Abstract
Continuous exercise and bright light affect brain function, particularly in seasonal affective disorders. However, the underlying mechanism of action remains unclear. In this study, we examined whether low-intensity treadmill exercise and 4 weeks of bright light exposure change the expression of brain-derived neurotrophic factor (BDNF), phosphoinositide 3-kinase/AKT (PI3K/AKT) pathway, protein kinase C (PKC), extracellular signal-regulated kinases (ERK1/2), cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB), vascular endothelial growth factor (VEGF), and glycogen synthase kinase-3β (GSK-3β) in the brain of 5-week old Sprague–Dawley male rats by performing western blot analysis. We demonstrated that BDNF expression significantly increased with exercise and light exposure compared to a control group. Moreover, we found that the expression levels of PI3K, PKC, p-ERK1/2, p-CREB, p-AKT, and VEGF increased significantly in the hippocampus and cortex with exercise and light exposure compared to the levels from a control group. However, phosphorylated GSK-3β level was unchanged, and even slightly decreased with exercise and light exposure. These results suggest that low-intensity treadmill exercise and bright light exposure induce BDNF expression and activate its downstream kinase signaling pathway, which in turn activate CREB-mediated transcription of neurotrophic factors and may stimulate neurogenesis and improve neuronal functions.
Introduction
Exercise is known to increase the neurogenesis through induction of neurotrophic factors in the hippocampus, which is associated with improved cognitive function (Neeper et al. Citation1995; van Praag et al. Citation1999; Trejo et al. Citation2001). Bright light can relieve the symptoms of seasonal mood disorders through neurobiological changes (Kasper et al. Citation1990; Partonen & Lonnqvist Citation2000; Lau et al. Citation2011). It is well accepted that physical activity and phototherapy can regulate hippocampal neurogenesis and neuronal cell function. We previously showed that low-intensity treadmill exercise and/or bright light promote neurogenesis and brain-derived neurotrophic factor (BDNF) expression in an animal model (Kwon et al. Citation2013). However, their molecular mechanisms of action remain largely unknown.
BDNF is a nerve growth factor and critical for survival, growth, and differentiation of neurons and thus, is critical to maintain proper neuronal functions. BDNF is active in the hippocampus and cortex where it regulates neuronal excitability, synaptic plasticity, long-term potentiation (LTP), and neurogenesis (Acheson et al. Citation1995; Huang & Reichardt Citation2001; Ninan et al. Citation2010). BDNF is known to bind the tyrosine kinase receptor B (TrkB) receptor, modulating neurotransmitter receptors to activate various kinase signaling pathways of phosphoinositide 3-kinase/AKT (PI3K/AKT), extracellular signal-regulated kinases (ERK1/2), mitogen-activated protein kinase (MAPK), or calcium/calmodulin dependent protein kinase (CaMKII), and inducing phospho-CREB-mediated transcription (Mai et al. Citation2002; Garoflos et al. Citation2005; Luikart et al. Citation2008). Phosphorylation of GSK-3β mediated by PI3K/AKT inactivates GSK-3β which is known to inhibit cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) transcriptional activity (Grimes & Jope Citation2001; Mai et al. Citation2002). The CREB target genes include neurotrophic factors such as BDNF and VEGF (Gass & Riva Citation2007; Lee et al. Citation2009) and many neuropeptides, which play important roles in neuronal plasticity and memory formation (Suzuki et al. Citation2011).
Here, we investigated the combined effect of exercise and light exposure on expression of BDNF and downstream signaling proteins to support their beneficial effect on brain functions.
Materials and methods
Animals
Male Sprague–Dawley rats weighing 160 ± 10 g (5-week old) were obtained from a commercial breeder (KOATECH, Gyeonggi, Korea). Experimental procedures were performed in accordance with the animal care guidelines of National Institutes of Health (NIH). Animals were housed under controlled temperature (22 ± 2°C) with an alternating 12-hour light/dark cycle (lights were turned on between 6 am and 6 pm), and provided food and water ad libitum. Rats were randomly divided into four experimental groups; control group (CG), exercise group (EG), light group (LG), and exercise plus light group (ELG; n = 5 per group).
