific function. We present three hypotheses for the function of the precopulatory bite. First, the precopulatory bite might allow the male to take the best position for observing the female's condition during a brief rest. The males would then obtain the best opportunity for successful copulation. A short precopulatory pause during successive mating attempts has been reported in the Pictus gecko (Paroedura pictus) (Brillet Citation1993). Second, we observed that copulation by the male followed only if the female was inactive during the precopulatory bite. Therefore, the precopulatory bite might function to increase female receptivity. Third, it might be possible that during the precopulatory bite, the males are sexually stimulated by chemical cues from the females, as suggested by Martín and López (Citation2011).
Copulation by males began with the copulatory bite and ended with the release bite. It is well known that the males bite the females’ lower trunk region during copulation in several lizards (Fearnley Citation2002; Du and Yao Citation2007). However, detailed descriptions of the process of terminating copulation have not yet been reported, although males have been found to push females frequently when terminating copulation (Du and Yao Citation2007). Because the release bite in E. argus caused the male's body to fold, the subsequent unfolding movement of the body facilitated the separation of the male's genitalia from the female's cloaca. Tail thrusting has also been found in L. kulzeri and in the Lebanon lizard (Lacerta laevis) (In den Bosch and Zandee Citation2001). It is possible that this behavior facilitates the delivery of sperm into the female cloaca (Greenberg Citation1943).
The sizes of E. argus females and males affected the temporal features of the mating process. In our study, the males performed their precopulatory bite more quickly after the first mating bite if they mated with larger females. In lizards, males and females generally prefer large mates (Martín and López Citation2011), and mating failures often occur because the female escapes from the male's initial grasp (Fearnley Citation2002). These results suggest that the rapid and tight hold on a large female produced by a rapidly achieved precopulatory bite could increase the likelihood of successful mating with the large females. In this study, the males adjusted their copulation time and the number of tail thrusts based on their body size or the female's body size. E. argus females produce at least two separate clutches (Kim et al. 2010a) so that it is possible that female lizards mate more than once. If so, saving energy or sperm in a mating through a briefer copulation time and fewer tail thrusts could be beneficial for the male's subsequent mating (Olsson et al. Citation1996).
Mate guarding is frequently observed in lizards, and prolonged postcopulatory bite found in male E. argus might prevent subsequent copulation by the female with other males. The Lebanon lizard and L. kulzeri performed postcopulatory bites for 0–20 sec (In den Bosch and Zandee Citation2001), and the Sand lizard (L. agilis) remained near the mated female for up to five days (Olsson et al. Citation1996). Although the causes and consequences of these behaviors still remain unclear for most lizards, it is suggested that these behaviors might decrease the possibility that a female would perform multiple matings (Sherman Citation1989). In this study, males that were not involved in mating showed prolonged interference behaviors during the postcopulatory stage. It seems that mating competition is the strongest during this stage than during the precopulatory and copulatory stages. In light of these results and the results of previous studies, it is possible that the function of postcopulatory bite in E. argus, with a mean duration of 1 hr 17 min, is for mate guarding and to prevent the female mating with a second male.
In the study, we described 12 different mating behaviors and suggested several possible functions of conserved behaviors such as precopulatory and postcopulatory bites. Future studies of these topics could further increase our understanding of the natural history of the endangered E. argus. The importance of conservation-oriented behavioral studies for in situ and ex situ conservation programs is increasing (Caro Citation2007), and our results can be used as a foundation for future behavioral studies in the field and for developing captive breeding programs for E. argus.
Acknowledgements
This study was approved by the Geomgang Regional Environmental Office and supported by the Korean Ministry of Environment as “The Eco-Technopia 21 Project (#052-091-080).” We thank Kwan-Su Yook in Taeanhaean National Park and Dae-In Kim and Il-Hoon Kim for their valuable help during the study.
Additional information
Notes on contributors
Ja-Kyeong Kim
These authors contributed equally to this articleReferences
- Brillet , C. 1993 . Behavioural cues in sex recognition by two species of nocturnal lizards: Eublepharis macularius and Paroedura pictus . Amphibia-Reptilia , 14 : 71 – 82 .
- Burghardt GM , Milostan M. 1995 . Ethological studies on reptiles and amphibians: lessons for species survival . In : Demarest J , Durrant B , Gibbons E , Captive conservation of endangered species: an interdisciplinary approach . New York , NY : State University of New York Press . p. 187 – 203 .
- Caro , T. 2007 . Behavior and conservation: a bridge too far? . Trends Ecol Evol , 22 : 394 – 400 .
- Carpenter CC , Ferguson GW. 1977 . Variation and evolution of stereotyped behavior in reptiles . In : Gans C , Tinkle DW , Biology of reptilia , Vol. 7 . New York , NY : Academic Press . p. 335 – 554 .
- Cooper , WE , Garstka , WR and Vitt , LJ. 1986 . Female sex pheromone in the lizard Eumeces laticeps . Herpetologica , 42 : 361 – 366 .
