Abstract
Mesenchymal stem cells (MSCs) derived from rabbit bone marrow are routinely investigated in cardiovascular and orthopedic models for regenerative medicine application. However, classical medium used for generating rabbit MSCs (rbMSCs) was supplemented with fetal bovine serum (FBS), which would raise several safety concerns and inconsistencies in the generation of MSCs. Here, we tested a serum-free medium for culture of rbMSCs through the investigation of multiple parameters including cell morphology, expansion, phenotype, and trilineage differentiation. RbMSCs were isolated using the gradient centrifugation method, and expanded in StemPro® MSC SFM in the presence of substrates and basic fibroblast growth factor (bFGF). Cells were then characterized by phase contrast microscopy observation, cell proliferation analysis, immunocytochemistry staining, RT-PCR, and qPCR analysis. Similar to that achieved with FBS-containing culture medium, the viability and proliferation of rbMSCs were improved in serum-free medium supplemented with bFGF and pre-coated with CELLstartTM. Serum-free expanded rbMSCs showed the fibroblastic spindle-shape morphology, and were positive for CD29, CD44, and CD73, and negative for CD34, CD45, and CD166. After the corresponding differentiation, rbMSCs from serum-free condition were demonstrated to differentiate into adipogenic, osteogenic, and chondrogenic lineages. In addition, differences were also observed between rbMSCs cultured with or without serum in terms of their morphology, proliferation, and gene expression levels of early differentiation transcription factors. In conclusion, this study demonstrates that rbMSCs can be expanded in a serum-free condition while maintaining the original characteristics of MSCs, which provides a useful tool to understand the basic biological properties of rbMSCs in culture.
1. Introduction
Based on their ability to proliferate and capacity to differentiate into specific cell types, mesenchymal stem cells (MSCs) have been proposed as a potential candidate for cell therapies and tissue engineering. Clinical studies employing human MSCs (hMSCs) derived from different sources have been initiated for the treatment of several diseases and injuries including myocardial infarction, osteogenesis imperfecta, graft-versus-host disease, diabetes, and other diseases (http://www.clinicaltrials.gov/). Rabbits were used widely in preclinical models for the evaluation of regenerative medicine in humans (Yanni Citation2004; Mapara et al. Citation2012). In in vivo models, the rabbit has the advantage due to its relatively large size compared with the rat and mouse. In addition, it is inexpensive and relatively easy to handle compared with other large animal models (Gupta & Lee Citation2007; Amini et al. Citation2012).
The use of rabbit MSCs (rbMSCs) has been described in previous studies (Lapi et al. Citation2008; Boo et al. Citation2011; Wasel et al. Citation2013). Recently, published literature compared in detail the characteristics of rbMSCs biology with their counterparts in humans, using established methods meant for hMSCs (Bakhtina et al. Citation2014; Tan et al. Citation2013). Similar to hMSCs, rbMSCs were able to adhere to plastic surfaces, exhibit spindle-fibroblast morphology, express surface markers, such as CD29, CD44, CD73, and CD81, and undergo trilineage differentiation, which meets the minimal criteria to define MSC populations by the International Society for Cellular Therapy (ISCT; Bakhtina et al. Citation2014; Tan et al. Citation2013). These important studies suggest that rbMSCs are feasible to be a translational model to assess human regenerative medicine. In addition, the use of rbMSCs is not subject to ethical issues compared to hMSCs.
Traditionally, MSCs have been expanded in medium supplemented with fetal bovine serum (FBS) with or without the addition of growth factors (Pittenger et al. Citation1999). FBS contains a high content of attachment and growth factors as well as nutritional and physiochemical compounds required for cell maintenance and growth. However, the use of FBS is not desirable, raising several safety and other concerns; for example, FBS existed in a high degree of lot-to-lot variation causing inconsistencies in the generation of quality-assured cells and also hampering the analysis of cell biological mechanisms that control cell behavior. Platelet lysates enhanced the expansion of MSCs when compared with FBS-supplemented medium (Fekete et al. Citation2012). However, there is still the issue of significant batch-to-batch variability. Thus, the development of a serum-free medium for the expansion of MSCs represents a necessary step in developing the tools required to study MSCs in a consistent and reproducible manner.