Treadmill exercise regimen
Animals from exercise groups were subjected to treadmill (PARK TECH, Daegu, Korea) exercise for 30 min per day for 5 consecutive days, each week for 4 weeks. The exercise load consisted of a running speed of 2 m/min for the first 5 min, 5 m/min for the next 5 min, and 8 m/min for the last 20 min at 0° inclination. The exercise was performed in the morning (between 10 am and 12 pm).
Bright light exposure
Light therapy was administered at an intensity of 10,000 lx (Gagné et al. Citation2007). A lamp for artificial light of 10,000 lx illumination was obtained from a commercial company (Danbee, Gyeonggi, Korea), and placed at a distance of 80 cm from animals during the experiments. For the LG, the bright light was exposed for 30 min, which is the matched time period with the EG. ELG was treated at the same time for 30 min.
Tissue preparation
Animals were sacrificed 24 hours after 4 weeks of treatment. Rats were anesthetized with an intraperitoneal injection of 10 mg/kg of Zoletil 50 (Virbac, Carros, France). The brains were quickly removed and hippocampus and cortex were dissected and frozen. The frozen samples were transferred to sterile 1.5 mL microcentrifuge tubes containing 550 µL of lysis buffer (T-buffer; Themo Scientific) with protease inhibitors. Homogenized tissues were incubated for 10 min on ice and sonicated. Samples were centrifuged at 4°C for 30 min at 12,000 rpm and supernatants were collected. Protein concentration was determined using a bicinchoninic acid protein assay (Bio-Rad, Hercules, CA, USA), and samples were stored at –80°C.
Western blot analysis
Protein extracts were separated by SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were incubated with the following primary antibodies (diluted at 1:1000); BDNF (Santa Cruz), PI3K (Santa Cruz), PKCα (Santa Cruz), PKCδ (Santa Cruz), ERK1/2 (Cell Signaling Technology), AKT (Cell Signaling Technology), p-AKT (Cell Signaling Technology), CREB (Cell Signaling Technology), p-CREB (Cell Signaling Technology), GSK3β (Cell Signaling Technology), p-GSK3β (Cell Signaling Technology), and VEGF (Santa Cruz). After washing in Tween 20 in Tris-buffered saline (0.5% TBST), the membranes were incubated with their corresponding secondary antibodies. The proteins were detected with the enhanced chemiluminescence (ECL) solution (Pierce, Rockford, IL) and images were captured by the LAS-4000 system (Fujifilm, Tokyo, Japan). The protein levels were normalized to those of α-tubulin (Sigma).
Statistical analysis
Differences between control and experimental groups were determined by using unpaired Student's t-test, and one-way repeated measures analysis of variance were used to analyze the differences among all the groups. Multiple comparisons were performed with Duncan's method. Values are expressed as mean ± standard error of the mean (SEM) and a p value <0.05 was considered as statistically significant.
Results
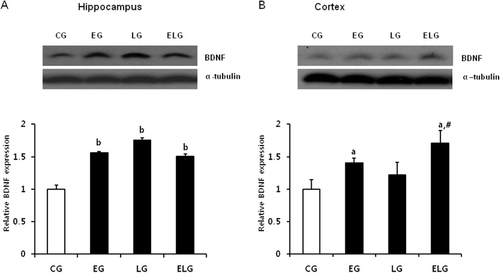
Exercise and light exposure increased BDNF protein levels
We examined whether BDNF expression in the hippocampus and cortex is altered by low-intensity treadmill exercise and bright light by performing western blot analysis. BDNF expression was increased in the EG, LG, and ELG compared to the CG in both hippocampus and cortex (). Moreover, there was an additive effect on BDNF expression in the ELG in cortex (). The statistical significance was indicated.
