Abstract
Targeted degradation of proteins through ubiquitin-mediated proteolysis is an important control mechanism in various cellular processes. The process of ubiquitin conjugation is achieved by three enzyme complexes, among which the ubiquitin ligase complex (E3) is in charge of substrate specificity. The SCF (SKP1-CUL1-F-box) family portrays the largest and the most characterized member of the E3 ligases. For each SCF complex, the ubiquitination target is recognized by the F-box protein subunit, which interacts with the substrate through a unique C-terminal domain. We have characterized a novel F-box protein CFL-1 that represents a single LRR-type F-box (FBXL) in the Caenorhabditis elegans genome. CFL-1 is highly homologous to FBXL20 and FBXL2 of mammals, which are known to regulate synaptic vesicle release and cell cycle, respectively. A green fluorescence protein (GFP)-reporter gene fused to the cfl-1 promoter showed restricted expression around the amphid and the anus. Modulation of CFL-1 activity by RNAi affected the time interval between defecations. RNAi-treated worms also exhibited reduced tendency to form dauer when exposed to daumone. The potential involvement of CFL-1 in the control of defecation and pheromone response adds to the ever expanding list of cellular processes controlled by ubiquitin-mediated proteolysis in C. elegans. We suggest that CFL-1, as a single LRR-type F-box protein in C. elegans, may portray a prototype gene exerting diverse functions that are allocated among multiple FBXLs in higher organisms.
Introduction
Targeted degradation of proteins through ubiquitin-mediated proteolysis is an important control mechanism in various cellular functions (Jung et al. Citation2009). The procedure for ubiquitin conjugation is achieved by three enzyme complexes, among which the ubiquitin ligase complex (E3) is in charge of determining the substrate. The SCF (SKP1-CUL1-F-box) family portrays the largest and the most characterized member of E3 ligases (Willems et al. Citation2004). In each SCF complex, the subunit containing an F-box motif is responsible for the recognition of ubiquitination target. These F-box proteins are known to interact with the target through unique C-terminal domains and are further classified into subtypes such as FBXW (with WD repeats) or FBXL (with Cys-containing leucine-rich-repeat; LRR_cc) according to their C-terminal structures.
The SCF-type E3 complex of the nematode Caenorhabditis elegans displays many characteristics that are distinct from higher eukaryotes. First of all, C. elegans genome encodes more than 20 SKP-related proteins (SKRs), while mammals have merely 4–5 SKP proteins (Kipreos and Pagano Citation2000). Such expansion of SKRs may have facilitated versatile interactions between CUL-1 and various F-box proteins. Indeed C. elegans has hundreds of F-box members with C-terminal domains that are not found in other species. In contrast, C. elegans appears to encode only one F-box protein with the authentic LRR_cc domain, which is the subject of present study. The solitary presence of FBXL in C. elegans demonstrates a stark contrast to vertebrates equipped with more than 20 FBXLs. Regarding the diverse functions carried out in mammals by these FBXLs (Chen, Coon, et al. Citation2011; Chen, Glassier, et al. Citation2011; Yao et al. Citation2007), the role of single FBXL in C. elegans is highly intriguing.
The presence of C. elegans FBXL was identified through a homology search using mammalian FBXLs and named CFL-1. CFL-1 is highly homologous to the FBXL2 and FBXL20 of mammals, which are involved in cell cycle arrest and synaptic vesicle release, respectively (Chen, Glassier, et al. Citation2011; Yao et al. Citation2007). Expression site of the GFP reporter fused to the promoter region of cfl-1 overlapped to the amphid and anal sphincter. The RNAi-based knockdown of CFL-1 affected daumone response and defecation frequency. We suggest that CFL-1 is a prototype of multiple LRR-type F-box proteins in higher organisms, which carries out functions that are delegated to multiple FBXLs in mammals.
Materials and methods
Worm maintenance
Caenorhabditis elegans was maintained on modified Yongren's medium only with bactopeptone (MYOB) plates (Church et al. Citation1995) seeded with OP50 bacteria, according to the standard culture protocol (Brenner Citation1974).
Sequence comparisons of FBXL proteins
The degree of homology between CFL-1 and human FBXLs was assessed by the BLOSUM 62 matrix of the BLASTP protein algorithm analysis program (Altschul et al. Citation1997). Multiple alignments of protein sequences were done by the ClustalW program of MEGA4 software using standard parameters (Tamura et al. Citation2007). Phylogenetic relationship among multiple families of human FBXLs was analyzed by the neighbor-joining method (Saitou and Nei Citation1987) of the MEGA4 suit.