Attempts have been made to develop a defined serum-free condition for hMSCs growth and expansion. Until now, several commercially (Mesencult-XF, StemPro® SFM Xeno-Free, MSCGM-CD, BD Mosaic hMSC SF, etc.) (Chase et al. Citation2010; Miwa et al. Citation2012; Crapnell et al. Citation2013; Mark et al. Citation2013) and lab developed (i.e. PPRF-msc6; Jung et al. Citation2010) serum-free media have recently been introduced for the successful expansion of hMSCs. Based on the available literature, these defined hMSCs serum-free media were demonstrated to support the expansion of hMSCs while maintaining their original properties. One of these media, StemPro® MSC SFM from Invitrogen, supports the serial cultivation of undifferentiated hMSCs in the absence of serum, and thus provides an experimental system for elucidating cellular response to specific environmental stimuli. However, to our knowledge, there is no report on the successful expansion of rbMSCs using defined serum-free medium. In view of the rbMSCs having properties similar to hMSCs, we speculated that rbMSCs should be able to grow in a similar culture condition as hMSCs. In the present study, we demonstrate that a defined StemPro® hMSC SFM medium supports the expansion of undifferentiated rbMSCs in the presence of substrate and basic fibroblast growth factor (bFGF). Cells obtained in this serum-free condition met all criteria for MSCs, including spindle-shaped morphology, surface marker expression, and differentiation capability into three mesenchymal lineages. This work sets the stage for serum-free rbMSCs cell culture and thereby provides a useful tool to understand the basic biological characteristics of rbMSCs.
2. Materials and methods
2.1. Isolation and culture of rabbit bone marrow-derived MSCs
Eight New Zealand female rabbits were included in this study under guidelines determined by the Local Ethical Committee. Bone marrow from three-month-old rabbits (2.0–2.5 kg in weight) was extracted from femur bones. The mononuclear cells from the bone marrow were separated using Ficoll Premium 1.077 density gradient centrifugation technique, then suspended and cultured in the growth medium described as follows. Cells were incubated in serum containing medium [alpha modification of Eagle medium (αMEM) (Gibco) + 10% FBS (Hyclone) + 1% Penicillin/Streptomycin (Gibco)]. Cells were fed twice a week with fresh medium and incubated until reaching a confluence of 80–90%.
Upon reaching 80–90% confluence, cells were then detached with 0.05% Trypsin-EDTA (Gibco), counted and seeded. For serum-free medium culture, cells were cultured at 5000 cells/cm2 in StemPro® hMSC SFM medium with or without the presence of substrates and 10 ng/mL bFGF (Protech). CELLstartTM (1:100 dilution) or fibronectin (5 µg/cm2) was coated on the culture vessel according to the manufacturer's instruction before cells seeding. Considering StemPro®, hMSC condition with the presence of CELLstartTM and bFGF showed extensive propagation with retained phenotypic potential; the MSCs from this system were employed for further study.
2.2. Cell growth curve assay
Briefly, 5000 cells/cm2 in the logarithmic growth phase were harvested and seeded on 4-well plates (NUNC) in StemPro® hMSC SFM medium with or without the presence of substrates (CELLstartTM or fibronectin) and bFGF. Cells were further incubated for six days. After incubation, cell viability was determined by trypan blue dye exclusion. A growth curve was drawn according to the logarithmic number of cells/L with incubation time. Multiplication time and growth saturation density were calculated based on the curve.
2.3. Immunocytochemistry staining
Cells were fixed with 4% paraformaldehyde in phosphate buffer saline (PBS) for 10 min and washed with PBS three times, followed by permeabilization with 0.2% Triton X-100 for 10 min and blockage with 4% goat serum for 30 min at 25°C. Subsequently, cells were incubated with antibodies of CD29 (Chemicon), CD44 (GeneTex), or CD45 (Gentaur Molecular) in staining solution (PBS containing 4% goat serum) for 40 min at 37°C, and then incubated with the appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibody in staining solution for 30 min at 37°C. For CD73 analysis, the rbMSCs were incubated with PE-Cy7 conjugated with CD73 (BD Biosciences) for 30 min. After incubation, cells were washed with PBS and detected.