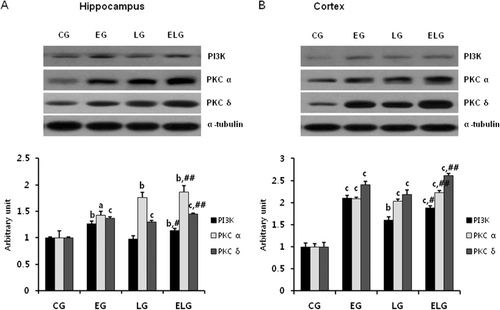
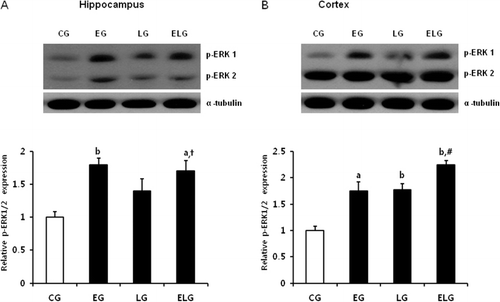
Exercise and light exposure increased the protein levels of PI3K, PKC, and p-ERK
Once BDNF binds to its receptor, TrkB, it activates downstream targets leading to various neuronal processes. The signaling cascade is mediated through PLC/PKC, PI3K/AKT, and ERK1/2 (p44/p42 MAPK) and regulates synaptic plasticity, proliferation, differentiation, and neuronal survival. Thus, we examined the expression of PI3K, PKCα, PKCδ (), and phosphorylated Erk1/2 (p44/p42; ) in the hippocampus and cortex by western blot analysis. The expression of PI3K, PKCα, PKCδ, and p-ERK was significantly increased in the EG, LG, and ELG compared to the CG. Our results suggest that exercise and light exposure activate various kinase signaling molecules, downstream of BDNF.
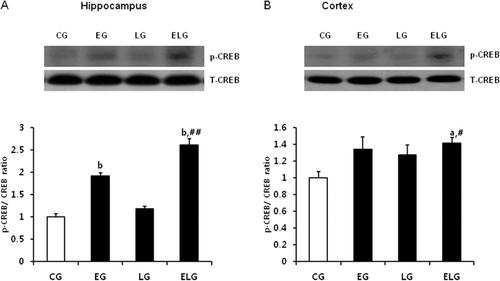
Exercise and light exposure increased the level of p-CREB
The activated protein kinases are translocated to nucleus and phosphorylate CREB to activate p-CREB-mediated transcription (Gonzalez & Montminy Citation1989; Karin & Hunter Citation1995; Mayr & Montminy Citation2001). CREB in the brain has well-known role in neuronal plasticity and LTP. Thus, we examined p-CREB level in the hippocampus and cortex by western blot analysis. We found that p-CREB protein levels were increased in the EG, LG, and ELG compared to the CG (). Notably, the significance of increase was higher in the combined stimulus group (ELG) than in EG or LG with a single stimulus.
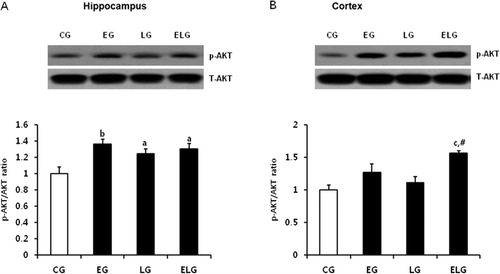
Exercise and light exposure increased p-AKT levels, but not p-GSK-3β levels
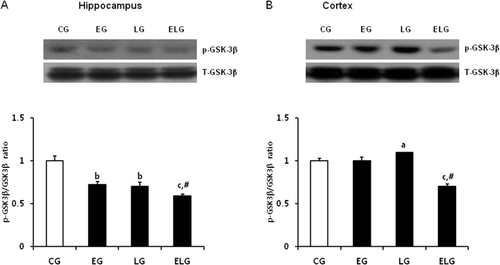
GSK-3β is a critical downstream target of the PI3K/AKT pathway and its activity is inhibited by AKT-mediated phosphorylation. GSK-3β is known to inhibit CREB binding activity. Thus, we examined whether exercise or light exposure affects the levels of p-AKT, activated AKT, by western blot analysis. We found p-AKT levels were significantly increased in the EG, LG, and ELG compared to the CG in the hippocampus (), and further, we observed an additive effect by exercise and light exposure in the cortex (). Subsequently, we examined expression of p-GSK-3, which is phosphorylated by AKT. While p-AKT level was enhanced by exercise and light exposure, p-GSK-3 level was not changed, and even slightly decreased in the hippocampus of EG, LG, and ELG and in the cortex of ELG (), indicating that GSK-3β may be not the direct target of BDNF-PI3K/AKT-mediated signaling pathway in our regimen of exercise and light exposure.