Expression analysis of cfl-1 promoter::GFP fusion reporter
The 2.4 kb upstream region of the cfl-1 gene was cloned by PCR amplification of N2 genomic DNA using primers 5′-aacc AAGCTT GGTGATCGAGGACTCAAAGC-3′ (forward) and 5′-ggaa GGATCC AACGAAGCGCATGTCTTTCT-3′ (reverse). Nucleotides written in underlined Italic letters represent the built-in restriction sites for HindIII and BamHI, and lowercase letters indicate additional sequences attached to ensure full digestion by restriction enzymes. The PCR product was double-digested with HindIII and BamHI, and inserted into the corresponding restriction sites of the pPD95.75 plasmid. Purified recombinant plasmids were microinjected into the gonad of young adult according to the procedure in Mello and Fire (Citation1995). pRF4 plasmid containing the rol-6 gene was co-injected as a phenotype marker. F1 worms with roller phenotype was transferred to a new plate and screened for continued production of roller mutants in following generations. Expression of GFP reporter was examined under the Olympus BX60 Fluorescence Photomicroscope equipped with a Hamamatsu Orea 12-bit digital CCD camera. Images were taken and analyzed with Openlab software (Improvision, Lexington, MA, USA).
RNAi knockdown of CFL-1 activity
To generate the RNA interference (RNAi) construct of cfl-1 transcript, 1.2 kb region of the cDNA spanning both the F-box and LRR repeat was PCR amplified using primers 5′-TACGACGCTTTCACCAGCTC-3′ (forward) and 5′-TGATCCGTTGGTGGAGTGAC-3′(reverse), then inserted into the L4440 plasmid. Both recombinant and original L4440 plasmid were transformed into Escherichia coli HT115 strain. Feeding RNAi was performed according to the protocol of Kamath and Ahringer (Citation2003) with minor modifications (Min and Lee Citation2007). Briefly, 200 µl of overnight HT115 culture was seeded onto MYOB and induced with IPTG for 48 hours. L4 worms were placed on the HT115 lawn and cultured at 16°C. F1 worms were retrieved after 48 hours and transferred onto fresh RNAi plate to produce F2 worms, which was subjected to required analyses.
Measurement of defecation frequency
F2 generation worms were picked from RNAi plate (MYOB seeded with HT115 containing original or recombinant L4440) at the young adult stage and placed onto a fresh MYOB plate that is lightly seeded with OP50 (e.g., 40 µl of overnight culture per plate). Worms were allowed to settle for 10 minutes at room temperature before starting defecation analysis. Movement of each individual worm was monitored under the dissection microscope, and time was measured starting from the first incident of defecation up to the 11th defecation. Average time interval between defecation motor program was calculated by dividing the recorded time by 10. Each set of experiment recruited 10 worms that were individually monitored.
Dauer formation assay
About 20 adult F2 generation worms were picked from RNAi plate (NGM without peptone, seeded with HT115 containing original or recombinant L4440) and transferred onto a fresh RNAi plate containing daumone (the heptanoid type, kind gift from Dr Y-K Paik at Yonsei University), dissolved in EtOH and added to the media at final concentrations of 38 µM (Jeong et al. Citation2005). Worms placed on the daumone plate were allowed to lay eggs at 20°C for two hours. After removing adults, the plates were incubated for three days at 25°C. The rate of dauer formation was calculated by dividing the number of dauer by the total number of worms hatched on the plate.
Statistical analysis
Statistical significance of differences between data from each experimental group was determined by t-test using the SAS program version 9.1.3. (SAS Institute Inc., USA).