2.4. Measurement of cell length and width
Photographs were taken at representative areas of culture plates. The length and the maximum width (cell body width) perpendicular to the long axes of individual cells were measured using Zeiss LSM image Examiner software. At least 20 representative cells were measured from each independent experiment.
2.5. MSC differentiation assay
RbMSCs were differentiated into trilineages (adipogenic, osteogenic, and chondrogenic lineages) using commercially available standard differentiation induction medium, namely StemPro® adipogenesis differentiation kit, StemPro® osteogenesis differentiation kit, and StemPro® chondrogenesis kit (Invitrogen-Gibco), in accordance with the manufacturer's protocol. Medium was changed twice a week. Differentiation potential was assessed by staining at day 10 of differentiation induction: Oil-red-O staining for adipogenic differentiation, alkaline phosphatase staining for osteogenic differentiation, and alcian blue for chondrogenic differentiation. Corresponding differentiation staining kits (Genmed) were employed and used in accordance with the manufacture's protocol.
2.6. Quantitative reverse transcription polymerase chain reaction
Samples of total RNA were isolated from cells and analyzed using SYBR Green-based quantitative reverse transcription polymerase chain reaction (qRT-PCR). Briefly, 0.5 µg of RNA was primed by Oligo(dT)16 (Promega, USA) and reverse transcribed with avian myeloblastosis virus reverse transcriptase in a 20-µL reaction system. From a twofold dilution of this cDNA solution, 0.5 µL was used in a 20-µL PCR solution containing 10 µL SYBR premix Ex Taq, 0.4 µL Rox (TaKaRa, Japan), and 10 µmol/L forward and reverse primers. All primers () were designed by submitting RefSeq sequences to Primer Premier 5.0 software; amplicons were approximately 100–200 bp. The PCR amplifications were conducted in 96-well BIOplastics (Axygen, USA) on an Applied Biosystems PRISM 7300 Sequence Detection System under the following conditions: 10 seconds denaturation and enzyme activation at 95°C, followed by 40 cycles of denaturation (95°C for 10 seconds), and annealing/extension (60°C for 30 seconds). Results were normalized to β-actin to control for differences in RNA loading, quality, and cDNA synthesis. Amplicon size and reaction specificity were confirmed by agarose gel electrophoresis. Each sample was assayed in triplicate and median threshold cycle values were used to calculate the fold change between treated and control samples.
Table 1. Primers used for RT-PCR and qPCR analyses.
2.7. Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). Student's t-test was used to determine the significance of differences in multiple comparisons (P < 0.05 was considered statistically significant).
3. Results and discussion
3.1. Isolation of primary rbMSCs
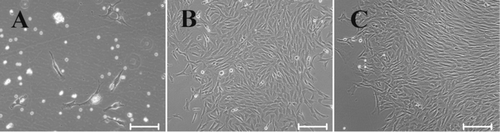
Bone marrow has been the traditional source of MSCs for basic research and therapeutic use because bone marrow harvest is a routine and safe procedure. In order to ensure the purity of bone marrow-derived MSCs, we used density gradient centrifugation to extract cells. The bone marrow mononuclear cells were collected and seeded. In our initial study, we attempted to expand rbMSCs in StemPro® MSC SFM from freshly isolated bone marrow mononuclear cells. Unfortunately, the rbMSCs failed to expand in this system alone, or even in the presence of a substrate and bFGF. This is consistent with other reports about the serum-free culture of MSCs (Chase et al. Citation2010; Hudson et al. Citation2011; Mimura et al. Citation2011). In these reports, successful expansion of MSCs in serum-free culture comes from in vitro expanded MSCs, which may be due to in vitro expanded MSCs that have better adapted the in vitro condition which made it feasible for the further expansion of MSCs in the serum-free condition. Therefore, 10% FBS-containing medium was used for primary rbMSC culture. Following change of medium after three to four days, rounded and fusiform cells with spindle-like cytoplasmic projection were observed adhering to the tissue culture flasks in FBS-containing medium (). Cells then formed distinct small colonies on the flask surface after seven to eight days of culture (). Large colonies were observed after 14 days of culture (). Cell and colony morphology were consistent with hMSCs (Colter et al. Citation2000), mouse (Nadri et al. Citation2007), rat (Fu et al. Citation2012), and rabbit (Lapi et al. Citation2008; Boo et al. Citation2011), which implied that cells adhering to the tissue culture flasks might be MSCs. Upon reaching 80–90% confluence, cells were detached and subcultured, and rbMSCs were maintained and subcultured up to six passages in FBS-containing medium.