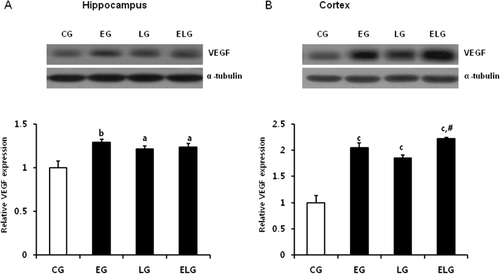
Exercise and light exposure increased VEGF protein levels
CREB activation increases expression of neurotrophic factors, such as BDNF and VEGF. Therefore, we examined whether exercise or light exposure affects VEGF level by western blot analysis. We found that VEGF levels were significantly increased in the EG, LG, and ELG compared to the CG in the hippocampus and cortex (). We propose a model that exercise and light exposure stimulate BDNF receptors and the downstream kinase signaling pathways. The following activated CREB increases expression of various neurotrophic factors, possibly leading to neurogenesis.
Discussion
The main finding of this study was that low-intensity treadmill exercise and bright light activate signaling pathways leading to VEGF and BDNF induction, which are closely related to neurogenesis. We showed increased p-ERK1/2, p-AKT and activation of PI3K and PKC pathway, as well as increased p-CREB and VEGF in the hippocampus and cortex of rats after exercise and light exposure.
Exercise stimulates the sympathetic nervous system causing a neuronal and hormonal stress response which induces calcium influx and cAMP signaling cascades resulting in activation of BDNF transcription (Zheng et al. Citation2012). A recent study showed that exercise affects epigenetic regulation of BDNF, inducing promoter demethylation and activation of methyl-CpG-binding protein 2 in rat hippocampus (Gomez-Pinilla et al. Citation2011).
Light received by the retina stimulates hypothalamic orexinergic neurons in the hypothalamic suprachiasmatic nucleus (SCN) and in the serotonergic nucleus in the dorsal raphe (DRN; Adidharma et al. Citation2012). The SCN is a principal circadian clock and orexin is a neuropeptide regulating the sleep/wake cycle. Serotonin production directly correlates with bright sunlight exposure and is often decreased in people with depression. These neuronal and hormonal changes stimulate neurotransmission, inducing calcium influx and cAMP signaling cascades and resulting in activation of BDNF transcription (Zheng et al. Citation2012).