- Du , WG and Yao , ZJ. 2007 . Mating behavior of the Northern grass lizard, Takydromus septentrionalis . Chinese J Zool , 42 : 7 – 12 .
- Fearnley , H. 2002 . A photographic study of reproductive behavior in the Sand lizard, Lacerta agilis, on a Dorset nature reserve . Herpetol Bull , 82 : 10 – 19 .
- Gillingham , JC and Miller , TJ. 1991 . Reproductive ethology of the Tuatara Sphenodon punctatus: applications in captive breeding . Int Zoo Yb , 30 : 157 – 164 .
- Greenberg , B. 1943 . Social behavior of the Western banded gecko, Coleonyx variegatus . Physiol Zool , 16 : 110 – 122 .
- He , B , Liu , Y , Shi , H , Zhang , J , Hu , M , Ma , Y , Fu , L , Hong , M , Wang , J Fong , JJ . 2010 . Captive breeding of the Four-eyed turtle (Sacalia quadriocellata) . Asian Herpetol Res , 1 : 111 – 117 .
- In den Bosch , HAJ and Zandee , M. 2001 . Courtship behavior in lacertid lizards: phylogenetic interpretations of the Lacerta kulzeri complex (Reptilia: Lacertidae) . Neth J Zool , 51 : 263 – 284 .
- Kim , JK , Lee , JH , Ra , NY , Lee , HJ , Eom , J and Park , D. 2009 . Reproductive function of the body and tail undulations of Hynobius leechii (Amphibia: Hynobiidae): a quantitative approach . Anim Cells Syst , 13 : 71 – 78 .
- Kim , BN , Kim , IH , Kim , JK and Park , D. 2010a . Characteristics of oviposition and egg hatching of the Mongolian racerunner (Eremias argus) in the laboratory and field . Korean J Herpetol , 2 : 27 – 34 .
- Kim , JK , Song , JY , Lee , JH and Park , D. 2010b . Physical characteristic and age structure of Mongolian racerunner (Eremias argus; Larcertidae; Reptilia) . J Ecol Field Biol , 33 : 325 – 331 .
- Lee , JH , Min , MS , Kim , TH , Baek , HJ , Lee , H and Park , D. 2010 . Age structure and growth rates of two Korean salamander species (Hynobius yangi and Hynobius quelpaertensis) from field populations . Anim Cells Syst , 14 : 315 – 322 .
- Lemm , JM , Steward , SW and Schmidt , TF. 2004 . Reproduction of the critically endangered Anegada island iguana . Int Zoo Yb , 39 : 141 – 152 .
- Liu , YX , He , B , Shi , HT , Murphy , RW , Fong , JJ , Wang , JC , Fu , LR and Ma , YG. 2008 . An analysis of courtship behavior in the Four-eyed spotted turtle, Sacalia quadriocellata (Reptilia: Testudines: Geoemydidae) . Amphibia-Reptilia , 29 : 185 – 195 .
- Luo , LG , Qu , YT and Ji , X. 2006 . Thermal dependence of food assimilation and sprint speed in a lacertid lizard Eremais argus from Northern China . Acta Zool Sinica , 52 : 256 – 262 .
- Martín J , López P. 2011 . Pheromones and reproduction in reptiles . In : Norris DO , Lopez KH , Hormones and reproduction of vertebrates , Vol. 3 . Reptiles . London : Elsevier . p. 146 – 157 .
- Mason , RT. 1992 . “ Reptilian pheromones ” . In Hormones, brain, and behavior , Edited by: Gans , C and Crews , D . 114 – 228 . Chicago , IL : The University of Chicago Press .
- Mesa-Avila , G and Molina-Borja , M. 2007 . Behavior as a tool for welfare improvement and conservation management in the endangered lizard (Gallotia bravoana) . J Appl Anim Welf Sci , 10 : 193 – 206 .
- Olsson , M , Gullberg , A and Tegelström , H. 1996 . Mate guarding in male Sand lizards (Lacerta agilis) . Behaviour , 113 : 367 – 386 .
- Qi , Y , Li , S , Suo , L , Li , H and Wang , Y. 2011 . An ethogram of the Toad-headed lizard Phrynocephalus vlangalii during the breeding season . Asian Herpetol Res , 2 : 110 – 116 .
- Rodríguez-Domínguez , MA and Molina-Borja , M. 1998 . Reproduction of the endangered Hierro giant lizard Gallotia simonyi machadoi . J Herpetol , 32 : 498 – 504 .
- Schofield , G , Katselidis , KA , Dimopoulos , P , Pantis , JD and Hays , GC. 2006 . Behaviour analysis of the Loggerhead sea turtle Caretta caretta from direct in-water observation . Endang Species Res , 2 : 71 – 79 .
- Sherman , PW. 1989 . Mate guarding as paternity insurance in Idaho ground squirrels . Nature , 338 : 418 – 420 .
- Zhao , Q , Wang , Z , Liu , LL , Zhoa , WG and Ji , X. 2008 . Selected body temperature, surface activity and food intake in tailed versus tailless Mongolian racerunners Eremias argus from three populations . Acta Zool Sinica , 54 : 60 – 66 .