Results
CFL-1 may represent a prototype of multiple LRR-type F-box proteins of mammals
The presence of a single, authentic LRR-type F-box protein encoded by the C. elegans genome (named CFL-1) was originally identified by a BLASTP search using the amino acid sequences of human FBXLs as queries. CFL-1 shows significant homology to all mammalian FBXLs within the conserved F-box domain and multiple LRR_cc motifs (not shown), but is most similar to FBXL2 (Chen, Coon, et al. Citation2011; Chen, Glassier, et al. Citation2011) and to the FBXL20 (known as Scr, SCRAPPER in mouse) (Yao et al. Citation2007) (). Notably, the homology between CFL-1 and FBXL2/FBXL20 is extended beyond the limit of these domains and maintained throughout the entire length of the protein (A). The three proteins share the C-terminal ‘CAAX’ box, which is known to be required for the attachment of lipid moieties (Wang et al. Citation2005). The overall homology assessment using BLASTP program shows that CFL-1 is slightly more similar to FBXL20 than to FBXL2 (BLASTP score 432 vs. 429). Interestingly, the phylogenetic analysis of human FBXLs revealed that their homology to CFL-1 tends to decrease in proportion to the phylogenetic distance from FBXL20 (B). This result suggests a possibility that the multiple members of mammalian FBXLs had been derived from a prototype such as FBXL20 and/or FBXL2, which retain the highest homology to the worm protein.
Figure 1. Evolutionary conservation between CFL-1 and mammalian FBXLs. (A) Protein alignment of CFL-1, FBXL2 and FBXL20. Regions marked with underlying arrows indicate; (a): F-box, (b): potential CaM binding site (see Discussion section), (c–g): LRR_cc's, (h) CAAX box. The GenBank accession numbers for the proteins used for the alignment are NP_741248 for CFL-1, NP_082425 for FBXL20, and NP_848739 for FBXL2. (B) Phylogenetic relation among human FBXLs and their similarity to CFL-1. The tree is drawn to scale, with branch lengths in the same units as of the evolutionary distance used to infer the phylogenetic relation. Numbers in parenthesis indicate homology to CFL-1 within the F-box domain of each protein, given as BLASTP scores.
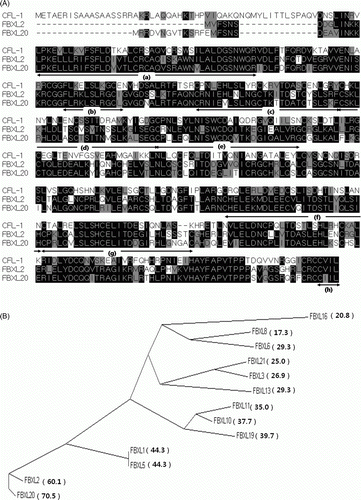
Table 1. Degree of amino acid homology between Caenorhabditis elegans and mouse FBXLs in the F-box and LRR_cc domains.
The cfl-1 promoter::GFP fusion reporter is expressed around the pharynx and anus
To investigate the tissue where CFL-1 would be expressed, a GFP reporter fused to the promoter region of the cfl-1 was constructed. The genomic locus of cfl-1 is located on the Chr.3 (cosmid/cDNA number C02F5.7) and appears to be the first gene of an operon since it has an SL1 trans-splice acceptor right in front of its first exon (). Based on this, the 2.4 kb upstream region up to the end of the previous gene (rfs-1) was presumed to be the promoter region of the gene and used for the construction of GFP reporter. Worms injected with the cfl-1 promoter::GFP fusion construct, together with the rol-6 marker, produced 10 independent lines with germ-line transmitted roller phenotype. In all 10 lines, expression of GFP fusion reporter was confined to the region around pharynx and anus ().
Figure 2. Genomic structure of cfl-1. The numbers on the tick mark represent the nucleotide on Chr. 3.
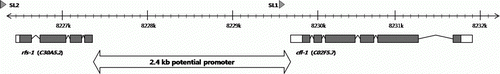
Figure 3. Expression of cfl-1 promoter::GFP fusion reporter. (A) Expression around the pharynx. The GFP fusion reporter was expressed in regions where structures associated with amphid neurons, such as cell body (a), dendrite (b) and its ciliated end (c), and axon (f). It may also be expressed in the socket cell (d, d’) and sheath cell (e). Expression in the rear end of the anterior pharyngeal bulb (g) roughly coincides with the location of neurosecretory motor neuron. (B) Expression around the anal sphincter. Strong and restricted expression of GFP fusion reporter was detected at the junction of intestine and rectum in hermaphrodites (a). Male worms showed a distin ct pattern of expression (b), probably due to the male specific anatomy in this part of the body.