3.2. Proliferation and morphology analysis of subcultured rbMSCs in serum-free condition
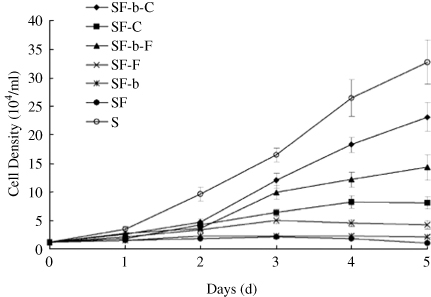
In order to optimize the culture system for expansion of rbMSCs in serum-free condition, subcultured cells were cultured in serum-free medium composed of StemPro® hMSC SFM with different combinations of substrates and growth factors. Cell growth factors, such as platelet-derived growth factor, transforming growth factor, epidermal growth factor, and fibroblast growth factor, have been described as mitogens for MSCs. Among them, bFGF is most representative and effective, and the greatest effect was observed at 10–20 ng/ml (Ahn et al. Citation2009; Mimura et al. Citation2011; Ramasay et al. Citation2012). The substrate component including fibronectin and CELLstart proved to be supportive of the attachment and expansion of MSCs (Hudson et al. Citation2011). Therefore, we investigate the use of fibronectin, CELLstart, and bFGF-based medium for the expansion of rbMSCs in a defined serum-free condition. As shown in , cells cultured in serum-free condition without the presence of a substrate (CELLstartTM or fibronectin) on the culture flasks were unable to achieve sufficient adherence after seeding and failed to proliferate in culture. In serum-free condition with a substrate, cell proliferation was significantly enhanced by bFGF. The growth rate of cells in a serum-free condition in the presence of CELLstartTM and bFGF was faster than other serum-free groups, although it was slower than the FBS-containing group. In addition, these rbMSC-like cells in serum-free condition were maintained and subcultured up to P4 for analysis. Results further revealed that serum-free grown rbMSCs in the presence of CELLstartTM and bFGF had significantly longer population-doubling times compared with FBS-grown rbMSCs (28.5 ± 1.8 h and 25.5 ± 1.6 h, respectively). This is not surprising because the current serum-free system is designed especially for hMSCs, and may have a different effect on rbMSCs. In spite of this, our results demonstrate that rbMSCs expansion in serum-free medium in the presence of CELLstartTM and bFGF have similar growth profiles compared to rbMSCs grown in FBS.
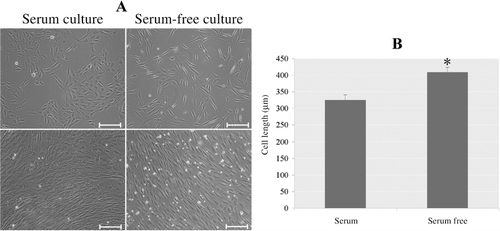
In general, rbMSCs expanded in FBS-containing and serum-free media shared similar fibroblast-like morphologies. However, a subtle difference was also observed. The majority of rbMSCs cultured in serum-free condition showed fibroblastic-shaped morphology with long and thin processes at P1 and P2; however, more flattened and larger size cells were observed in serum condition medium at P1 and P2 (). Length of the cells from serum-free condition was significantly longer than those of in FBS-containing condition (). This is in accordance with previous reports that MSCs cultured in serum-free medium tended to grow in a long spindle morphology (Chase et al. Citation2010; Crapnell et al. Citation2013; Mark et al. Citation2013). In these studies, MSCs cultured in serum-free system presented a longer morphology compared to their counterparts cultured in FBS-containing medium. The reason for cell morphology difference may be a result of change of medium. Lange et al. reported that cell grown in Alpha Modification of Eagle Medium (αMEM) showed a flattened morphology compared to Dulbecco's Modified Eagle's medium (DMEM) or LP02. Their results further demonstrated that until senescence cells grown in LP02 + platelet lysate preserved their spindle-like morphology (Lange et al. Citation2007). A study reported that the mTeSR-expanded cells exhibited smaller morphology compared to FBS-expanded cells (Mimura et al. Citation2011). Our study supported the opinion that in vitro cultivation in a different system may affect MSCs morphology.