BDNF promotes neuronal growth, development, and survival, and is also associated with learning and memory through regulation of synaptic plasticity (Bibel & Barde Citation2000; Mizuno et al. Citation2000). A previous study has shown that learning in water maze increases BDNF mRNA expression in the rat hippocampus (Kesslak et al. Citation1998). In agreement with our study, exercise such as treadmill running increased neurogenesis in hippocampus and enhanced spatial learning and LTP (Neeper et al. Citation1995; van Praag et al. Citation1999; Kwon et al. Citation2013). A recent study has found that the improved spatial learning and memory by exercise was correlated with an increase in the number of cholinergic neurons (Ang et al. Citation2006). Thus, our study supports that behaviors such as physical exercise and learning protect neurons at risk in aging and neurodegenerative diseases by BDNF signaling (Binder & Scharfman Citation2004). Additionally, BDNF was shown to produce an antidepressant effect in animal behavioral models of depression through MAPK kinase signaling (MAPK/ERK). BDNF heterozygote mice exhibited a depressive phenotype when combined with a low-dose MEK inhibitor or stress exposure (Shirayama et al. Citation2002; Duman et al. Citation2007). Thus, environmental challenge such as light deprivation might be treated with BDNF expression or by activating its downstream signaling molecules. All of these support that BDNF induced by exercise and light exposure stimulates neuronal functions and alleviates neuropathological symptoms.
Activation of CREB is known to increase the proliferation and survival of newborn neurons, and enhance the differentiation and maturation of neurons in the adult mouse hippocampus (Bender et al. Citation2001; Nakagawa et al. Citation2002; Fujioka et al. Citation2004). Previous studies demonstrated that BDNF induced by exercise and antidepressant treatment activates PI3K/AKT, GSK-3β, and CREB leading to neuronal survival (Mai et al. Citation2002; Chen & Russo-Neustadt Citation2005). In this study, we exposed rats to bright light along with treadmill exercise, expecting antidepressant and further synergistic effects. We propose here that PI3K/AKT signaling mediates these effects via CREB transcriptional activation. Although GSK-3β was not directly associated with the signaling pathway in our study, other downstream targets may involve CREB activation and remain to be further studied.
VEGF has well-known roles in angiogenesis during development and in various diseases, and moreover, recent reports showed its neurotrophic and neuroprotective effects in neurons and glial cells. Thus, VEGF has therapeutic potentials for treating ischemia, traumatic injury as well as chronic neurodegenerative diseases perhaps through an increase in angiogenesis and stimulation of neuronal functions (Greenberg & Jin Citation2004; Rosenstein & Krum Citation2004; Zachary Citation2005). In our study, we found that VEGF was significantly increased after exercise and light exposure, supporting its promising roles in treating cognitive diseases, depression, or mood disorders through its neurotrophic and neuroprotective effects.
In summary, low-intensity treadmill exercise and bright light exposure activate BDNF and its downstream biochemical signaling leading to induction of neurotrophic factors. We also found that the combined treatment of exercise and light therapy more effectively stimulates BDNF-mediated neuronal functions.
Acknowledgments
We would like to thank Lauren Shields at the Gladstone Institutes affiliated with the University of California, San Francisco (UCSF) for editing our manuscript. This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea [No. 2005-0049415].
References
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. 1995. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 374:450–453. 10.1038/374450a0
- Adidharma W, Leach G, Yan L. 2012. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 220:201–207. 10.1016/j.neuroscience.2012.06.020
- Ang ET, Dawe GS, Wong PT, Moochhala S, Ng YK. 2006. Alterations in spatial learning and memory after forced exercise. Brain Res. 1113:186–193. 10.1016/j.brainres.2006.07.023
- Bender RA, Lauterborn JC, Gall CM, Cariaga W, Baram TZ. 2001. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur J Neurosci. 13:679–686. 10.1046/j.1460-9568.2001.01432.x
- Bibel M, Barde YA. 2000. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14:2919–2937. 10.1101/gad.841400
- Binder DK, Scharfman HE. 2004. Brain-derived neurotrophic factor. Growth Factors. 22:123–131. 10.1080/08977190410001723308