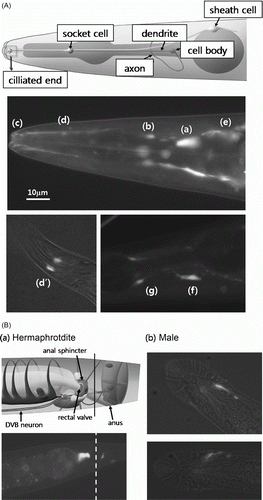
According to the reference pictures in WormAtlas™, expression sites of the GFP reporter in the pharyngeal region roughly overlapped to structures associated with the amphid (A). For example, positive areas may represent parts of the neurons including the cell body (a), dendrite (b) and its ciliated end (c) and the axon (f). The reporter may also be expressed in neuroglial cells such as the socket cell (d, d’) and the sheath cell (e). Expression on the rear side of the pharynx anterior bulb (g) may indicate the neurosecretory motor neuron. Similar pattern of expression was also observed in the pharynx of male and L4 hermaphrodites (data not shown).
In the posterior end of the body, GFP reporter was expressed in a confined region at the junction between intestine and rectum (B-a). The expression site may represent the rectal valve or anal sphincter muscle, or both. Possible involvement of the D-type ventral neuron B (DVB) neuron cannot be excluded, if the expression is targeted to the DVB cell body which is contacting the sphincter muscle. The GFP reporter was expressed in similar regions in L4 hermaphrodites (not shown), while the expression pattern appears distinct in males (B-b) probably due to male-specific anatomy in the tail.
The RNAi knockdown of cfl-1 affected the interval between defecations
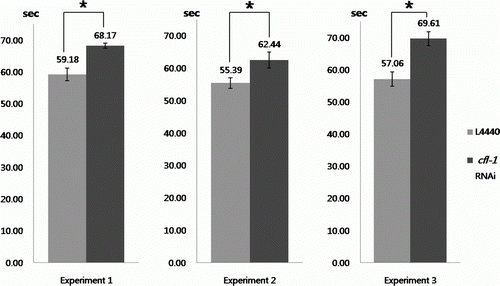
The strong and confined expression of GFP reporter in the region around the rectal valve and anal sphincter suggested that CFL-1 might be involved in the regulation of fecal discharge. To examine if inhibiting the expression of cfl-1 would affect the defecation behavior, RNAi was performed by feeding worms with HT115 bacteria transformed with either empty L4440 plasmid or recombinant L4440 with cfl-1 insert. Feeding worms with cfl-1 RNAi construct did not cause apparent abnormality in the overall defecation behavior. However, when the average time interval between defecations was measured, the worms fed with cfl-1 RNAi plasmid exhibited approximately 10–20% increase compared to the control group fed with empty L4440 (). The difference was not huge, but it was statistically significant and observed repeatedly from three independent experiments each performed with 10 worms.
Figure 4. Effect of cfl-1 RNAi on defecation frequency. Worms treated with RNAi construct targeted to cfl-1 showed a slight increase in average time interval between defecations. Each bar represents average value (in seconds) calculated from 10 worms ± SD (*p<0.01). Data from three independent experiments are shown.
Intrigued by the distinct expression pattern of the GFP fusion reporter around anal sphincter in males, we also attempted similar analysis in this sex. Unfortunately, defecation monitoring in male worms was not feasible due to busy movement of the tail.
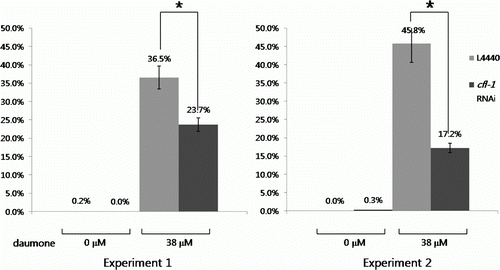
Worms treated with cfl-1 RNAi showed decreased dauer formation after daumone treatment
Next we examined if the expression of cfl-1 around the amphid is associated with activities assigned to this region. The majority of neurons in the amphid are known to govern chemosensory responses (Schackwitz et al. Citation1996; Shakir et al. Citation1993). List of candidate chemicals detected by these neurons includes the dauer-inducing pheromone (daumone), although the exact mechanism of its cellular response is not fully understood. We tested whether modulation of CFL-1 activity would influence daumone response either positively or negatively. The feeding RNAi protocol was used again to inhibit CFL-1 activity. When treated with same concentration of daumone (38 µM), the worms fed with cfl-1 RNAi construct showed up to 2.7 fold decreases in dauer number compared to the control worms fed with empty L4440 plasmid (). These data indicate that normal CFL-1 activity may be required for the proper response toward dauer-inducing pheromone.