In conclusion, these results supported our hypothesis that rbMSCs can be expanded using established serum-free system for hMSCs. CELLstart and fibronectin promoted rbMSC attachment and survival, and bFGF significantly promoted rbMSC proliferation.
3.3. MSCs surface antigen and lineage transcription factor expression analysis
As proposed by the ISCT, a population of multipotent cells must possess a specific cell-surface antigen expression profile. According to the published reports, rbMSCs were positive for CD29, CD44, CD73, and CD90, and negative for CD34 and CD45. To determine whether expansion of cells in serum-free cultures was compatible with the maintenance of phenotypic properties, we analyzed the expression of surface marker antigens and lineage transcription factors for MSCs. Similar to previous reports in rabbit, murine, human, and canine MSCs, expanded rbMSCs in serum-free condition were positively stained by known MSC markers CD29, CD44, and CD73 at P1 or P2 (). qPCR analysis further verified a high-level expression for CD29 and CD44 in rbMSCs at P1 and P2 (). The expression of MSC markers CD29 and CD44 was similar in cells cultured in serum-free condition compared with cells cultured in FBS-containing condition (). And RT-PCR analysis demonstrated a negative expression for CD34 and CD166 in rbMSCs (). The rbMSCs are CD29 and CD44 positive, which is consistent with that reported in rodent (Nadri et al. Citation2007) and human (Mödder et al. Citation2012). CD34 is a hematopoietic stem cells marker. The negative expression of CD166 in rbMSCs is in accordance with previous report that CD166 has been described as an inconsistent marker (Cheng et al. Citation2012). Thus, our results demonstrated that rbMSCs obtained from serum-free condition are consistent with the criteria set for MSCs.
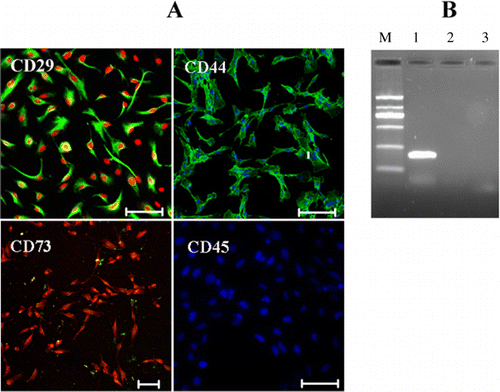
Table 2. Quantitative RT-PCR analysis of expanded rbMSCs in both serum-free and FBS containing conditions.
3.4. rbMSCs trilineage differentiation assay
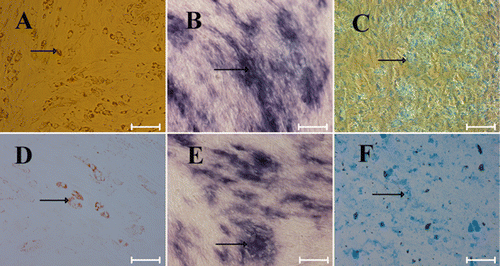
One key requirement for any stem cell expansion is that it should preserve its phenotypic characteristics and multipotentiality of the cells. Differentiation potential into adipogenic, osteogenic, and chondrogenic lineage is commonly used to demonstrate the multipotency of MSCs. RbMSCs expanded in serum-free condition were compared with those cultured in FBS-containing condition in their ability to differentiate into osteoblasts, chondrocytes, and adipocytes under conditions favorable for adipogenic, osteogenic, and chondrogenic differentiation for 10 days. Using the adipogenic differentiation assay, the presence of intracellular lipid droplets was clearly observed by Oil-red-O staining (). Although adipoctes were clearly detected, induction of adipogenic differentiation of cells grown in FBS-containing medium was more efficient compared to growth condition without FBS (, ). Our results is similar to a recent study in which adipogenic differentiation appears to be reduced when hMSCs are expanded using serum-free medium (Hudson et al. Citation2011) or platelet lysates (Lange et al. Citation2007). Osteogenesis of rbMSCs was detected by staining intracellular alkaline phosphatase in the osteogenic-induced rbMSC cultures (). Chondrogenic differentiation of rbMSCs was demonstrated by alcian blue staining (). Thus, our results indicated that rbMSCs obtained from serum-free condition retained their capacity to differentiate into the adipogenic, osteogenic, and chondrogenic lineages.