- Chen MJ, Russo-Neustadt AA. 2005. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 135:181–193. 10.1016/j.molbrainres.2004.12.001
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. 2007. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 61:661–670. 10.1016/j.biopsych.2006.05.047
- Fujioka T, Fujioka A, Duman RS. 2004. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci. 24:319–328. 10.1523/JNEUROSCI.1065.03.2004
- Gagné AM, Gagné P, Hébert M. 2007. Impact of light therapy on rod and cone functions in healthy subjects. Psychiatry Res. 151:259–263. 10.1016/j.psychres.2006.09.004
- Garoflos E, Stamatakis A, Mantelas A, Philippidis H, Stylianopoulou F. 2005. Cellular mechanisms underlying an effect of “early handling” on pCREB and BDNF in the neonatal rat hippocampus. Brain Res. 1052:187–195. 10.1016/j.brainres.2005.06.032
- Gass P, Riva MA. 2007. CREB, neurogenesis and depression. Bioessays. 29:957–961. 10.1002/bies.20658
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. 2011. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 33:383–390. 10.1111/j.1460-9568.2010.07508.x
- Gonzalez GA, Montminy MR. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 59:675–680. 10.1016/0092-8674(89)90013-5
- Greenberg DA, Jin K. 2004. VEGF and ALS: the luckiest growth factor? Trends Mol Med. 10:1–3. 10.1016/j.molmed.2003.11.006
- Grimes CA, Jope RS. 2001. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem. 78:1219–1232. 10.1046/j.1471-4159.2001.00495.x
- Huang EJ, Reichardt LF. 2001. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 24:677–736. 10.1146/annurev.neuro.24.1.677
- Karin M, Hunter T. 1995. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 5:747–757. 10.1016/S0960-9822(95)00151-5
- Kasper S, Rogers SL, Madden PA, Joseph-Vanderpool JR, Rosenthal NE. 1990. The effects of phototherapy in the general population. J Affect Disord. 18:211–219. 10.1016/0165-0327(90)90038-A
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. 1998. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 112:1012–1019. 10.1037/0735-7044.112.4.1012
- Kwon SJ, Park JS, Park SY, Song KS, Jung ST, Jung SB, Park IR, Choi WS, Kwon SO. 2013. Low-intensity treadmill exercise and/or bright light promote neurogenesis in adult rat brain. Neural Regen Res. 8:922–929.
- Lau BW, Ren C, Yang J, Yan SW, Chang RC, Pu M, So KF. 2011. Light deprivation induces depression-like behavior and suppresses neurogenesis in diurnal mongolian gerbil (Meriones unguiculatus). Cell Transplant. 20:871–881. 10.3727/096368910X539065
- Lee JS, Jang DJ, Lee N, Ko HG, Kim H, Kim YS, Kim B, Son J, Kim SH, Chung H, et al. 2009. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J Neurosci. 29:8493–8505. 10.1523/JNEUROSCI.1321-09.2009
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. 2008. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci. 28:7006–7012. 10.1523/JNEUROSCI.0195-08.2008
- Mai L, Jope RS, Li X. 2002. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J Neurochem. 82:75–83. 10.1046/j.1471-4159.2002.00939.x
- Mayr B, Montminy M. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2:599–609. 10.1038/35085068
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. 2000. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 20:7116–7121.
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. 2002. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 22:9868–9876.
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. 1995. Exercise and brain neurotrophins. Nature. 373:109. 10.1038/373109a0
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. 2010. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 30:8866–8870. 10.1523/JNEUROSCI.1405-10.2010
- Partonen T, Lonnqvist J. 2000. Bright light improves vitality and alleviates distress in healthy people. J Affect Disord. 57:55–61. 10.1016/S0165-0327(99)00063-4
- Rosenstein JM, Krum JM. 2004. New roles for VEGF in nervous tissue – beyond blood vessels. Exp Neurol. 187:246–253. 10.1016/j.expneurol.2004.01.022
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. 2002. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 22:3251–3261.
- Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, et al. 2011. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 31:8786–8802. 10.1523/JNEUROSCI.3257-10.2011
- Trejo JL, Carro E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 21:1628–1634.
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. 1999. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 96:13427–13431. 10.1073/pnas.96.23.13427
- Zachary I. 2005. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 14:207–221. 10.1159/000088637
- Zheng F, Zhou X, Moon C, Wang H. 2012. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol. 4:188–200.