Discussion
The nematode C. elegans postulates an interesting case for the work of SCF-type E3 complex in ubiquitin-mediated proteolysis. The C. elegans genome encodes more than 20 SKRs and equally diverse inventory of F-box proteins, most of which are found only in nematodes and performs functions unique to this species (Kipreos and Pagano Citation2000). For example, the FTH-domain F-box protein FOG-2 is required for spermatogenesis in hermaphrodites (Clifford et al. Citation2000). In contrast, C. elegans has rather limited member of F-box families that are also found in higher organisms, such as FBXW and FBXL. An intriguing paradox is that CFL-1 appears to be the only F-box protein with authentic LRR domain in C. elegans, while mammals have over 20 FBXLs.
The single FBXL in the C. elegans genome is reminiscent of Grr-1 in fungi (Fey and Lanker Citation2007; Han et al. Citation2007), while BLASTP search revealed that there are at least four FBXLs in the Drosophila genome (data not shown). The differential expansion of F-box proteins may reflect the diversion of evolutionary pathways taken by nematodes and higher animals. Yet, the strong homology between CFL-1 and mammalian FBXLs suggests that they still may share part of cellular functions. For example, yeast Grr-1 is much less homologous to mammalian FBXLs than CFL-1, but its involvement in cell cycle progression bears a resemblance to FBXL2 (Chen, Glassier, et al. Citation2011).
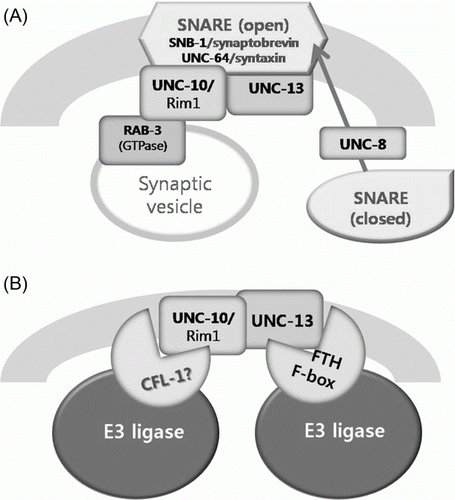
Regarding the evolutionary distance between nematodes and mammals, the conservation between CFL-1 and FBXL20/FBXL2 draws attention particularly in that the homology extends beyond the F-box and LRR domain. The restricted expression of cfl-1 promoter::GFP reporter around the amphid and anus suggests that this protein may partake in specific functions, rather than house-keeping duties. According to the results from our study, CFL-1 might be involved in defecation and daumone detection, either directly or indirectly. Both of these functions could be explained by its homology to FBXL20 of mammals. The mouse FBXL20 is known to control synaptic vesicle release by degrading Rim1, a docking protein for Rab3 GTPase on the synaptic vesicle (Yao et al. Citation2007). In C. elegans, the Rim1 homolog UNC-10 has also been shown to interact directly with RAB-3 (Gracheva et al. Citation2008). UNC-10, together with UNC-13, is associated with a releasing complex called SNARE on the synaptic membrane (A). UNC-13 itself has been shown to interact with a novel FTH-type F-box in a yeast 2-hybrid screening (Polinsky et al. Citation2006). It would be intriguing to find out whether the activity of nearby UNC-10 is also targeted for ubiquitination, presumably by CFL-1(B). Prompt degradation of synaptonemal complex proteins would insure normal propagation of signals at the synapse. Interestingly, the e102 unc-10 mutant strain also exhibited slight increase in defecation intervals compared to the wild type N2 strain (Supplement Figure S1).Footnote1 These data suggest that CFL-1 may affect defecation frequency by via ubiquitination of UNC-10, although the possible existence of targets other than UNC-10 should not be excluded. For both e102 mutant and cfl-1 RNAi, the degree of increase in defecation interval was rather small compared to what was observed with known defecation mutants such as itr-1 and shn-1 (Jee et al. Citation2004; Walker et al. Citation2002); however, it was statistically significant and repeatedly observed in independent trials ( and Figure S1).Footnote2
Figure 6. Hypothetical representation of CFL-1 function in synaptic vesicle release based on its homology to FBXL20. (A) The closed SNARE complex is modified into the open state through the activation by UNC-18, and associates with UNC-10 and UNC-13 on the presynaptic membrane. (B) Once the contents of the synaptic vesicle are released by the engagement or RAB-3 and UNC-10, proteins in the synaptonemal complex are subjected to ubiquitin-mediated proteolysis by F-box containing E3 ligases.