3.5. Gene expression quantification analysis of stemness and differentiation markers in rbMSCs under differentiation
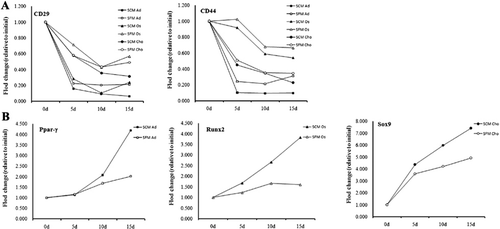
In order to track rbMSC differentiation, qPCR was utilized to analyze the expression levels of stemness (CD29 and CD44) and lineage markers (adipogenic marker Ppar-γ, osteogenic marker Runx2, and chondrogenic marker Sox9) in cells under differentiation. The expressions of the above-mentioned genes were analyzed on day 0, 5, 10, and 15 during rbMSC differentiation. The mRNA levels (CD29, CD44, Ppar-γ, Runx2, and Sox9) normalized to day 0 are illustrated in . In general, results from qPCR analyses were in line with the results from the staining assay. The mRNA expression levels of CD29 and CD44 were decreased during differentiation in a time-dependent manner and the lowest expression levels of CD29 and CD44 were observed on day 15 (). In addition, the mRNA expression levels of CD29 and CD44 were higher in cells cultured in serum-free condition as compared with those cultured in FBS-containing condition (). Consistent with the results of downregulation of CD29 and CD44 during differentiation, adipogenic marker Ppar-γ, osteogenic marker Runx2, and chondrogenic marker Sox9 were upregulated during rbMSC differentiation as expected (). Further, cells cultured in FBS-containing condition exhibited a higher degree of differentiation as compared with those cultured in serum-free condition ().
4. Conclusion
In summary, we have demonstrated that StemPro® MSC SFM in the presence of CELLstart and bFGF provides an alternative to FBS expansion of rbMSCs. Although rbMSCs cultured in serum-free condition did not exhibit completely unbiased properties compared with those cultured in FBS-condition, they exhibited spindle-shaped morphology, were positive for MSC surface markers, were negative for hematopoietic markers, and were capable to differentiate into adipogenic, osteogenic, and chondrogenic lineages, which meets the minimal criteria to define MSCs recommended by the ISCT. This work sets the stage for serum-free culture of rbMSCs, provides a platform for future modification to the formulation, and also provides a useful tool to understand the basic biological characteristics of rbMSCs.
Acknowledgment
This work was supported by research grants from Zhejiang Province Science and Technology Project of China [grant number 2013C33189 and grant number 2014C37030].
References
- Ahn HJ, Lee WJ, Kwack K, Kwon YD. 2009. FGF-2 sitmulates the proliferation of human mesenchymal stem cells through the transient activation of JNK signaling. FEBS Lett. 583:2922–2926. 10.1016/j.febslet.2009.07.056
- Amini AR, Laurencin CT, Nukavarapu SP. 2012. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res. 30:1507–1515. 10.1002/jor.22097
- Bakhtina A, Tohfafarosh M, Lichtler A, Arinzeh TL. 2014. Characterization and differentiation potential of rabbit mesenchymal stem cells for translational regenerative medicine. In Vitro Cell Dev Biol Anim. 50:251–260.