CFL-1 also exhibits high homology to FBXL2 throughout the entire length of the protein. Recently, FBXL2 has been reported to trigger mitotic arrest by mediating ubiquitination of cyclin D3 (Chen, Glassier, et al. Citation2011). In our RNAi analysis of cfl-1, we did not observe changes in cell-cycle associated aspects such as hatching rate or lifespan (data not shown). Another report from the same group showed that FBXL2 degrades an enzyme for membrane lipid biosynthesis (Chen, Coon, et al. Citation2011). This activity is antagonized by calmodulin (CaM), which binds to FBXL2 through the 79FLRKLSLRGCI89 region. The CaM binding motif is copied in FBXL20 as well, and also found in CFL-1 with a few homologous changes in amino acids (A). Like a variety of other cellular pathways of C. elegans, both defecation and chemosensation are governed by the antagonistic interplay between Ca+ + and CaM (Branicky and Hekimi Citation2006; Lans and Jansem Citation2006). Perhaps the role CFL-1 partakes in these processes as an inducer of protein ubiquitination is additionally subjected to the control exerted by Ca+ + and CaM.
The inhibitory effect of cfl-1 knock-down on daumone-induced dauer formation raises several questions. In the absence of pheromone, chemosensory neurons in the amphid strive to repress dauer formation, and as a result the L1 larva progress into the L2 stage (Bargmann and Horvitz Citation1991). Daumone is believed to lift this repression by blocking signals that lead to normal development. If CFL-1 is indeed involved in daumone signaling pathway, it is likely to target one or more of these blockers, since RNAi mediated inhibition of cfl-1 has reduced the dauer formation (). Finding the cellular target of CFL-1 would enable the elucidation of daumone signaling network in chemosensory neurons, which remains largely unknown. CFL-1 might also affect dauer formation in the absence of daumone. To test this possibility, dauer formation needs to be monitored in the heat or starvation induced system. However, quantitative monitoring of dauer formation is challenging in these methods.
In summary, we have identified and characterized CFL-1, a novel C. elegans protein with strong homology to mammalian LRR-type F-box proteins. In accordance with the specific expression of GFP fusion reporter in the amphid and anal sphincter, cfl-1 RNAi affected defecation frequency and daumone response. Further investigation on the molecular function of CFL-1, including the identification of its ubiquitination target(s), may provide better understanding toward neuronal regulation in C. elegans.
Acknowledgements
We thank Prof. Yoongki Paik at Yonsei University for the gift of daumone. The e102 strain of unc-10 mutant was kindly provided by Prof. Joohong Ahnn at Hanyang University. We are also deeply indebted to Prof. Leslie Rose at the University of California at Davis for her help with microinjection and imaging analyses. This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ008196), Rural Development Administration, Republic of Korea.
Additional information
Notes on contributors
Sang-Ho Jang
Current address: Sang-Ho Jang, Department of Infectious and avian diseases, College of Veterinary Medicine, Chunbuk National University, Jeonju, KoreaNotes
1. Supplementary material can be found by clicking on the Supplementary Content tab at http://dx.doi.org/10.1080/19768354.2012.665612.
2. See note 1.
References
- Altschul , SF , Thomas , LM , Alejandro , AS , Zhang , J , Zhang , Z , Miller , W and Limpman , DJ. 1997 . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Res , 25 : 3389 – 3402 .
- Bargmann , CI and Horvitz , HR. 1991 . Control of larval development by chemosensory neurons in Caenorhabditis elegans . Science , 251 : 1243 – 1246 .
- Branicky , R and Hekimi , S. 2006 . What keeps C. elegans regular: the genetics of defecation . Trends Genet , 22 : 571 – 579 .
- Brenner , S. 1974 . The genetics of Caenorhabditis elegans . Genetics , 77 : 71 – 94 .
- Chen BB , Coon TA , Glasser JR , Mallampalli RK 2011 . Calmodulin antagonizes a calcium-activated SCF ubiquitin E3 ligase subunit, FBXL2, to regulate surfactant homeostasis . Mol Cell Biol . 9 : 1905 – 1920 .
- Chen , BB , Glassier , JR , Coon , TA and Mallampalli , RK. 2011 . FBXL2 is an ubiquitin E3 ligase subunit that triggers mitotic arrest . Cell Cycle , 10 : 3487 – 3494 .
- Church , DL , Guan , KL and Lambie , EJ. 1995 . Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans . Development , 121 : 2525 – 2535 .
- Clifford , R , Lee , MH , Nayak , S , Ohmachi , M , Giorgini , F and Shedle , T. 2000 . FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline . Development , 127 : 5265 – 5276 .
- Fey , JP and Lanker , S. 2007 . Delayed accumulation of the yeast G1 cyclin Cln1 and Cln2 and the F-box protein Grr1 in response to glucose . Yeast , 24 : 419 – 429 .
- Gracheva , EO , Hadwiger , G , Nonet , M and Richmond , J. 2008 . Direct interaction between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density . Neuroscience Lett , 444 : 137 – 142 .
- Han , YK , Kim , MD , Lee , SH , Yun , SH and Lee , YW. 2007 . A novel F-box protein involved in sexual development and pathogenesis in Gibberella zeae . Mol Microbiol , 63 : 768 – 779 .
- Jee , C , Lee , J , Lee , JI , Lee , WH , Park , B-J , Yu , J-R , Park , E , Kim , E and Ahnn , J. 2004 . SHN-1, a Shank homologue of C. elegans, affects defecation rhythm via the inositol-1,4,5-trisphosphate receptor . FEBS Lett , 561 : 29 – 36 .
- Jeong , PY , Jing , M , Yim , YH , Kim , H , Park , M , Hong , E , Lee , W , Kim , K and Paik , YK. 2005 . Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone . Nature , 433 : 541 – 545 .
- Jung , T , Catalgol , B and Grune , T. 2009 . The proteasomal system . Mol Asp Med , 30 : 191 – 296 .
- Kamath , RS and Ahringer , J. 2003 . Genome-wide RNAi screening in Caenorhabditis elegans . Methods , 30 : 313 – 321 .
- Kipreos , ET and Pagano , M. 2000 . The F-box protein family . Genome Biol , 1 : 1 – 7 .
- Lans , H and Jansem , G. 2006 . Noncell- and cell-autonomous G-protein-signaling converges with Ca2 +/mitogen-activated protein kinase signaling to regulate str-2 receptor gene expression in Caenorhabditis elegans . Genetics , 173 : 1287 – 1299 .
- Mello , C and Fire , A. 1995 . DNA transformation . Methods Cell Biol , 48 : 451 – 482 .
- Min , K and Lee , J. 2007 . RNA interference in C. elegans: history, application and perspectives . Integr Biosci , 11 : 99 – 104 .
- Polinsky , C , Houston , C , Vado , J , Shaikh , A and Kohn , RE. 2006 . Synaptic protein UNC-13 interacts with an F-box protein that may target it for degradation by proteasomes . Acta Biochim Pol , 53 : 145 – 148 .
- Saitou , N and Nei , M. 1987 . The neighbor-joining method: a new method for reconstruction phylogenetic tree . Mol Bio Evol , 4 : 406 – 425 .
- Schackwitz , WS , Inoue , T and Thomas , JH. 1996 . Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans . Neuron , 17 : 719 – 728 .
- Shakir , MA , Miwa , J and Siddiqui , SS. 1993 . A role of ADF chemosensory neurons in dauer formation behavior in C. elegans . Neuroreport , 4 : 1151 – 1154 .
- Tamura , K , Dudley , J , Nei , M and Kumar , S. 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Mol Biol Evol , 24 : 1596 – 1599 .
- Walker , DS , Grower , NJD , Ly , S , Bradley , GL and Batlis , HA. 2002 . Regulated disruption of Inositol 1,4,5-trisphosphate signaling in Caenorhabditis elegans reveals new functions in feeding and embryogenesis . Mol Biol Cell , 13 : 1329 – 1337 .
- Wang , C , Gale , M Jr , Keller , BC , Huang , H , Brown , MS , Goldstein , JL and Ye , J. 2005 . Identification of FBL2 as a genanylgeranylated cellular protein required for hepatitis C virus RNA replication . Mol Cell , 18 : 425 – 434 .
- Willems , AR , Schwab , M and Tyers , M. 2004 . A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin . Biochem Biophys Acta , 1695 : 133 – 170 .
- Yao , I , Takagi , H , Ageta , H , Kahyo , T , Sato , S , Hatanaka , K , Fukuda , Y , Chiba , T , Morone , N Yuasa , S . 2007 . SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release . Cell , 103 : 943 – 957 .