- Boo L, Selvaratnam L, Tai CC, Ahmad TS, Kamarul T. 2011. Expansion and preservation of multipotentiality of rabbit bone-marrow derived mesenchymal stem cells in dextran-based microcarrier spin culture. J Mater Sci Mater Med. 22:1343–1356. 10.1007/s10856-011-4294-7
- Chase LG, Lakshmipathy U, Solchaga LA, Rao MS, Vemuri MC. 2010. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 1:1–8. 10.1186/scrt8
- Cheng CC, Lian WS, Hsiao FS. 2012. Isolation and characterization of novel murine epiphysis derived mesenchymal stem cells. Plos One. 7:e36085. 10.1371/journal.pone.0036085
- Colter DC, Class R, Digirolamo CM, Prockop DJ. 2000. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 98:7841–7845. 10.1073/pnas.141221698
- Crapnell K, Blaesius R, Hastings A, Lennon DP, Caplan AI, Bruder SP. 2013. Growth, differentiation capacity, and function of mesenchymal stem cells expanded in serum-free medium developed via combinational screening. Exp Cell Res. 19:1409–1418. 10.1016/j.yexcr.2013.04.004
- Fekete N, Rojewski MT, Fürst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H. 2012. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSCs. Plos One. 7:e43255. 10.1371/journal.pone.0043255
- Fu WL, Zhang JY, Fu X, Duan XN, Leung KK, Jia ZQ, Wang WP, Zhou CY, Yu JK. 2012. Comparative study of the biological characteristics of mesenchymal stem cells from bone marrow and peripheral blood of rat. Tissue Eng Part A. 18:1793–1803. 10.1089/ten.tea.2011.0530
- Gupta R, Lee TQ. 2007. Contribution of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 16:S149–S157. 10.1016/j.jse.2007.05.002
- Hudson JE, Mills RJ, Firth JE, Brooke G, Jaramillo-Ferrada P, Wolvetang EJ, Cooper-White JJ. 2011. A defined medium and substrate for expansion of human mesenchymal stromal cell progenitors that enriches for osteo- and chondrogenic precursors. Stem Cells Dev. 20:77–87. 10.1089/scd.2009.0497
- Jung S, Sen A, Rosenberg L, Bechie LA. 2010. Identification of growth and attachment factors for the serum-free isolation and expansion of human mesenchymal stromal cells. Cytotherapy. 12:637–657. 10.3109/14653249.2010.495113
- Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. 2007. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 213:18–26. 10.1002/jcp.21081
- Lapi S, Nocchi F, Lamanna R, Passeri S, Iorio M, Paolicchi A, Urciuoli P, Coli A, Abramo F, Miragliotta V, et al. 2008. Different media and supplements modulate the lonogenic and expansion properties of rabbit bone marrow mesenchymal stem cells. BMC Res Notes. 28:1–7.
- Mapara M, Thomas BS, Bhat KM. 2012. Rabbit as an animal model for experimental research. Dent Res J. 9:111–118. 10.4103/1735-3327.92960
- Mark P, Kleinsorge M, Gaebel R, Lux CA, Toelk A, Pittermann E, David R, Steinhoff G, Ma N. 2013. Human mesenchymal stem cells display reduced expression of CD105 after culture in serum-free medium. Stem Cell Int. 2013:1–8; Article ID: 698076. doi:10.1155/2013.698076.
- Mimura S, Kimura N, Hirata M, Tateyama D, Hayashida M, Umezawa A, Nikawa H, Okamoto T, Furue MK. 2011. Growth factor-defined culture medium for human mesenchymal stem cells. Int J Dev Biol. 55:181–187. 10.1387/ijdb.103232sm
- Miwa H, Hashimoto Y, Tensho K, Wakitani S, Takagi M. 2012. Xeno-free proliferation of human bone marrow mesenchymal stem cells. Cytotechnology. 64:301–308. 10.1007/s10616-011-9400-7
- Mödder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG, Khosla S. 2012. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 50:804–810. 10.1016/j.bone.2011.12.014
- Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. 2007. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 51:723–729. 10.1387/ijdb.072352ns
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999. Multilineage potential of adult human mesenchymal stem cells. Sciences. 284:143–147. 10.1126/science.284.5411.143
- Ramasay M, Tong CK, Yip WK, Vellasamy S, Tan BC, Seow HF. 2012. Basic fibroblast growth factor modulates cell cycle of human umbilical cord-derived mesenchymal stem cells. Cell Prolif. 45:132–139. 10.1111/j.1365-2184.2012.00808.x
- Tan SL, Ahmad TS, Selvaratnam L, Kamarul T. 2013. Isolation, characterization and multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cell. J Anat. 222:437–450. 10.1111/joa.12032
- Wasel OG, Badria AF, Mohamed AA, Karei MK. 2013. Hepatogenic differentiation of rabbit BM-MSC using non-coated flask. Electron J Biol. 9:1–7.
- Yanni A. 2004. The laboratory rabbit: an animal model of atherosclerosis research. Lab Anim. 38:246–256. 10.1258/002367704323133628